ABSTRACT
Background
The present study was designed to investigate the inhibition role of two polyamine biosynthesis inhibitors, i.e., D-arginine (D-Arg) and DL-α-difluoromethylornithine (DFMO), in polyamine biosynthesis under chilling stress in different tissues of two maize inbred lines – Huang C (chilling-tolerance) and Mo17 (chilling-sensitive).
Results
The results showed that exposure to the lower concentration of polyamine biosynthesis inhibitors improved seedlings growth, such as the root length, root and shoot fresh weight, chlorophyll a (chl a). The effectiveness of 10 µM D-Arg treatments was more prominent than those of 10 µM DFMO. However, the higher concentration of inhibitors suppressed seedlings growth, and the exposure to 100 µM DFMO caused stronger decreases in the photosynthetic pigments, such as chlorophyll a (chl a), chlorophyll b (chl b), total chlorophyll and carotenoids, than the other treatments. Meanwhile, the inhibitor treatments caused the lower content of putrescine (Put) in roots, mesocotyls and coleoptiles in both maize inbred lines as compared with untreated plants. However, the lower concentration (10 µM) of polyamine biosynthetic inhibitors improved the Spd content, except 10 µM D-Arg in root of Huang C, and 10 µM DFMO in coleoptiles of both Mo17 and Huang C. The correlation analysis found that Spd was positively significantly correlated with root length and shoot fresh weight of seedling.
Conclusion
It was showed that the Spd played an important role in seedling growth improvement. At the same concentration of polyamine biosynthetic inhibitors, the Put contents in different tissues of the seedlings treated with DFMO were generally lower than those treated with D-Arg, except for Put contents in root of Mo17 with 10 µM treatment. Moreover, the treatments of 100 µM were more prominent than those of 10 µM treatments. Exposure to 100 µM D-Arg and 100 µM DFMO could each decrease the activities of Arginine decarboxylase (ADC), Ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (SAMDC) in all maize tissues. However, the decrease of the ADC activity was more prominent in 100 µM D-Arg-treated seedlings, while the decrease of SAMDC and ODC activities was prominent in 100 µM DFMO-treated seedlings. Genes involved in polyamine biosynthesis, such as ADC, ODC, SAMDC, and PAO, showed different expression patterns in response to chilling stress and polyamine biosynthesis inhibitors. This study suggested that Put was synthesized via both the ADC and ODC pathways after chilling stress, with the ODC pathway being the major one.
Introduction
Maize (Zea mays L.) is one of the most important field crops in the world. Its total world production ranks the third following wheat and rice, and it is a staple food in many countries, especially those in the tropics and sub-tropics.Citation1-3 Chilling is one of main abiotic stresses that limit the productivity and quality of maize.Citation4,Citation5 Previous studies showed that both tropical and subtropical maize species are sensitive to chilling stress.Citation6,Citation7 Low temperature, especially occurred during seed germination and early seedling growth, would severely inhibit seed germination, delay seedling growthCitation8-10 and result in the serious reduction of seed emergence and seedling establishment in the field.Citation11-13 Therefore, it is of great importance to study the physiological events during seed germination under chilling stress.
Plants activate their defense system in response to low temperature by altering gene expression, protein metabolism, metabolite profile and cell membrane lipid composition.Citation14,Citation15 Under chilling stress, plants accumulate polyamines (PA), such as Spd, spermine (Spm) and Put, which are considered as modulators of biological processes, including plant growth, development, and senescence.Citation6,Citation16 These polyamines exhibited a strong defense role under chilling stress, as reported earlier by Sheteiwy et al.Citation8 They each play a pivotal role in the regulation of plant developmentCitation17 and physiological processes.Citation18 Moreover, PA also has a function as stress messengers in plant responses to different stress signals.Citation19,Citation20 Their contents have been found to increase during chilling stress in several plants, such as maize,Citation21 cucumber,Citation22 riceCitation23 and chickpea.Citation24 Several studies have also reported that endogenous polyamines levels, especially Put content, increased in maize seedlings under chilling stress.Citation6,Citation21
The biosynthetic pathways of PA in higher plants have been studied in some researches.Citation6,Citation9,Citation25,Citation26 Put synthesis proceeds through either ADC via agmatine (Agm) or ODC. Spd is synthesized by spermidine synthase (SPDS) from Put with the addition of an amino propyl moiety, which is donated by decarboxylated S-adenosylmethionine (dcSAM) formed by SAMDC.Citation9 Spm is synthesized by Spermine synthase (SPMS) from Spd with the addition of an amino propyl, which is donated by dcSAM.Citation6,Citation9 It has been previously documented that ADC is more important than ODC in the production of Put in plants.Citation25,Citation26 Inhibitors of PA biosynthesis, such as D-Arg and DFMO have been found to inhibit main PA biosynthetic enzymes, such as ADC, ODC, and SAMDC.Citation27-30 As such, the activity of ADC can be inhibited by D-ArgCitation27 and ODC can be inhibited by DFMO.Citation28 Previously, a study of polyamine inhibitors on maize polyamines was reported, which mainly used calluses.Citation29,Citation30 Similarly, Torne et al.Citation31 found that DFMO pretreatment improved the regeneration ability of maize callus under normal temperatures, while it increased the membrane permeability under chilling stress. However, the negative effect of PA biosynthesis inhibitors could be diminished by adding Put.Citation32 Similarly, treatment with inhibitors of PA biosynthesis reduces the plant stress tolerance, whereas addition of exogenous polyamines restores successfully stress acclimation.Citation33-35 Therefore, PA are thought to play an essential role in the environmental stress tolerance of plants. Meanwhile, the specific or nonspecific inhibitors have been used to elucidate the role of PA in plant developments under abiotic stresses.Citation36-39 For example, DFMO at 10–1,000 µm concentrations significantly reduced the number of Panax ginseng somatic embryos,Citation37 and caused an 83% decrease in the embryogenic response.Citation38
In the present study, both chilling-tolerant and chilling-sensitive maize inbred lines are used to determine the polyamine contents in different tissues, i.e., roots, mesocotyls, and coleoptiles, in response to chilling stress. We attempted to elucidate the response of the biosynthetic pathway of PA in maize seedlings, as well as the differences among different tissues under chilling stress. The underlying mechanism of the response of PA biosynthetic pathway to chilling stress in maize is still not fully studied. Hence, further investigations are required to disclose the mechanisms of PA biosynthetic pathway in chilling-stressed maize plants.
Materials and methods
Plant materials and growth condition
Seeds of two maize inbred lines, Huang C (chilling-tolerance) and Mo 17 (chilling-sensitivity),Citation6 were used in the present study. The seeds were surface sterilized with 0.5% NaClO for 5 min.Citation40 The seeds were germinated on a wet paper towel in a plastic germination box (12 cm×18 cm) for 3 days in dark at 25°C (control temperature), in which 50 seeds for each treatment with three replications were used. Then, three days-old seedlings were uniformed and incubated for 1 day in different Hoagland solutions including the PA biosynthesis inhibitors (DFMO and D-Arg) at different concentrations, i.e., 0, 10, and 100 µM. After, the incubated seedlings grown in Hoagland solution with PA inhibitors and the controls plants (without PA inhibitors) were exposed to low temperature (5°C) for 3 days. During the entire growing duration in nutrition liquids, seedlings were grown under 12 h light/12 h dark (LD) cycles with 250 μmol·m−2·s−1 illumination. The physiological parameters such as root and shoot height, root, and shoot fresh weight, photosynthetic pigments, polyamine contents, polyamines biosynthetic and degrading enzymes and their expression levels of polyamines’ biosynthetic genes were determined at the end of low temperature treatment.
Seedling growth and photosynthetic pigment measurements
The shoot and root lengths of 10 randomly selected seedlings were manually measured and used for the fresh weight measurements.Citation41 The photosynthetic pigments including chl a, chl b, total chl. and carotenoids were measured according to the methods of Salah et al.Citation42 In brief, fresh leaves (0.2 g) were grinded thoroughly in liquid nitrogen using a pestle and mortar and homogenized with 10 mL of 95% ethanol. Then, the extract was centrifuged at 5,000 × g for 10 min. The obtained extract was diluted by adding 4 mL of 95% ethanol to 1 mL of extract, after which the mixture was determined by monitoring the absorbance using the spectrophotometer (752 (N) UV-Vis Spectrophotometer, China) at the wave lengths 470, 649, and 665 nm.
Polyamine contents of maize inbred lines under chilling stress
The polyamine contents in the root, mesocotyls, and coleoptiles and their transcript levels were measured immediately after the chilling stress treatment. Polyamine contents were measured according to the method of Gao et al.Citation6 Fresh tissue (0.1 g) was homogenized with 1 mL of 5% (w/v) cold HClO4, incubated in ice for 1 h, and centrifuged at 23,000 × g for 30 min at 4°C. Then, the supernatant was stored at −70°C for PA measurements. 10 mL of 2 M NaOH and 10 μL of benzoyl chloride were added to 0.5 mL of the obtained supernatant and the mixture was vortex-mixed vigorously and incubated in 37°C water for 20 min. Then, 2 mL of saturated NaCl solution and 2 mL of diethyl ether were added to the mixture. After 1,500 × g centrifugation for 5 min at 4°C, 1 mL of the ether phase was obtained and allowed to evaporate with a warm air-stream. The dried materials were dissolved in 100 μL methanol and its filtration through a 0.22 μm filter was subjected to reading using an HPLC, which included a 3.9 × 150 mm, 4 μm particle size reverse-phase (C18) column (Waters Nova-Pak) and a Waters 2487 dual λ absorbance detector. The mobile phases were consisted of methanol-water (64: 36, v/v) at a flow rate of 1 mL min−1. Three PA standard samples for Put, Spd and Spm were prepared at different contents for the development of standard curves.
Polyamines biosynthetic enzymes under chilling stress
The enzymes involved in PA biosynthesis were measured according to our previous study.Citation8 In brief, 0.5 g of root, mesocotyls, and coleoptiles (each) were ground at 4°C into fine powder and homogenized with 3 mL of extraction buffer (pH 8.0) containing 25 mM potassium phosphate, 50 μM EDTA, 100 µM phenylmethylsulphonyl fluoride, 1 mM 2-mercaptoethanol and 25 mM ascorbic acid.Citation34 The homogenate was then centrifuged at 5,000 × g for 20 min at 4°C and the supernatant dialyzed at 4°C against extraction buffer for 24 h in darkness. Dialyzed extract (50 ml) was used in the enzyme assay. The reaction buffers for ADC, ODC, and SAMDC assays were 0.1 ml of 200 mM Tris-HCl (pH 8.5) buffer, 0.1 ml of 200 mM Tris-HCl (pH 8.0) buffer and 0.1 ml of 200 mM potassium-phosphate (pH 7.5) buffer, respectively. The reaction was stopped by injecting 0.2 ml 10% (w:v) trichloroacetic acid with a syringe and then trapping for a further 60 min. Then, the activities of ADC, ODC, and SAMDC enzymes were determined by measuring CO2 evolution.Citation8 Spd synthase activity was measured according to the method of Kasukabe et al.Citation43
PA degrading enzymes under chilling stress
The degrading enzymes such as Polyamine oxidase (PAO) and Diamine oxidase (DAO) activity were determined according to the method of Sheteiwy et al.Citation8 The plant tissues (0.5 g) were homogenized in 0.5 mL of 0.2 M phosphate buffer (pH 6.5) and centrifuged at 8000 × g for 15 min at 4°C. The reaction was initiated by the addition of 0.2 mL of 20 mM of Spd for the determination of these degradative enzyme activities.
Expression levels of PA biosynthetic genes
In order to analyze the expression levels of PA biosynthetic genes, frozen tissues samples (200 mg) were ground thoroughly in liquid nitrogen using a mortar and pestle. Total RNA was isolated from the treated and untreated seedlings by using RNA isolation kit (Takara, Japan). The concentration of the RNA was determined using NanoDrop (Thermo Scientific, USA).Citation44-46 The RNA purity was checked spectrophotometrically by means of the 260/280 nm ratio. The primers of genes used in real-time PCR (RT-PCR) are shown in supplementary Table 1. cDNA was synthesized using Primer Script RT reagent Kit (Takara, Japan) from 1 µg of total RNA in a 20 µ L reaction, and diluted 4-fold with water.
Quantitative real-time RT-PCR was performed using SYBR premix EX Taq (Takara, Japan). ACT1 was used as an endogenous control gene to normalize expression of the other genes. The PCR program was as follows: 30 s at 95°C, followed by 40 cycles of 10 s at 95°C, 30 s at 60°C.
Statistical analysis
Treatments were arranged in factorial experiments in completely randomized design. All values described in Results section are mean of three replicates ± standard deviation (SD). All data were subjected to an analysis of variance (ANOVA). When a significant (P < .05) F ratio occurred for treatment effects, a least significant difference (LSD) was calculated. Before ANOVA, the data of percentage was transformed according to y = arcsin [sqrt (x/100)]. The correlation analysis was performed using SPSS v16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Physiological response of maize to PA inhibitors and chilling stress
The present study depicted that D-Arg at 10 µM and 100 µM concentrations significantly increased root length in Mo17 inbred line as compared with untreated plants (). Conversely, DFMO at 100 µM concentration significantly decreased the root length of Mo17, while there were no significant differences between 10 µM DFMO and the control. The root length of Huang C treated with 10 µM D-Arg was significantly higher than the control, 10 µM DFMO, and 100 µM DFMO-treated plants. For Mo17 and Huang C, there were no significant differences in the shoot height between both treated and untreated seedlings, except for seedlings treated with 100 µM DFMO (). The seedling shoot height treated with 100 µM D-Arg was significantly higher than those treated with10 µM DFMO, and 100 µM DFMO for two maize inbred lines ().
Figure 1. Effects of D-Arg and DFMO on root length (a), shoot height (b), root fresh weight (c), shoot fresh weight (d), chlorophyll a (e), chlorophyll b (f), total chlorophyll (g) and carotenoids (h) of two maize inbred lines under chilling stress
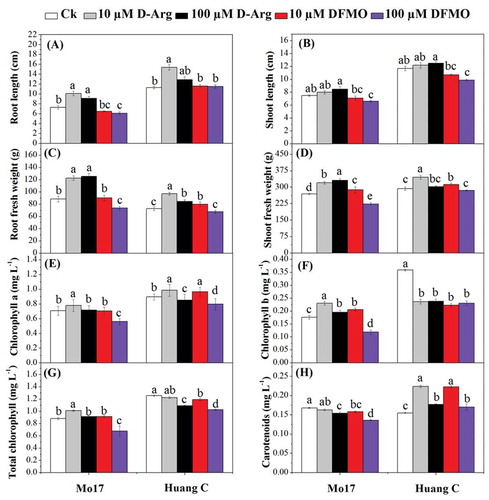
There was a significant increase in the root fresh weight of Mo17 treated with D-Arg as compared to the control. Moreover, 100 µM DFMO significantly reduced root fresh weight of both inbred lines as compared to 10 µM DFMO, 10 µM D-Arg and 100 µM D-Arg (). The root fresh weight of Huang C was not significantly affected by 100 µM DFMO (); while it was significantly improved by 10 µM D-Arg, 100 µM D-Arg and 10 µM DFMO as compared with the control plants. The similar results were also observed in Mo17, except the 100 µM DFMO significantly reduced the root fresh (). Additionally, both DFMO and D-Arg at 10 µM concentration significantly increased shoot fresh weight in Huang C as compared with the control plants.
Chl a content was significantly increased in Mo17 by 10 µM D-Arg and decreased by 100 µM DFMO (), while it was not significantly affected by 100 µM D-Arg and 10 µM DFMO. For Huang C, both D-Arg and DFMO at 10 µM concentration resulted in an increase of chl a content. On the contrary, it significantly decreased at both D-Arg and DFMO at higher concentration (100 µM) (). Chl b content of Mo17 was significantly increased by D-Arg at both 10 µM and 100 µM concentrations and 10 µM DFMO. In contrast, 100 µM DFMO significantly decreased chl b in Mo17 inbred line (). In addition, all inhibitors treatments significantly reduced the chl b content in Huang C as compared with control. The total chl contents were significantly increased in Mo17 by 10 µM D-Arg treatment and significantly decreased by 100 µM DFMO treatment. While the total chl content of Mo17 was not significantly affected by 100 µM D-Arg and 10 µM DFMO. Except 10 µM D-Arg, the total chl content of the other three treatments was significantly lower than those of untreated seedlings of Huang C (). The carotenoids content was significantly decreased by all inhibitors treatments except 10 µM D-Arg in Mo17 as compared with the control plants (). For Huang C, all polyamines inhibitors significantly increased the carotenoids content, and the carotenoids content of the seedling treated with both D-Arg and DFMO at 10 µM treatments was significantly higher than those of 100 µM treatments.
Polyamine content of maize in response to PA inhibitors and chilling stress
The contents of Put in the roots were significantly reduced by D-Arg and DFMO treatments in both maize inbred lines (). There were no significant differences in Put content in the roots of plants treated with 10 µM and 100 µM D-Arg, but the content of Put in the root of the plants treated with 100 µM DFMO were significantly lower than those in the plants treated with 10 µM DFMO. The Spd content in the roots of Mo17 inbred line was significantly increased by D-Arg and DFMO treatments (). However, for Huang C, only 10 µM DFMO significantly increased the Spd content in the roots. Additionally, both D-Arg and DFMO at 100 µM concentration resulted in a significant decrease in Spd content in the roots of Huang C. There were no significant effects of D-Arg and DFMO treatments on Spm content in the roots of Mo17 (). For Huang C, except 100 µM DFMO, the other three treatments significantly increased Spm content in the roots (). The total PA contents in the roots were decreased after exposure to D-Arg and DFMO inhibitors in Huang C, and the decrease was only significant upon exposure to 100 µM DFMO as compared to other three inhibitor treatments (). On the other hand, D-Arg improved the total PA content in the roots of Mo17, and this increase was only significant in the 10 µM D-Arg-treated seedlings. However, total PA content in the roots was significantly decreased with increasing concentration of both PA biosynthesis inhibitors in both maize inbred lines ().
Table 1. Effects of D-Arg and DFMO on polyamine contents (n mol/g FW) in the roots of two maize inbred lines under chilling stress
The data related with Put content in mesocotyls of two maize inbred lines after inhibitor treatments are presented in . The results showed that D-Arg and DFMO induced a significant decrease in Put content in the mesocotyls of both maize inbred lines as compared with untreated plants. Moreover, Put content with DFMO treatments was lower than those of D-Arg treatments at the same concentration. On the contrast, Spd content in the mesocotyls of Mo17 was significantly increased by D-Arg and DFMO treatments as compared to the untreated plants (). D-Arg and DFMO at 10 µM concentration significantly increased Spd content in the mesocotyls of Huang C as compared with untreated seedlings (), while the content of Spd in the mesocotyls of Huang C was significantly decreased by 100 µM DFMO. Except 10 µM DFMO, Spm content significantly increased in the mesocotyls of Mo17 after being treated with D-Arg and DFMO. For Huang C, inhibitor treatments improved the Spm content in the mesocotyls, which was significantly increased only with 100 µM DFMO (). Total PA content in mesocotyls was significantly decreased in Huang C inbred line after being treated with D-Arg and DFMO (). In addition, total PA content in the mesocotyls of Mo17 inbred line was significantly decreased when exposed to all inhibitor treatments, except 10 µM D-Arg.
Table 2. Effects of D-Arg and DFMO on polyamine contents (n mol/g FW) in the mesocotyls of two maize inbred lines under chilling stress
Put contents in coleoptiles of both maize inbred lines were similar to those in mesocotyls. Put content significantly decreased by D-Arg and DFMO treatments, but the decrease was more prominent in DFMO at the same concentration-treated (). The Spd content in the coleoptile of both maize inbred lines significantly increased by 10 µM D-Arg treatments, however, decreased by 100 µM DFMO treatments. There were no significant differences in Spd content of both maize inbred lines between 10 µM DFMO and the control seedlings (). In addition, Spm content in the coleoptiles of Mo17 was significantly decreased when the seedlings treated with 10 µM D-Arg and 100 µM DFMO (). Furthermore, D-Arg and DFMO resulted in a significant decrease of Spm and total polyamine content in the coleoptiles of Huang C. Except 10 µM D-Arg, the other three treatments significantly decreased total polyamine content in the coleoptiles of Mo17 ().
Table 3. Effects of D-Arg and DFMO on polyamine contents (n mol/g FW) in the coleoptiles of two maize inbred lines under chilling stress
Polyamine biosynthetic and degradative enzymes under chilling stress
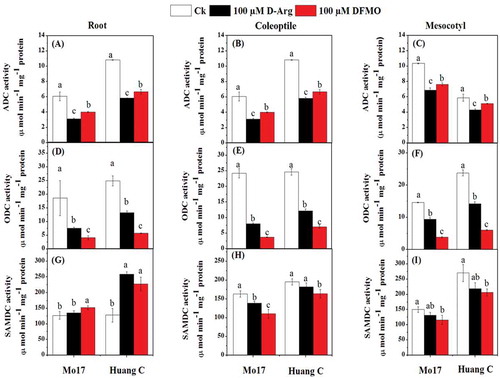
Under chilling stress, ADC and ODC activity was significantly decreased in the roots, coleoptiles, and mesocotyls of both Mo17 and Huang C inbred lines when treated with 100 µM D-Arg and 100 µM DFMO (). The decrease in ADC was more prominent in the seedlings those treated with 100 µM D-Arg as compared to the untreated seedlings and 100 µM DFMO (). On the contrary, plants treated with 100 µM DFMO showed a significant reduction of ODC content as compared to plants those treated with 100 µM D-Arg and plants without polyamines inhibitors treatments in both inbred lines under chilling stress (). Except 100 µM D-Arg treatment in Mo17 inbred lines roots, the activity of SAMDC was improved in the roots of both maize inbred lines when treated with 100 µM D-Arg and 100 µM DFMO as compared to the untreated seedlings under chilling stress (). Interestingly, the SAMDC activity in the coleoptiles and the mesocotyls of both inbred lines was significantly decreased in response to 100 µM DFMO as compared to the untreated seedlings under chilling stress ().
Figure 2. Effects of D-Arg and DFMO on ADC activity (a-c), ODC activity (d-f) and SAMDC activity (g-i) in the root, coleoptiles and mesocotyls of two maize inbred lines under chilling stress
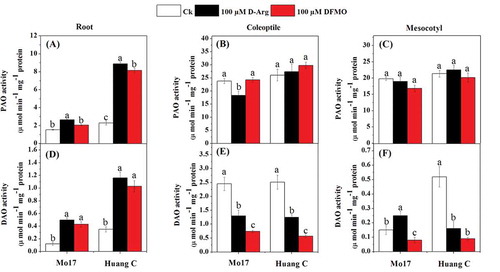
Except 100 µM DFMO treatment in Mo17 inbred lines roots, PAO and DAO activities were significantly increased in the roots of both Mo17 and Huang C inbred lines with 100 µM D-Arg and 100 µM DFMO under chilling stress (). The results showed that the PAO activity was significantly inhibited only in the coleoptiles of Mo17 inbred line upon treated with 100 µM D-Arg in under chilling stress, however, there were no differences among all polyamines inhibitors in PAO activity in the coleoptiles of Huang C or in mesocotyls of both inbred lines (). The DAO activity was significantly inhibited in the coleoptiles and mesocotyls of both maize inbred lines when treated with 100 µM D-Arg and 100 µM DFMO, except that the DAO activity in the mesocotyls of Mo17 was significantly increased by 100 µM D-Arg treatment ().
Polyamine biosynthetic genes in response to PA biosynthesis inhibitors under chilling stress
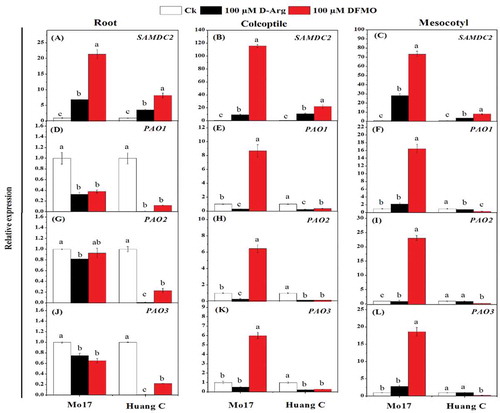
The mean data of the gene expression related to the PA biosynthesis in response to chilling stress and inhibitors are shown in . The transcript level of ADC1 was down-regulated in the root of both maize inbred lines treated with 100 µM D-Arg and 100 µM DFMO under chilling stress (). In comparison, treatment with 100 µM D-Arg resulted in up-regulation of ADC1 in the coleoptile and mesocotyls in Mo17 inbred line, but ADC1 expression was down-regulated in Huang C inbred line when treated with 100 µM DFMO under chilling stress (). Interestingly, the same trend was observed for ADC2 in roots, coleoptiles, and mesocotyls of both inbred lines upon treatment with 100 µM D-Arg and 100 µM DFMO under chilling stress (). ODC expression was inhibited in the roots of both inbred lines when treated with 100 µM D-Arg and 100 µM DFMO under chilling stress (). Similar expression pattern of ODC was observed in the coleoptiles and mesocotyls of Huang C inbred line (). But for Mo17 inbred line, the expression of ODC in coleoptiles and mesocotyls was significantly up-regulated with 100 µM DFMO as compared to 100 µM D-Arg and the untreated seedlings ().
Figure 4. Effects of D-Arg and DFMO on the relative expression of ADC1 (a-c), ADC2 (d-f), ODC (g-i) and SPDS (j-l) in the root, coleoptiles and mesocotyls of two maize inbred lines under chilling stress
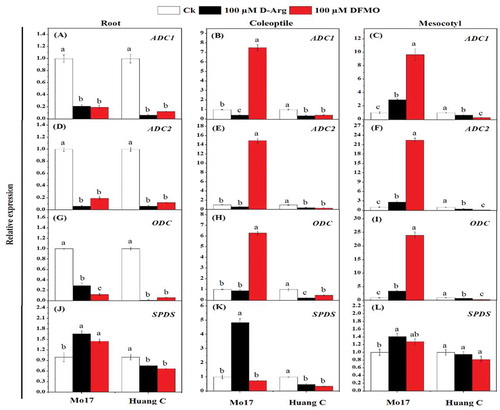
The SPDS expression was significantly down-regulated in the roots and coleoptiles of the Huang C when treated with 100 µM D-Arg and 100 µM DFMO under chilling stress (). On the other hand, the expression level of SPDS in the roots, coleoptiles, and mesocotyls of Mo17 inbred line was significantly up-regulated upon treatment with 100 µM D-Arg under chilling stress ().
SAMDC2 expression was significantly up-regulated in roots, coleoptiles, and mesocotyls of both inbred lines with 100 µM D-Arg and 100 µM DFMO under chilling stress (). PAO1 expression was significantly down-regulated in roots, coleoptiles, and mesocotyls of both inbred lines when treated with inhibitors under chilling stress, except that PAO1 expression in the coleoptiles and mesocotyls of Mo17 was significantly up-regulated with 100 µM DFMO (). Except the expression of PAO2 with 100 µM DFMO treatment in Mo17 inbred lines roots, the similar trend was observed for the expression of PAO2 and PAO3 in the roots, coleoptiles, and mesocotyls of both inbred lines exposed to the inhibitors under chilling stress ().
Correlation between maize seedling growth indexes and polyamines pathway physiological parameters under chilling stress
The correlation analysis between the maize seedling growth indexes, polyamines pathway and physiological parameters under chilling stress are presented in . The correlation analysis showed that Spd was positive significantly correlated with root length and shoot fresh weight in the seedling treated with DFMO and D-Arg both at 100 µM concentration (). While Spm was only positive significantly correlated with root length in the seedlings treated with 100 µM of DFMO and D-Arg. On the other hand, the correlation analysis showed a different pattern for the enzymes involved in both polyamines synthesis and polyamines degradation and their transcript levels in the seedling treated with DFMO and D-Arg at 100 µM concentration ().
Table 4. Correlation analysis between maize seedling growth indexes, polyamines pathway and physiological parameters under chilling stress
Discussion
PA acted as secondary metabolites, participating in the local allergic reaction of plants against abiotic stress,Citation47 and also in plant morphogenesis.Citation48-50 Nowadays, the exogenous PA, PA synthesis inhibitors and even transgenic methods have been used to intensively investigate the role of PA in plant development and their mechanism of action.Citation51 D-Arg is the most important PA biosynthesis inhibitors which were shown to promote root length, root, and shoot fresh weight, but these effects occurred in a dose-dependent manner. A recent study reported that applying PA inhibitors to the growth medium reduced the Spd content in Arabidopsis, and inhibited bolting and flowering.Citation51 Another study also reported that treatment with low concentration of D-Arg reduced the chilling damage.Citation52 In the current study, D-Arg at a lower concentration (10 µM) positively improved plant growth higher than those caused by DFMO at the same concentration (). The increase of maize seedling growth by D-Arg might be due to the inhibition of ethylene and the improvement of the zeatin, zeatin-riboside and ABA hormones as a result of increasing Spd and Spm and reduction of Put content as reported previously by Liu et al.Citation53 These findings also are consistent with those observed in oat (Avena sativa L.) leavesCitation54 and chickpeas (Cicer arietinum L.).Citation55 Another study reported that Put was positively correlated to the expression levels of genes regulating ABA biosynthesis but down-regulated those of ethylene, jasmonates, and gibberellin biosynthesis, and the action of spermidine were found to be exactly opposite.Citation56 A similar observation was reported by Bais and Sudha GravishankarCitation39 who found that the addition of DFMA + DFMO (1 mM each) resulted in a minimum tissue response in terms of shoot multiplication and length of shoots. These results are also consistent with those obtained by Tiburcio et al.Citation57 and Bharti Rajam.Citation58 The effectiveness of D-Arg comparing to DFMO might be due to the capability of D-Arg to reduce PAO activity and thus can reduce the H2O2 accumulation in the plants organs under chilling stress.Citation59 As previously reported, PA are a source of reactive oxygen species due to their catabolism produces the strong oxidizers H2O2 and acrolein, and thus can potentially be the cause of cellular harm under stress conditions.Citation60 However, H2O2 can play as a signaling molecule that can enter the stress signal transduction chain and activate an antioxidant defense response to the abiotic stress.Citation61 Thus, it seems that PA is regulators of redox homeostasis that play a dual role in plant oxidative stress.Citation62 Similar reports also stated that PAOs catalyze the production of metabolic end-products of PA to H2O2 in wheat plants.Citation63,Citation64 In the current study, higher values of chlorophyll-b, and carotenoids were observed in untreated seedlings of Huang C than those of Mo17, but higher chlorophyll-a and total chlorophyll were observed in the Huang C plants treated with 10 µM D-Arg. A previous study reported that there was a positive correlation between the PA accumulation and chlorophyll content.Citation65 The enhanced chlorophyll content might be related to increased reactive oxygen metabolism and photosynthesis due to the PA accumulation, which improved plant growth and reduced the inhibitory effects of abiotic stress.Citation66,Citation67 The effective role of PA biosynthesis inhibitors at low concentration (10 µM) on chlorophyll production under cold stress might be related to the original content of chlorophyll in the two maize inbred lines.Citation65 In the present study, D-Arg at 10 µM concentration improved chlorophyll-b and carotenoid content of Mo17 inbred line, but decreased them in Huang C inbred line (). Bharti and RajamCitation58 reported that DFMO at 5 mM had no significant effects on the wheat growth, chlorophyll content, PA level in the cell. However, the DFMO at high concentration (10 mM) reduced the seedling growth and PA levels.
In the present study, the Put levels were significantly decreased by D-Arg and DMFO in the different tissues of both maize inbred lines, and both inhibitors caused a sharp inhibition at 100 µM as compared with 10 µM concentration (, and ). This study indicated that the inhibition of Put biosynthesis under both PA inhibitors might be due to the inhibition of ADC and ODC pathways by the application of D-Arg and DMFO under chilling stress. The inhibition of Put biosynthesis may contribute to increase antioxidant enzyme activity, enhance ROS scavenging ability, and reduce membrane lipid peroxidation which ultimately increased the plants stress tolerance.Citation68,Citation69 Our findings are consistent with a previous study reporting that several PA inhibitors have the function to repress different PA biosynthetic enzymes such asADC and ODC, thereby inhibiting endogenous PA synthesis and ultimately affect the role of PA in stress tolerance.Citation36 Another study has also reported that D-Arg an inhibitor of ADC, was shown to be effective in reducing Put synthesis and its application to apple callus reduce their tolerance to salt stress; and this effect was diminished upon exogenous Put was applied, suggesting a role for Put in combating salt stress.Citation36
DFMO decreased Put percentages in all maize tissues as compared with the same concentration of D-Arg treatment (, , and ). These results reported that the biosynthesis of Put might be mainly through ODC pathway for both maize inbred lines under chilling stress. These results are in accordance with those obtained by HiattCitation65 who reported that free Put content was mainly synthesized by ODC in maize callus tissue. However, Hummel et al.Citation27 stated that the pathway of Put biosynthesis was mainly through ADC in Kerguelen cabbage (Pringlea antiscorbutica) seedlings under low temperature stress. In addition, Lee et al.Citation70 and LeeCitation71 indicated that Put biosynthesis pathways in rice seedlings were mainly through ADC and ODC enzymes under chilling stress. This evidence suggested that the Put biosynthetic pathway is intensively dependent on the plant species.
Compared with other tissues of maize seedlings, our study found that the roots of the chilling-sensitive Mo17 inbred line had increased percentages of Put content when treated with DFMO at 10 µM concentration. These findings explored that the inhibitory effects of DFMO on PA biosynthesis varied considerably according to the intrinsic genetic differences of maize species and the sensitivity of different seedling tissues to low temperatures. These findings are consistent with those obtained by Moschou et al.Citation72 who revealed that PA biosynthesis may vary between tissues/organs, reporting that the shoot apical meristem of tobacco serves as the predominant site of Spd and Spm synthesis, while Put was mostly synthesized in roots. Moreover, as the Put biosynthesis was inhibited by D-Arg and DMFO at 10 µM concentration, Spd, and Spm contents in the roots were increased correspondingly (). Hence, this study explored that the lower ADC and ODC activity levels caused by D-Arg and DFMO might promote the activities of SAMDC by a feedback mechanism. Among the PA tested in the different tissues of both inbred lines, Spm content was the lowest. In this respect, different types of PA also show different localization patterns within cells. As such, Put was found to accumulate in the cytoplasm and Spm in the cell wall in carrot.Citation73 The distribution patterns of PA may be related to their unique functions.Citation51 These findings are consistent with those obtained by Nemeth et al.Citation21 who reported that the Spm content was decreased after low temperature treatments comparing to other PA contents. Furthermore, Shen et al.Citation22 considered the contribution of Spm to the chilling tolerance of cucumber to be much lower than Put and Spd. Thus, the function of Spm in the chilling tolerance of maize seedlings still needs further study.
A previous study has reported that ADC, ODC and SAMDC are the key enzymes in polyamine metabolism of plants under the chilling stress.Citation74 In addition, ADC has been considered to be the enzyme responsible for the abiotic stress tolerance via accumulation of Put in plants.Citation8 In the present study, ADC was significantly decreased in different tissues of both maize inbred lines in response to the higher concentrations of two inhibitors under chilling stress (). These results are consistent with the PA contents in roots, coleoptiles, and mesocotyls of both inbred lines (). Meanwhile, the current study suggested that PA, especially Spd, has a positive correlation with the physiological parameters of maize under the chilling stress (). The positive correlation might be due to the ODC, ADC, and SPDS enzymes are responsible for the expansion of root tip and hypocotyle cells.Citation75 Moreover, many studies have reported that genetic transformation with polyamine biosynthetic genes encoding ADC, ODC, SAMDC, and SPDS improved environmental stress tolerance in various plant species.Citation20 Also Duan et al.Citation74 reported that Put can be synthesized directly by ADC via agmatine and N-carbamoylputrescine intermediates under the stress condition. Our results showed that PA biosynthesis inhibitors treatment inhibited the activities of both ADC and ODC under the chilling stress (). These results are supported by the findings of Bouchereau et al.Citation76 who reported that the accumulation of PA contents and polyamine biosynthetic enzymes, mainly ADC, ODC, and SAMDC, has been observed under different salt-stressed cultivars. In the present study, the PA biosynthesis inhibitors significantly reduced the DAO activities of coleoptile and mesocotyl of both maize inbred line in response to chilling stress (), and reduced PAO activity in mesocotyl of Mo 17 inbred line. The current findings showed that the relative expression of PAO1, PAO2, and PAO3, the main genes encoding the PA degradative enzymes were down-regulated in the root when the plants treated with PA biosynthesis, however, it was up-regulated in the coleoptile and mesocotyl under chilling stress. These findings the relative expression of PAO and DAO showed different patterns in the different tissues of maize exposed to both PA biosynthesis and chilling stress. These findings are consistent with a previous study reporting that the relative expression of genes involved in the PA degrading enzymes, such as OsPAO3 and OsPAO4, was differentially altered under the chilling stress and PA biosynthesis inhibitors.Citation8 Another study reported that the accumulation of different organic compounds including polyamines and their related gene expression was part of the plant signaling and defense system against salinity stress.Citation77,Citation78
Conclusions
Based on the obtained results, we concluded that higher concentration PA biosynthetic inhibitors inhibited the growth of both maize inbred lines under chilling stress, and the inhibition was more accentuated when treated with 100 µM DFMO. The growth inhibition might be related to the decreased Put accumulation in the different tissues of maize plants treated with DFMO. Treated maize inbred line with lower concentration inhibitor positively improved maize seedling growth under chilling stress, which might be related to the higher accumulation of Spd in PA biosynthetic inhibitors treated seedling as compared with untreated plants. In addition, the activities of ADC and ODC enzymes were inhibited in all maize tissues when the seedlings were exposed to 100 µM D-Arg and 100 µM DFMO. Moreover, DFMO decreased Put percentages in all maize tissues as compared with the same concentration of D-Arg treatment. The decrease of the activity of ADC enzyme was more prominent in 100 µM D-Arg-treated seedlings, while the decrease of ODC and SAMDC activities was prominent in 100 µM DFMO-treated seedlings. These results showed that the biosynthesis of Put might be mainly through ODC pathway for both maize inbred lines under chilling stress. However, considering that D-Arg is a competitive and reversible inhibitor of ADC, and DFMO is an irreversible inhibitor of ODC, the observed effect on Put biosynthesis might also be due to the nature of the inhibitors, so it still needed to be further studied. Finally, the relative expression of genes involved in PA biosynthesis, such as ADC, ODC, SAMDC, and PAO, showed different patterns in response to chilling stress and PA biosynthesis inhibitors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (18.1 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Mohammadi NK, Pankhaniya RM, MP J, Patel KM. Influence of inorganic fertilizer, vermicompost and biofertilizer on yield & economic of sweet corn and nutrient status in soil. Int J Applied Res. 2017;3:1–10.
- Gao C, El-Sawah AM, Ali DFI, Alhaj Hamoud Y, Shaghaleh H, Sheteiwy MS. The Integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agronomy. 2020;10:319.
- Abdoulaye AO, Lu H, Zhu Y, Alhaj Hamoud Y, Sheteiwy M. The global trend of the net irrigation water requirement of maize from 1960 to 2050. Climate. 2019;7:124.
- Li Z, Xu J, Gao Y, Wang C, Guo G, Luo Y, Huang Y, Hu W, Sheteiwy MS, Guan Y, et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during Maize (Zea mays L.) seed germination. Front Plant Sci. 2017;8:1153.
- He F, Shen H, Lin C, Fu H, Sheteiwy MS, Guan Y, Huang Y, Hu J. Transcriptome analysis of chilling-imbibed embryo revealed membrane recovery related genes in maize. Front Plant Sci. 2017;7:1978.
- Gao CH, Hu J, Zhang S, Zheng YY, Knapp A. Association of polyamines in governing the chilling sensitivity of maize genotypes. Plant Growth Regul. 2009;57:31–38.
- Hola D, Kocova M, Rothova O, Wilhelmova N, Benesova M. Recovery of maize (Zea mays L.) inbreds and hybrids from chilling stress of various duration: photosynthesis and antioxidant enzymes. J Plant Physiol. 2007;164:868–877.
- Sheteiwy M, Shen H, Xu J, Guan Y, Song W, Hu J. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ Exp Bot. 2017a;137:58–72.
- Gill SS, Tuteja N. Polyamines and abiotic stress tolerance in plants. Plant Signaling Behavior. 2010;5:26–33.
- Allen DJ, Ort DR. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001;6:36–42.
- Farooq M, Aziz T, Basra SMA, Wahid A, Khaliq A, Cheema MA. Exploring the role of calcium to improve chilling tolerance in hybrid maize. J Agron Crop Sci. 2008;194:350–359.
- Guan YJ, Hu J, Wang XJ, Shao CX. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B. 2009;10:427–433.
- Imran S, Afzal I, Basra SMA, Saqib M. Integrated seed priming with growth promoting substances enhances germination and seedling vigour of spring maize at low temperature. Int J Agric Biol. 2013;15:1251–1257.
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000;124:1854–1865.
- Shinozaki K, Dennis ES. Cell signalling and gene regulation: global analyses of signal transduction and gene expression profiles. Curr Opin Plant Biol. 2003;6:405–409.
- Koetje DS, Kononowicz H, Hodges TK. Polyamines metabolism associated with growth and embryogenic potential of rice. J Plant Physiol. 1993;141:215–221.
- Cao DD, Hu J, Zhu SJ, Hu WM, Knapp A. Relationship between changes in endogenous polyamines and seed quality during development of sh2 sweet corn (Zea mays L.) seed. Sci Horticult. 2010;123:301–307.
- Kusano T, Yamaguchi K, Berberich T, Takahashi Y. Advances in polyamine research. J Plant Res. 2007;120:345–350.
- Zhang W, Jiang B, Li W, Song H, Yu Y, Chen J. Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Sci Hort. 2009;122:200–208.
- Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007;24:117–126.
- Nemeth M, Janda T, Horva´th E, Pa´ldi E, Szalai G. Exogenous salicylic acid increases polyamines content but may decrease drought tolerance in maize. Plant Sci. 2002;162:569–574.
- Shen WY, Nada K, Tachibana S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000;124:431–439.
- Lee TM, Huu SL, Chu C. Absisic acid and putrescine accumulation in chilling-tolerant rice cultivars. Crop Sci. 1995;35:502–508.
- Nayyar H, Chander S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci. 2004;190:355–365.
- Schneider J, Wendisch VF. Putrescine production by engineered Corynebacterium glutamicum. Application Microbiol Biotech. 2010;88:859–868.
- Montague MJ, Armstrong TA, Jaworski EG. Polyamine metabolism in embryogenic cells of Daucuscarota. II. Changes in ADC activity. Plant Physiol. 1979;63:341–345.
- Hummel I, Couee I, Amrani AE, Martin-Tanguy J, Hennion F. Involvement of polyamines in root development at low temperature in the subantarctic cruciferous speciecs Pringlea antiscorbutica. J Exp Bot. 2002;53:1463–1473.
- Wallace HM, Fraser AV. Inhibitors of polyamine metabolism: review article. Amino Acids. 2004;26:353–365.
- Guergue A, Claparols I, Santos M, Torne JM. Modulator effect of DL-α-difluoromethylarginine treatments on differentiation processes of young maize calluses. Plant Growth Regul. 1997;21:7–14.
- Bernet E, Claparols I, Santos MA, Torne JM. Role of putrescine metabolic pathways in the differentiation process of maize meristematic callus. Plant Physiol Biochem. 1998;36:759–766.
- Torne JM, Claparols I, Figueras X, Santos M. Effect of DL-Alpha-Difluoromethylornithine pretreatments in maize callus differentiation. Plant Cell Physiol. 1993;34:371–374.
- Songstad DD, Duncan DR, Widholm JM. Proline and polyamine involvement in chilling tolerance of maize suspension cultures. J Exp Bot. 1990;41:289–294.
- He L, Nada K, Kasukabe Y, Tachibana S. Enhanced susceptibility of photosynthesis to lowtemperature photoinhibition due to interruption of chillinduced increase of S-adenosylmethionine decarboxylase activity in leaves of spinach (Spinacia oleracea L.). Plant Cell Physiol. 2002;43:196–206.
- Lee TM, Lur HS, Chu C. Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings. Modulation of free polyamine levels. Plant Sci. 1997;126:1–10.
- Liu HP, Dong BH, Zhang YY, Liu ZP, Liu YL. Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Sci. 2004;166:1261–1267.
- Liu JH, Wang W, Wu H, Gong X, Moriguchi T. Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci. 2015;6:827.
- Kevers C, Gal NL, Monteiro M, Al E. Somatic embryogenesis of Panax ginseng in liquid cultures: a role for polyamines and their metabolic pathways. Plant Growth Regul. 2000;31:209–214.
- Kumar V, Giridhar P, Chandrashekar A, Al E. Polyamines influence morphogenesis and caffeine biosynthesis in vitro cultures of Coffea canephora P. Acta Physiol Plant. 2008;30:217–223.
- Bais HP, Sudha GGA. Influence of putrescine, silver nitrate and polyamine inhibitors on the morphogenetic response in untransformed and transformed tissues of Cichorium intybus and their regenerants. Plant Cell Rep. 2001;20:547–555.
- Sheteiwy MS, Gong D, Gao Y, Pan R, Hu J. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ Exp Bot. 2018a;153:236–248.
- Sheteiwy MS, Dong Q, An J, Song W, Guan Y, He F, Huang Y, Hu J. Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul. 2017b;1–15.
- Salah MS, Guan Y, Cao D, Li J, Nawaz A, Hu Q, Hu W, Ning M, Hu J. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep. 2015;5:14278.
- Kasukabe Y, He LX, Nada K, Misawa S, Ihara I, Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004;45:712–722.
- Li G, Zhang H, Shao H, Wang G, Zhang Y, Zhang Y, Zhao L, Guo X, Sheteiwy M. ZmHsf05, a new heat shock transcription factor from Zea mays L. improves thermotolerance in Arabidopsis thaliana and rescues thermotolerance defects of the athsfa2 mutant. Plant Sci. 2019;283:375–384.
- Sheteiwy MS, Fu Y, Hu Q, Nawaz A, Guan Y, Li Z, Huang Y, Hu J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ Sci Pollut Res. 2016;1–14.
- Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int J Mol Sci. 2019;20:5709.
- Kumar A, Taylor M, Altabella T, Al E. Recent advances in polyamine research. Trends Plant Sci. 1997;2:124–130.
- de Oliveira LF, Elbl P, Navarro BV, Al E. Elucidation of the polyamine biosynthesis pathway during Brazilian pine (Araucaria angustifolia) seed development. Tree Physiol. 2016;37:116–130.
- De Oliveira LF, Navarro BV, Cerruti G, Al E. Polyamines and amino acid related metabolism: the roles of arginine and ornithine are associated with the embryogenic potential. Plant Cell Physiol. 2018;59:1084–1098.
- Mustafavi SH, Badi HN, Sekara A, Al E. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant. 2018;40:102.
- Chen D, Shao Q, Yin L, Younis A, Zheng B. Polyamine function in plants: metabolism, regulation on development and roles in abiotic stress responses. Front Plant Sci. 2019;9:1945.
- Sun X, Wang Y, Tan J, Al E. Effects of exogenous putrescine and D-Arg on physiological and biochemical indices of anthurium under chilling stress. Jiangsu J Agric Sci. 2018;34:152–157.
- Liu Y, Gu D, Wu W, Al E. The relationship between polyamines and hormones in the regulation of wheat grain filling. PLoS ONE. 2013;8:e78196.
- Fuhrer J, Kaur-Sawhney R, Shih LM, Galston AW. Effects of exogenous 1, 3-diaminopropane and spermidine on senescence of oat leaves. Plant Physiol. 1982;70:1597–1600.
- Bueno M, Matilla A. Abscisic-acid increases the content of free polyamines and delays mitotic-activity induced by spermine in isolated embryonic axes of chickpea seeds. Physiol Plant. 1992;85:531–536.
- Anwar R, Mattoo AK, Handa AK. Polyamine interactions with plant hormones: crosstalk at several levels. In: Kusano T, Suzuki H, editors. Polyamines. 2015. p. 1–37.
- Tiburcio AF, Kaur-sawhney R, Galston AW. Effect of polyamine biosynthesis inhibitors on alkaloids and organogenesis in tobacco callus cultures. Plant Cell Tissue Organ Cult. 1987;9:111–120.
- Bharti RMV. Effects of the polyamine biosynthesis inhibitor difluoromethylornithine on growth, polyamine levels, chromosome behaviour and polygenic traits in Wheat (Triticum aestivum L.). Annals Bot. 1995;76:297–301.
- Xu L, Xing ST, Sun X. Effects of polyamines on hormones contents and the relationship with the flower bud differentiation in chrysanthemum. Plant Physiol J. 2014b;50:1195–1202.
- Minocha R, Majumdar R, Minocha SC. Polyamines and abiotic stress in plants: a complex relationship. Front Plant Sci. 2014;5:175.
- Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34:35–45.
- Saha J, Brauer EK, Sengupta A, Al E. Polyamines as redox homeostasis regulators during salt stress in plants. Front Environ Sci. 2015;3:21.
- Cona A, Rea G, Angelini R, Al E. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;11:80–88.
- Liu T, Kim DW, Niitsu M, Al E. Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 2014;55:1110–1122.
- Hiatt A. Polyamine synthesis in maize cell lines. Plant Physiol. 1989;90:1378–1381.
- Meng D, Hou L, Yang S. Exogenous polyamines alleviating salt stress on peanuts (Arachis hypogaea) grown in pots. Chin J Plant Ecol. 2015;39:1209–1215.
- Baniasadi F, Saffari VR, Moud AAM. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci Hort. 2018;234:312–317.
- Jia Y, Guo S, Li J. Effects of exogenous putrescine on polyamines and antioxidant system in cucumber seedlings under root-zone hypoxia Stress. Acta Bot Boreali Occidentalia Sinica. 2008;28:1654–1662.
- Wu J, Shu S, Li C, Sun J, Guo S. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol Biochem. 2018;128:152–162.
- Lee TM, Lur HS, Lin YH, Chu C. Physiological and biochemical changes related to methyl jasmonate-induced chilling tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ. 1996;19:65–74.
- Lee TM. Polyamine regulation of growth and chilling tolerance of rice (Oryza sativa L.) roots cultured in vitro. Plant Sci. 1997;122:111–117.
- Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell. 2008;20:1708–1724.
- Cai Q, Zhang J, Guo C, Al E. Reviews of the physiological roles and molecular biology of polyamines in higher plants. J Fujian Educ Coll. 2006;7:118–124.
- Duan J, Li J, Guo S, Kang Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physio. 2008;165:1620–1635.
- Delis C, Dimou M, Efrose RC, Flemetakis E, Aivalakis G, Katinakis P. Ornithine decarboxylase and arginine decarboxylase gene transcripts are co-localized in developing tissues of Glycine max etiolated seedlings. Plant Physiol Biochem. 2005;43:19–25.
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140:103–125.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–499.
- Sairam RK, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–421.