ABSTRACT
Nitrate transporter 2.5 (NRT2.5) was originally characterized as the transporter for nitrogen (N) limitation. In Arabidopsis, NRT2.5 is expressed mainly under extremely low NO3− and N starvation conditions, and this must work in conjunction with NAR2.1. NRT2.5 is expressed both in the roots and leaves in Arabidopsis, poplars, tea trees and cassava. This is also expressed in the seeds of Arabidopsis and wheat. In wheat, NRT2.5 is expressed in the embryo and shell and plays a role in the accumulation of NO3− in the seeds. In maize, this is also expressed in silk, cobs and tassel husk leaves. In rice, OsNRT2.5 (also known as OsNRT2.3a) may help the species to remove NO3− from the roots to shoots. In addition, NRT2.5 may interact with TGA3, MYC1, LBD37, LBD38, TaNAC2 and other transcription factors and participate in the transmission of NO3− signals. The present review summarizes the functions of NRT2.5 in different plant species, which may help plant breeders and molecular biologists to improve crop yield.
Abbreviations: NRT, Nitrate transporter; NUE, nitrogen use efficiency; PTR, peptide transporter; NPF, nitrate peptide transporter family; CLC, chloride channel; LAC1/SLAH, slow anion channel-associated 1 homolog 3; LATS, low-affinity transporter systems; HATS, high-affinity transport systems; NNP, nitrate-nitrite-porter; MFS, major facilitator superfamily.
Introduction
Nitrogen (N) is the main component of proteins, nucleic acids and phospholipids, and these substances are important components of protoplasts, cell nuclei and biofilms. Therefore, N plays an extremely important role in life activities and is called the element of life. N affects physiological metabolic processes, such as photosynthesis, respiration and antioxidant system in plants.Citation1
N can be available to plant roots in several different forms, including nitrate (NO3−), ammonium (NH4+) and organic molecules such as amino acids. NO3− is one of the most important N sources for plants. Therefore, investigating the molecular mechanisms of NO3− utilization is critical for plants,Citation2 and this also provides a vital theoretical basis for the improvement of nitrogen use efficiency (NUE) in crops, thereby contributing to sustainable agriculture.
The concentration of NO3− in soils might extremely change from 10 µM in natural soils to 100 mM in fertilized soils.Citation3 Plants have a sophisticated network of membrane transporters for the sensing, absorption, storage and distribution of NO3− inside the plant organism. It has been reported that NO3− transporters belong to four families, that is, NRT1/PTR/NPF (nitrate transporter 1/peptide transporter/nitrate peptide transporter family), NRT2, chloride channel (CLC) and slow anion channel-associated 1 homolog 3 (SLAC1/SLAH).Citation4 NO3− is absorbed from the soil by plant roots and transported to the shoots, and reused between organs, tissues and cells. These processes are mediated by NRT1 and NRT2, coupled with proton (H+) transport, and subjected to the plasma membrane H+-ATPase-regulated active transport process.Citation5,Citation6
According to the difference in NO3− concentration in the soil, plants have evolved two types of NO3− transport systems, namely, low-affinity transporter system (LATS) and high-affinity transport system (HATS). LATS is activated when the external NO3− content is high, while HATS is activated when the external NO3− content is low.Citation6 There are different opinions about the critical point of the NO3− absorption mode transition. Tsay et al. suggested that when Km is within 10–300 μM, HATS works, and when Km is greater than 0.5 mM, LATS works.Citation7 However, Ho et al. suggested that HATS work when Km is less than 50 μM, and LATS work when Km is greater than 5 mM.Citation8
In Arabidopsis, the NRT1/PTR/NPF family consists of 53 members that are mainly characterized as low-affinity transporters, and seven members have been described in the NRT2 family characterized as high-affinity nitrate transporters.Citation4Citation9 The CLC family has seven members, and the SLAC/SLAH family has five members.Citation4 Overall, 35 of these 72 genes have been characterized in detail, and 24 of these have been shown to transport NO3−. However, only the NRT1/PTR/NPF and NRT2 families are involved in root N uptake. Nine NRT1 genes and seven NRT2 genes involved in NO3− transport have been identified and confirmed in Arabidopsis.Citation10
High-affinity NRT2 transporters belong to the Nitrate-Nitrite Porter family (NNP),Citation11 which is a member of the Major facilitator superfamily (MFS).Citation12 The first high-affinity nitrate transporter gene, NrtA (originally called crnA), was isolated from a filamentous fungus, Aspergillus nidulans,Citation13,Citation14 and the NRT2 genes, CrNR2.1, CrNR2.2 and CrNR2.3, in Chlamydomonas reinhardtii came afterward.Citation15 The first Nrt2 genes isolated from plants were HvNRT2.1 and HvNRT2.2 from Hordeum vulgarela,Citation16 followed by Nicotiana plumbaginifolia.Citation17 Glicine maxCitation18 and Arabidopsis.Citation19,Citation20
All NRT2s were high-affinity transporters and only used nitrate as a specific substrate. When the available nitrate was limited, the high-affinity transport system is activated and plays a leading role.Citation11 To date, in the model plant Arabidopsis thaliana, root NO3− uptake activities have been shown for four NRT2 transporters: NRT2.1, NRT2.2, NRT2.4 and NRT2.5.Citation21–24 In Arabidopsis, NRT2.1, NRT2.2 and NRT2.4 are localized in plasma membranes in the roots and show more than 87% amino acid similarity.Citation25,Citation26 The fourth is NRT2.5, which displays only 65% amino acid similarity, compared with NRT2.1, NRT2.2 and NRT2.4.Citation14 Under low N conditions, NRT2.1 and NRT2.2 play an important role in the NO3− uptake. The expression level would immediately increase after receiving the low N signal. NRT2.1 is the main force for nitrate uptake and transport in the roots, NRT2.2 displays expression patterns and properties similar to those of NRT2.1, and the NRT2.1 and NRT2.2 have complementary functions.Citation26–28 NRT2.4 and NRT2.5 are also involved in high-affinity nitrate uptake, but their contribution is only revealed under nitrogen starvation, and NRT2.4 transporter contributes to NO3− uptake at very low external NO3− concentrations. After a long period of starvation, the expression of NRT2.5 is highly induced, and this becomes the major transporter for high-affinity uptake.Citation23,Citation24 NRT2.7 is specifically expressed in seeds and is the only NRT2 transporter located in tonoplast for loading NO3− into vacuoles.Citation26 Kotur et al. demonstrated that all (expect NRT2.7) members of the NRT2 family are capable of nitrate uptake in Xenopus oocytes when co‐expressed with NAR2.1.Citation29 Recent studies have suggested that the spatio-temporal distribution of these four AtNRT2 transporters is critical for the efficient NO3− uptake to sustain growth under low N availability for plants.
NRT2.5 belongs to the NRT2 family, which widely exists across all organisms, and is initially characterized as the transporter for N limitation.Citation24 NRT2.5 is expressed in the roots and shoots, especially in the roots, and is repressed by the provision of NO3−Citation24,Citation30 However, the function of NRT2.5 has not been reported in detail. The present study summarized the multiple functions of NRT2.5 in different species, including coping with N starvation, participation in NO3− transport in the phloem of buds, seed NO3− accumulation, NO3− signal transduction guidance, etc.
Bioinformatics analysis of NRT2.5
NRT2.5 has been reported in higher plants, algae, yeast and bacteria. NRT2 belongs to the nitrate-nitrite-porter (NNP) family and is one of the major facilitator superfamily (MFS) assisting transporters.Citation12 The structure of a typical NRT2 protein has 12 transmembrane helixes. Every six transmembrane helixes are a group, and these groups are connected by a large hydrophilic ring. There is a conserved domain between the 2 and 3 transmembrane helixes, and a conserved domain exists within the 4 and 5 transmembrane helixes.Citation12
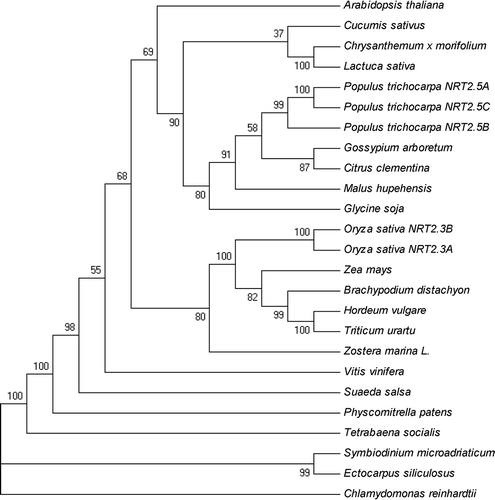
The NRT2.5 protein sequences of 24 species were queried in NCBI. The investigators used DNAman for the multi-alignment of the amino acid sequences of NRT2.5 in different species, 23 of these had conserved MFS and NNP domains (), with the typical domains of the NRT2 family. The number of amino acids was between 450 aa and 520 aa, except for C. reinhardtii, which had 910 aa. The prediction of transmembrane domains was performed using the TMHMM predictor. It was found that the transmembrane domains of different species were basically between 10 and 12, except that C. reinhardtii has six transmembrane domains (). The phylogenetic tree based on the entire amino acid sequence was constructed using the neighbor-joining method through the MEGA5 software after the ClustalW alignment with 1000 bootstrap trials on 24 sequences (12 sequences of dicotyledons, five sequences of monocots, two fungi, two bacteria and three algae) (). The phylogenetic analysis revealed that monocotyledonous plants and dicotyledonous plants have a common ancestor. However, the protein sequences were different and were clustered on the two major branches, except for Suaeda salsa. This indicates that the function of NRT2.5 in halophyte and non-halophyte may be different. The NRT2.5 among the fungi is similar, but the gap is large in algae. C. reinhardtii has a half-size transporter NRT2.5. The protein sequence of Zostera marina L. as the angiosperm also changed when the sequence was compared with algae (), indicating that its function may change. These above results indicate the co-evolutionary origin of monocotyledonous and dicotyledonous plants. However, due to the differences in species and environments, the function changed after the long-term evolution in different species.
Table 1. Summary of identified NRT2.5 in plants
Figure 1. Multi-alignment of NRT2.5 amino acid sequences in different plants. On the left is the MFS-conserved motif (MFS: G-XXX-D-XX-G-X-R), On the right is the nitrate/nitrite transporters family-conserved motif (NNP: G-W/L-G-N-M/A-G)
Figure 2. Phylogenetic analysis of NRT2.5 proteins. The amino acid sequences were aligned using ClustalW software and the phylogeny constructed using the neighbor-joining method with 1000 replicates through MEGA5 software. Database accession numbers of the amino acid sequences are as follows: Arabidopsis thaliana (OAP19210.1), Symbiodinium microadriaticum (OLQ11495.1), Ectocarpus siliculosus (CBJ31724.1), Physcomitrella patens (BAE45929.1), Chlamydomonas reinhardtii (AME17970.1), Hordeum vulgare (KAE8819762.1), Malus hupehensis (ACN22073.1), Tetrabaena socialis (PNH10816.1), Chrysanthemum ×morifolium (AMR68973.1), Gossypium arboretum (KHG03895.1), Suaeda salsa, Zostera marina L. (KMZ59016), Oryza sativa (OsNRT2.3a (sp|Q94JG1-1), OsNRT2.3b (sp|Q94JG1-2)), Lactuca sativa (XP_023762233.1), Triticum urartu (EMS50263.1), Glycine soja (KHN08612.1), Zea mays (AQK97719.1), Gossypium arboretum (XP_017636365.1), Citrus clementine (XP_024042199.1), Populus trichocarpa (A (XP_024437706.1) B (XP_002321622.2) C (XP_002321624.2)), Cucumis sativus (NP_001295862.1), Brachypodium distachyon (XP_003569637)
NRT2.5 function in Arabidopsis thaliana
In Arabidopsis, NRT2.5 is a high-affinity transporter encoded by a single gene, and AtNRT2.5 was found on isolated BACs of chromosomes I in the first half of the chromosome.Citation25
The expression and subcellular localization of AtNRT2.5
NRT2.5 is the most abundantly expressed gene in the NRT2 family in the roots and leaves and is strongly induced by N starvation in Arabidopsis.Citation24,Citation47,Citation48
The subcellular localization of NRT2.5-GFP revealed that AtNRT2.5 is localized in the plasma membrane.Citation24 In the spatial expression pattern induced by N starvation, GUS staining in the ProNRT2.5: GUS and ProNRT2.5: GUS: NRT2.5–3ʹUTR present in the root hair zone of the primary roots and lateral roots of transgenic plants.Citation24 A cross-section of the lateral root revealed that the expression was mainly located in the epidermis and the cortex, while GUS staining was limited to higher-order veins in the shoots.Citation24
AtNRT2.5 transporters is involved in NO3− uptake
AtNRT2.5 was upregulated in WT, and the atnrt2.1–1 mutant and atnar2.1–1 mutant under low N conditions (0.2 mM NO3−).Citation47,Citation48 When AtNRT2.5 was overexpressed in the atnrt2.1–1 mutant, part of the mutant’s ability to absorb NO3− could be restored, while part of the phenotype could be restored.Citation24 This result was also confirmed in functional studies in Xenopus oocytes.Citation29,Citation49 These above results show that AtNRT2.5 plays a role in absorbing NO3−.
Okamoto et al. classified the identified NRT genes in Arabidopsis into NO3− inducible, NO3− inhibitory and NO3− constitutive types.Citation50 The expression of the AtNRT2.5 gene was inhibited by the high concentration of NO3−. After several hours of supplying NO3−, its expression in the root was only 25%.Citation25,Citation50,Citation51 This means that AtNRT2.5 is a high-affinity transporter.
HATS is divided into two types: constitutive (cHATS) and inductive (iHATS). The cHATS can be expressed in the absence of NO3− in the medium, while iHATS can be expressed only in the presence of NO3− induced in the medium.Citation6 At the same time, Kotur et al. reported that NO3− influx was significantly reduced in the Atnrt2.5 mutant (GK-213H10), and that the complementation of the Atnrt2.5 mutant and AtNRT2.5 cDNA led to a statistically significant increase in cHATS.Citation30 This shows that AtNRT2.5 represents the major component of cHATS influx, with the primary responsibility for NO3− uptake at low NO3− concentrations in nitrate-deprived plants.Citation30
Kotur et al. demonstrated that AtNRT2.5 is mainly expressed in the roots of nitrate-deprived WT plants and is only a 150 kDa molecular complex with AtNAR2.1.Citation30 In addition, Kotur et al. reported that the NO3− transport activity of NRT2.5 in oocytes was stimulated by the co-expression of NAR2.1 (NRT3.1).Citation29 Similarly, the activity of NRT2.1 depends on the presence of NAR2.1.Citation48,Citation52 During the long-term N starvation, the expression of NAR2.1 follows that of NRT2.5, suggesting that NRT2.5 activity depends on NAR2.1.Citation24
The expression pattern of NRT2.5 in response to N starvation
Under N starvation, AtNRT2.5 is mainly expressed in adult plants, rather than in young seedlings.Citation24 NRT2.5 transcripts are mainly present in the roots, especially in the epidermal cells of primary and lateral roots, and are also expressed in the buds, mainly in the parenchymal cells of the leaf phloem,Citation24 which confirms the earlier observations.Citation25,Citation50 Interestingly, Kechid et al. reported that the NRT2.5 expression in the buds was higher than that in the roots.Citation51 In contrast, in young primary and lateral root epidermal cells, the AtNRT2.5 transcription levels during N deprivation continued to increase over time.Citation23,Citation24,Citation30 These spatial expression patterns indicate that AtNRT2.5 is responsible for the NO3− uptake from the soil, and this is predominantly dominant in adult plants during N deprivation.Citation23,Citation24 The expression of NRT2.5 in the roots and shoots continued to increase from days two to 10 during the N starvation and became the highest among the analyzed NRT2 family member transcript in adult plants. However, after 10 d, the expression in the shoots reached far lower levels, when compared with that in the roots.Citation24 NRT2.5 was downregulated by exposure to NO3− after 3 h in the roots, and NO3− induction had little effect on the NRT2.5 expression in the shoot tissue.Citation30
Kechid et al. reported that Atnrt 2.5 had no difference in root weight and root growth, when compared to WT, but had a 23% reduction in root growth under low N conditions, indicating that NO3− deprivation was associated with the disruption in AtNRT2.5 and resulted in the increase in root to shoot ratio.Citation30,Citation51
The plant growth of the nrt2.4 nrt2.5 double mutants also remained unaltered, but the NO3− content in the phloem sap strongly decreased and reached approximately 20% of the WT.Citation24,Citation30 This indicates that the NO3− levels in the phloem are not limiting for the plant adaptation to N starvation conditions. The loss of function of NRT2.4 and NRT2.5 led to a sharp decrease in the NO3− content in the phloem exudates of the N-starved double mutant, which is specific for NO3− in phloem secretions. This occurred because the levels of amino acids in the phloem exudates and the whole leaf NO3− levels were unchanged in all mutant lines when compared with the WT.Citation23 It was demonstrated that NRT2.4 and NRT2.5 are complementarily involved in the phloem NO3− transport in the shoots.Citation23,Citation24
The fresh weight of the triple mutant nrt2.1-nrt2.2-nrt2.5 was reduced to approximately 10% of the WT. However, the seedling weight of the nrt2.1-nrt2.2-nrt2.4-nrt2.5 tetraploid mutant was significantly reduced to 2%, and the triple mutant nrt2.1–2 nrt2.5 and quadruple mutant displayed that HATS activity decreased to 7% and 3%, respectively, when compared with the WT. However, no significant further reduction was observed for the triple mutant nrt2.1–2 nrt2.4–1, when compared to the double mutant nrt2.1–2.Citation24 At a low external NO3− concentration, the simultaneous loss of NRT2.5, NRT2.1 and NRT2.2 functions led to the further reduction in NO3− uptake and affected the growth of the shoots.Citation24 This finding led to the conclusion that the interplay between NRT2.1, NRT2.2 and NRT2.5 was required for the optimal adaptation to N limitation in plants.
NRT2.5 functions beyond nitrate transporter
Clearly, the function of AtNRT2.5 is not limited to the NO3− influx associated with cHATS activity. Kechid et al. reported that AtNRT2.5 and AtNRT2.6 play an essential role in plant growth regulation in response to the growth promoted by the rhizospheric bacterium Phyllobacterium brassicacearum. Interestingly, this effect was NO3− independent.Citation51
Todd et al. reported that the expression of MYB-like genes in Arabidopsis under nitrogen and phosphorus stresses decreased, when compared to the expression of AtNRT2.5, in the WT and pho2 mutants, suggesting that AtNRT2.5 may be associated with nitrogen and phosphorus interactions.Citation53
Chopin et al. studied the expression of NRT2 family members in Arabidopsis dry seeds, found only AtNRT2.7 and AtNRT2.5, and that this reaches only 6% of the EF1α (control gene) level of ATNRT2.5, while the former gene expressed 20 times more strongly than the latter. During the seed imbibition, the expression of both NRT2 genes decreased and became hardly detectable for ATNRT2.5. However, the role of AtNRT2.5 in dried seeds has not been well studied.Citation26 It was also reported that AtNRT2.5 is both induced in sugar-regulated senescence and during developmental leaf senescence.Citation54 Therefore, NRT2.5 may not only express in the roots and shoots in response to N starvation but also express in the seeds, and that this may be correlated to the N and P interactions. However, the specific roles of NRT2.5 have not been clearly studied.
NRT2.5 function in maize
Plett et al. divided the NRT genes of maize (Zea mays) into NRT1, NRT2 and NRT3 (NAR2) by dichotomy after sequencing the maize genome sequence. Four ZmNRT2 genes have been identified in the maize genome: ZmNRT2.1, ZmNRT2.2, ZmNRT2.3 and ZmNRT2.5.Citation10
The research on ZmNRT2.5 has mainly focused on the transcript levels, and there has been no specific functional research. ZmNRT2.5 was the only NRT2 that was upregulated under low N conditions, supporting its possible participation in response to low N limitation.Citation24,Citation34,Citation55
The transcript levels for ZmNRT2.5 were the most N decrease responsive, and the ZmNRT2.5 expression was detectable only in plants grown after low N treatment, which had significant changes throughout its life cycle. ZmNRT2.5 reached its peak transcription level on the 25th d during N starvation.Citation55 The ZmNRT2.5 transcript was differently expressed after 2 h of exposure to NO3−Citation35 Under 0.5 mM of NO3−, ZmNRT2.5 revealed a peak expression after 29 d, which was very similar to the peak of corn NO3− uptake capacity.Citation56 In conclusion, ZmNRT2.5 was detected only under N starvation or low N condition, suggesting that this may play an important role in absorbing NO3−Citation35 under low NO3− conditions.
Studies on ZmNRT2.5 revealed that in addition to its expression in the roots, it was also expressed during the vegetative stage throughout the growth cycle.Citation36,Citation55 At the reproductive stage, no transcript could be detected in the roots or silks.Citation36 The gene was expressed in the leaves, cobs and tassel, and its expression was significantly higher in the husk leaves.Citation36 ZmNRT2.5 was the only putative NO3− transporter expressed at high levels in the husk leaves.Citation36 Since husk leaves play a central role in the distribution of N during the grain filling period, the role of ZmNRT2.5 in this process needs further study.Citation36
NRT2.5 function in wheat
To date, five nitrate transporter genes (TaNRT2.1, TaNRT2.2, TaNRT2.3, TaNRT2.4 and TaNRT2.5) have been found in wheat (Triticum urartu).Citation57–59 It has been reported that TaNRT2.5 not only played a role in N starvation but also played a role in seed NO3− accumulation.
In Xenopus oocyte assay, TaNRT2.5 requires the partner protein TaNAR2.1 to present NO3− transport activity.Citation31 The co-localization of TaNRT2.5–3B: GFP and TaNAR2.1–6B: mRFP in the tobacco leaf transient expression system has proven that this was localized on the tonoplast. When TaNRT2.5–3B: GFP was expressed alone, the GFP signal could not be detected under the same microscope conditions, and the TaNRT2.5–3B protein was unstable.Citation31 These results suggest that TaNRT2.5 might cooperate with TaNAR2.1 to play a role in NO3− uptake after anthesis. This shows that TaNRT2.5–3B plays a role in NO3− transport to the vacuole and affects intracellular NO3− distribution. Therefore, this presents an indirect function in NO3− acquisition from the soil.Citation31
N starvation greatly inhibits the growth of wheat seedlings and decrease in protein and NO3− contents. The changes in phenotypic and physiological parameters may be correlated to the altered transcription levels of TaNRT1 and TaNRT2 family genes.Citation59 In the study of TaNRT2.5 in wheat, it was found that the transcription level of TaNRT2.5 in the roots of the control wheat seedlings (normal N) remained almost stable. However, during N starvation in wheat seedlings, the transcription level of the TaNRT2.5 gene was strongly induced throughout the N starvation process.Citation59 In contrast, for TaNRT2.5 in the roots, an upregulation was observed under N starvation, followed by a decline under NO3− resupply, indicating that TaNRT2.5 is a nitrate-starvation-inducible gene.Citation60 In response to N starvation, TaNRT2.5 plays a more important role in wheat seedlings than in other NO3− transporters.Citation59,Citation60
In addition to the role of NO3− acquisition during N starvation, TaNRT2.5 was also expressed in leaf sheath, peduncle and developing seeds, especially in the husk and embryo.Citation59,Citation60 It was observed that the expression of TaNRT2.5 in the roots was much higher at the grain filling stage when compared with the seedling growth stage.Citation31,Citation59,Citation60 The TaNRT2.5–3B overexpression promoted seed germination and increased the grain NO3− concentration, seed vigor and seed yield, while the RNA interference of TaNRT2.5 had the opposite effects.Citation31 The TaNRT2.5 transgenic lines altered the TaAMY and TaUGPase expression and decreased in the RNAi lines, which may affect the seed germination rate.Citation31–33
In conclusion, TaNRT2.5 not only plays an important role during N starvation but also plays a specific role in seed NO3− accumulation, which is an important signal for seed vigor and crop establishment.
NRT2.5 function in rice
Five NRT2 genes have been identified in the rice genome. Yan et al. analyzed the rice nitrate transporters in EST, DDBJ and NCBI, and the results revealed that OsNRT2.3 has two transcripts, OsNRT2.3a and OsNRT2.3b, with 94.2% similarity in their putative amino acid sequences.Citation37,Citation38 The bioinformatics analysis of the closest relationship between OsNRT2.3a/b and AtNRT2.5 revealed that OsNRT2.3a may be an ortholog of AtNRT2.5.Citation39
However, only one of the two splice variants, OsNRT2.3a in rice, requires an interaction with OsNAR2.1 to mediate the NO3− uptake, and the absorption of NO3− by OsNRT2.3b is affected by pH.Citation38,Citation39,Citation61,Citation62 Indeed, when NAR2 was co-expressed in Xenopus oocytes, the nitrate transport activities of OsNRT2.3a were greatly enhanced. Nevertheless, NAR2 is not required for the nitrate transport activities of OsNRT2.3b.Citation37,Citation39 In rice, the orthologous gene OsNRT2.5 (also known as OsNRT2.3a) is mainly expressed predominantly in xylem parenchyma cells of the root stele and has been demonstrated to play a role in the transport of NO3− from the roots to the shoots, again under low NO3− conditions that are induced by NO3− and sucrose, and inhibited by ammonium ion and aspartic acidCitation37,Citation38,Citation39,, There was no significant difference in phenotype with the overexpression of OsNRT2.3a alone. However, the OsNRT2.3b overexpression was obviously higher and bigger than WT.Citation39 In terms of function, OsNRT2.3a plays a key role in the long-distance nitrate transport from the root to the shoot at a low nitrate supply level. Furthermore, the overexpression of rice OsNRT2.3b not only enhanced the NO3− uptake but also improved the yield and nitrogen use efficiency (NUE) under both low and high nitrogen conditions in the field.Citation39,Citation62 Meanwhile, OsNRT2.3b can improve the P uptake and accumulation in rice.Citation62
The differential transcriptome of rice in response to N supply revealed that under low NO3−, the nitrate transporter gene OsNRT2.5 was upregulated, while under high NO3−, OsNRT2.5 was downregulated.Citation63 The characterization shows that OsNRT2.5 has a specific role in transporting NO3− from the roots to the shoots in rice, and that this is different from NRT2.5 in Arabidopsis and wheat.Citation24,Citation31,Citation39
NRT2.5 function in other plants
Poplar (Populus trichocarpa) NRT2.5 is coded by three genes. Poplar NRT2.5A is expressed in the bark, and NRT2.5B is expressed in the leaves, while the expression of NRT2.5C is unknown.Citation43 In Populus tremula × tremuloides, PttNRT2.5B expression occurred in all investigated vegetative poplar organs and was the highest in fine roots.Citation44 Therefore, the strong PttNRT2.5B expression in the roots at low external NO3− concentrations could not be explained by N starvation. PttNRT2.5B function within the poplar roots remains obscure and need to be further analyzed in future studies.Citation44
Feng et al. reported that the CsNRT2.5 gene was expressed in different tissues of tea tree (Camellia sinensis), and the expression level was lower in the shoots and stems, and higher in mature leaves and roots, when induced by NO3−Citation45
Under low concentrations of NO3−, the cassava (Manihot esculenta Crantz) MeNRT2.5 gene can reach its peak within a short time in the roots, and it was speculated that MeNRT2.5 was the same as genes such as AtNRT2.1 as a NO3− receptor. However, this needs further experimental proof.Citation46
The function of NRT2.5 under salinity
Seagrasses are the only group of angiosperms that evolved from land plants to complete their life cycle submerged in marine environments. As far as NO3− is concerned, the concentration of NO3− in the seagrass environment is as low as 5 µM, suggesting that high-affinity transporters developed by terrestrial plants would work in seagrass, facing the challenge of salinity and alkaline conditions.Citation64 In terrestrial angiosperms, NRT2 encodes a high-affinity NO3− transporter that acts as an H+ cotransporter, but Zostera marina L. may use a Na+ -dependent high-affinity nitrate transporter to acquire NO3−. Interestingly, only one gene (Zosma70g00300.1; NRT2.1) in the chlorella genome has annotated this function. The analysis of this sequence predicted the presence of 12 transmembrane domains, including the MFS domain of the NNP transporter family and the “nitrate tag” that appears in all members of the NNP family.Citation41 The phylogenetic analysis revealed that the sequence had a greater relationship with NRT2.5 than with NRT2.1 and shared common ancestors with monocotyledonous and dicotyledonous plants.Citation41 The phylogenetic analysis revealed that there was only one NRT2 gene in the Z. marina genome, which was most relevant to AtNRT2.5. This finding supports the idea that ZosmaNRT2 encodes a high-affinity NO3− transporter that localized on the plasma membrane.Citation41 ZosmaNRT2 also requires the interaction of NAR2 to mediate NO3− transport, and the presence of NAR2 is important to stabilize the ZosmaNRT2 protein at the plasma membrane.Citation41 These molecular data, together with the previous electrophysiological results, support that ZosmaNRT2 would have evolved to use Na+ as a driving ion, which could provide important insights for NO3− uptake in plants under alkaline, NO3– limited and salinized environments.Citation62
S. salsa is an annual herbaceous halophyte with high salt tolerance during reproductiveCitation65–67 seed germinationCitation68–70 and seedling stages.Citation71–73 The species occurs in both intertidal zones and inland saline sites in China.Citation74–77 Under the growth conditions of 200 mM of NaCl, and compared with the inland population of S. salsa, the expression of nitrate transporter 2.5 (SsNRT2.5) in the old and mature leaves of the intertidal population was significantly upregulated during N starvation. Therefore, SsNRT2.5 may play an important role in NO3− migration during N starvation, and this trait may make S. salsa adapts to changeable low nitrogen environments.Citation40 However, further investigations are needed to determine whether SsNRT2.5 plays a role in NO3− absorption during N starvation.
Transcription factors participate in modulating the expression levels of NRT2.5
At present, few transcription factors involved in NO3− signaling with NRT2.5 were reported. Ruffel et al. identified two putative transcription factors, TGA3 and MYC1, which are NRT2.5 regulators that respond to N or C signals in Arabidopsis.Citation78 The functional analysis of mutants combined with yeast one-hybrid experiments confirmed that both transcription factors can bind to the promoter of NRT2.5, in response to N or C signals.Citation78
The transcription factors, LBD37 and LBD38, also regulate NO3− response genes. Rubin et al. reported that LBD37 and LBD38 are negative regulators of NO3−, which act as regulatory genes in N-free conditions.Citation79,Citation80
The nitrate-induced NAC transcription factor TaNAC2-5A controls the NO3− signaling in wheat.Citation81 ChIP-Seq and EMSA identified the downstream genes of TaNAC2, including TaNRT2.5–3B.Citation31 TaNAC2 could bind to the promoter of TaNRT2.5–3B and promote the expression of TaNRT2.5, which plays a key role in seed vigor.Citation31,Citation81 TaNAC2 is the transcription factor that regulates the “workhorse” TaNRT2.5 transporter, which drives NO3− accumulation and thereby seedling vigor.Citation31 The TaNAC2-NRT2.5 module plays a key role in regulating the accumulation of NO3− particles and seed vigor.Citation31
Concluding remarks and perspectives
The evolutionary analysis of 12 dicotyledons and five monocotyledons in the present study also obtained the same results, including the halophyte S. salsa and Z. marina (). The most highly conserved regions of the NRT2.5 transporters are found within the predicted transmembrane domains, the NNP and MFS domain, indicating that the role of the NRT2.5 generally appears to be the NO3− and NO2− influx.Citation11
Monocotyledonous and dicotyledonous plants have different nitrogen-responsive expression patterns, leading to changes in localization. For example, in Arabidopsis, Poplar, tea tree and cassava, NRT2.5 is expressed in the roots and leaves, only in the seeds of Arabidopsis, but the expression level is extremely low. However, in monocotyledons, in addition to the roots and leaves, this was also expressed in the embryo and shell in wheat, and in silk, cobs and tassel husk leaves in maize (). In Arabidopsis, NRT2.5 mainly responds to N starvation and N absorption under very low nitrogen conditions, but it must work in conjunction with NAR2.1.Citation23,Citation24,Citation30 However, in Poplar, the NRT2.5B expression appears in all investigated vegetative organs. Hence, N starvation cannot be used to explain the strong expression of NRT2.5B in the roots at low external NO3− concentrations.Citation43,Citation44 In monocotyledons, in addition to responding to N starvation, TaNRT2.5 also plays a role in seed NO3− accumulation in wheat,Citation31,Citation59 and NRT2.5 in bract leaves may play a central role in N distribution during grain filling in maize.Citation36 This means that the function of NRT2.5 between monocotyledonous and dicotyledonous plants should be different. Interestingly, in halophytes such as S. salsa and Z. marina, the function of NRT2.5 may have a certain relationship with Na+, but the specific function of NRT2.5 in halophytes needs to be further investigated.Citation40,Citation41
In summary, NRT2.5 not only responds to N starvation and NO3− absorption but also participates in the accumulation of NO3− in seeds and plays a role in seed vitality and NO3− distribution in the reproductive stage. The above results indicate that there are still many unknown functions of NRT2.5 that have not yet been tapped, and these functions may play roles in improving crop yield and participating in stress tolerance. Therefore, the physiological and molecular mechanisms of NRT2.5 involved in NO3− absorption and distribution, as well as adaptation to stress environments, such as salinity, are worthy of in-depth research.
Author contributions
Ranran Liu wrote the manuscript. Ting Jia and Bing Cui assisted in revising the manuscript. Jie Song revised and gave final approval of the manuscript.
Additional information
Funding
References
- Amtmann A, Armengaud P. Effects of N, P, K and S on metabolism: new knowledge gained from multi-level analysis. Curr Opin Plant Biol. 2009;12:1–10. doi:10.1016/j.pbi.2009.04.014.
- Wang YY, Cheng YH, Chen KE, Tsay YF. Nitrate transport, signaling, and use efficiency. Annu Rev Plant Biol. 2018;69:85–122. doi:10.1146/annurev-arplant-042817-040056.
- Miller AJ, Fan X, Orsel M, Smith S, Wells D. Nitrate transport and signalling. J Exp Bot. 2007;58:2297–2306. doi:10.1093/jxb/erm066.
- Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince A, Chaillou S, Ferrariomery S, Meyer C, Daniel-Vedele F. Nitrate transport and signalling in Arabidopsis. J Exp Bot. 2014;65:789–798. doi:10.1093/jxb/eru001.
- McClure PR, Kochian LV, Spanswick RM, Shaff JE. Evidence for cotransport of nitrate and protons in maize roots: i. effects of nitrate on the membrane potential. Plant Physiol. 1990;93:281–289. doi:10.1104/pp.93.1.281.
- Crawford NM, Glass ADM. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998;3:389–395. doi:10.1016/S1360-1385(98)01311-9.
- Tsay YF. Nitrate Transporters and Peptide Transporters. FEBS Lett. 2007;581:2290–2300. doi:10.1016/j.febslet.2007.04.047.
- Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi:10.1016/j.cell.2009.07.004.
- Léran S, Varala K, Boyer JC, Chiurazzi M, Crawford N, Daniel-Vedele F, David LC, Dickstein R, Fernandez E, Forde BG, et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014;19:5–9. doi:10.1016/j.tplants.2013.08.008.
- Plett D, Toubia J, Garnett T, Tester M, Kaiser BN, Baumann U. Dichotomy in the NRT gene families of dicots and grass species. Plos One. 2010;5:e15289. doi:10.1371/journal.pone.0015289.
- Forde B. Nitrate transporters in plants: structure, function and regulation. Acta Bioch Bioph Sin. 2000;1465:219–235. doi:10.1016/S0005-2736(00)00140-1.
- Pao SS, Paulsen IT, Saier MH. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi:10.1128/mmbr.62.1.1-34.1998.
- Johnstone IL, Mccabe PC, Greaves P, Gurr SJ, Cole GE, Brow MAD, Unkles SE, A J C, Kinghorn JR, Innis MA. Isolation and characterisation of the crnA-niiA-niaD gene cluster for nitrate assimilation in Aspergillus nidulans. Gene. 1990;90:181–192. doi:10.1016/0378-1119(90)90178-T.
- Unkles SE, Hawker KL, Grieve C, Campbell EI, Montague P, Kinghorn JR. crnA encodes a nitrate transporter in Aspergillus nidulans. Proc Natl Acad Sci USA. 1991;88:204–208. doi:10.1073/pnas.88.1.204.
- Quesada A, Galvan A, Fernandez E. Identification of nitrate transporter genes in Chlamydomonas reinhardtii. Plant J. 1994;5:407–419. doi:10.1111/j.1365-313X.1994.00407.x.
- Trueman LJ, Richardson A, Forde BG. Molecular cloning of higher plant homologues of the high-affinity nitrate transporters of Chlamydomonas reinhardtii and Aspergillus nidulans. Gene. 1996;175:223–231. doi:10.1016/0378-1119(96)00154-0.
- Quesada A, Krapp A, Trueman LJ, Danielvedele F, Fernandez E, Forde BG, Caboche M. PCR-identification of a Nicotiana plumbaginifolia cDNA homologous to the high-affinity nitrate transporters of the crnA family. Plant Mol Biol. 1997;34:265–274. doi:10.1023/A:1005872816881.
- Amarasinghe BHRR, De Bruxelles G, Braddon M, Onyeocha I, Forde BG, Udvardi MK. Regulation of GmNRT2 expression and nitrate transport activity in roots of soybean (Glycine max). Planta. 1998;206:44–52. doi:10.1007/s004250050372.
- Zhuo D, Okamoto M, Vidmar JJ, Glass ADM. Regulation of a putative high‐affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J. 1999;17:563–568. doi:10.1046/j.1365-313X.1999.00396.x.
- Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Danielvedele F, Gojon A. Molecular and functional regulation of two NO3− uptake systems by N‐ and C‐status of Arabidopsis plants. Plant J. 1999;18:509–519. doi:10.1046/j.1365-313X.1999.00480.x.
- Tsay Y, Schroeder JI, Feldmann KA, Crawford NM. The herbicide sensitivity gene CHL1 of arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi:10.1016/0092-8674(93)90399-B.
- Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 2001;489:220–224. doi:10.1016/S0014-5793(01)02096-8.
- Kiba T, Feria-Bourrellier AB, Lafouge F, Lezhneva L, Boutet-Mercey S, Orsel M, Bréhaut A, Miller A, Daniel-Vedele F, Sakakibara H, et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012;24:245–258. doi:10.1105/tpc.111.092221.
- Lezhneva L, Kiba T, Feria-Bourrellier AB, Lafouge F, Boutet-Mercey S, Zoufan P, Sakakibara H, Daniel-Vedele F, Krapp A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014;80:230–241. doi:10.1111/tpj.12626.
- Orsel M, Krapp A, Daniel-Vedele F. Analysis of the NRT2 nitrate transporter family in Arabidopsis. Structure and gene expression. Plant Physiol. 2002;129:886–896. doi:10.1104/pp.005280.
- Chopin F, Orsel M, Dorbe MF, Chardon F, Truong HN, Miller AJ, Krapp A, Daniel-Vedele F. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell. 2007;19:1590–1602. doi:10.1105/tpc.107.050542.
- Krouk G, Lacombe B, Bielach A, Perrinewalker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010;18:927–937. doi:10.1016/j.devcel.2010.05.008.
- Wang Y, Hsu P, Tsay Y. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012;17:458–467. doi:10.1016/j.tplants.2012.04.006.
- Kotur Z, Mackenzie N, Ramesh S, Tyerman SD, Kaiser BN, Glass ADM. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 2012;194:24–731. doi:10.1111/j.1469-8137.2012.04094.x.
- Kotur Z, Glass ADM. A 150 kDa plasma membrane complex of AtNRT 2.5 and AtNAR 2.1 is the major contributor to constitutive high‐affinity nitrate influx in Arabidopsis thaliana. Plant Cell Environ. 2015;38:1490–1502. doi:10.1111/pce.12496.
- Li WJ, He X, Chen Y, Jing YF, Shen CC, Yang JB, Teng W, Zhao XQ, Hu WJ, Hu MY, et al. A wheat transcription factor positively sets seed vigour by regulating the grain nitrate signal. New Phytol. 2020;225:1667–1680. doi:10.1111/nph.16234.
- Yu YL, Guo GF, Lv DW, Hu YK, Li JR, Li XH, Yan YM. Transcriptome analysis during seed germination of elite Chinese bread wheat cultivar Jimai 20. BMC Plant Biol. 2014;14:20. doi:10.1186/1471-2229-14-20.
- Ju LL, Deng GB, Liang JK, Zhang HL, Li Q, Pan ZF, Yu MQ, Long H. Structural organization and functional divergence of high isoelectric point α-amylase genes in bread wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.). BMC Genet. 2019;20:25. doi:10.1186/s12863-019-0732-1.
- Sabermanesh K, Holtham LR, George J, Roessner U, Boughton BA, Heuer S, Tester M, Plett D, Garnett T. Transition from a maternal to external nitrogen source in maize seedlings. J Integr Plant Biol. 2017;59:261–274. doi:10.1111/jipb.12525.
- Zamboni A, Astolfi S, Zuchi S, Pii Y, Guardini K, Tononi P, Varanini Z. Nitrate induction triggers different transcriptional changes in a high and a low nitrogen use efficiency maize inbred line. J Integr Plant Biol. 2014;56:1080–1094. doi:10.1111/jipb.12214.
- Dechorgnat J, Francis KL, Dhugga KS, Rafalski JA, Tyerman SD, Kaiser BN. Tissue and nitrogen-linked expression profiles of ammonium and nitrate transporters in maize. BMC Plant Biol. 2019;19:206. doi:10.1186/s12870-019-1768-0.
- Feng HM, Yan M, Fan XR, Li BZ, Shen QR, Miller AJ, Xu GH. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J Exp Bot. 2011;62:2319–2332. doi:10.1093/jxb/erq403.
- Yan M, Fan XR, Feng HM, Miller AJ, Shen Q, Xu GH. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011;34:1360–1372. doi:10.1111/j.1365-3040.2011.02335.x.
- Tang Z, Fan XR, Li Q, Miller AJ, Shen QR, Xu GH. Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiol. 2012;160:2052–2063. doi:10.1104/pp.112.204461.
- Ma YC, Yang Y, Liu RR, Li QQ, Song J. Adaptation of euhalophyte Suaeda salsa to nitrogen starvation under salinity. Plant Physiol Biochem. 2020;146:287–293. doi:10.1016/j.plaphy.2019.11.025.
- Rubio L, Díaz-García J, Amorim-Silva V, Macho AP, Botella MA, Fernández JA. Molecular characterization of ZosmaNRT2, the putative sodium dependent high-affinity nitrate transporter of Zostera marina L. Int J Mol Sci. 2019;20:3650. doi:10.3390/ijms20153650.
- Higuera JJ, Calatrava V, González Z, Mariscal V, Siverio JM, Fernández E, Galván A. NRT2.4 and NRT2.5 are two half-size transporters from the Chlamydomonas NRT2 Family. Agronomy. 2016;6:20. doi:10.3390/agronomy6010020.
- Bai H, Euring D, Volmer K, Janz D, Polle A. The nitrate transporter (NRT) gene family in poplar. Plos One. 2013;8:e72126. doi:10.1371/journal.pone.0072126.
- Willmann A, Thomfohrde S, Haensch R, Nehls U. The poplar NRT2 gene family of high affinity nitrate importers: impact of nitrogen nutrition and ectomycorrhiza formation. Environ Exp Bot. 2014;108:79–88. doi:10.1016/j.envexpbot.2014.02.003.
- Feng SH, Wang LY, Chen CS. Cloning and expressing analysis of a nitrogen transporter 2.5 gene from tea plant [Camellia sinensis (L.)]. J Tea Sci. 2014;4:364–370. [In Chinese].
- Ren N, Chen XZ, Xia YQ, Bai XY, Jiang XY, Zhou Y. Cloning and expression analysis of MeNRT2.5 gene in cassava. J Trop Biol. 2019;2:111–118. [In Chinese].
- Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F. Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta. 2004;219:714–721. doi:10.1007/s00425-004-1266-x.
- Orsel M, Chopin F, Leleu O, Smith SJ, Krapp A, Da-Vedele F, Miller AJ. Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis. Physiology and protein-protein interaction. Plant Physiol. 2006;142:1304–1317. doi:10.1104/pp.106.085209.
- Yong Z, Kotur Z, Glass ADM. Characterization of an intact two‐component high‐affinity nitrate transporter from Arabidopsis roots. Plant J. 2010;63:739–748. doi:10.1111/j.1365-313X.2010.04278.x.
- Okamoto M, Vidmar JJ, Glass ADM. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol. 2003;44:304–317. doi:10.1093/pcp/pcg036.
- Kechid M, Desbrosses G, Rokhsi W, Varoquaux F, Djekoun A, Touraine B. The NRT2.5 and NRT2.6 genes are involved in growth promotion of Arabidopsis by the plant growth‐promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM 196. New Phytol. 2013;198:514–524. doi:10.1111/nph.12158.
- Okamoto M, Kumar A, Li W, Wang Y, Siddiqi MY, Crawford NM, Glass ADM. High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 2006;140:1036–1046. doi:10.1104/pp.105.074385.
- Todd CD, Zeng P, Huete AMR, Hoyos ME, Polacco JC. Transcripts of MYB-like genes respond to phosphorous and nitrogen deprivation in Arabidopsis. Planta. 2004;219:1003–1009. doi:10.1007/s00425-004-1305-7.
- Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta. 2006;224:556–568. doi:10.1007/s00425-006-0243-y.
- Garnett T, Conn V, Plett D, Conn S, Zanghellini J, Mackenzie N, Enju A, Francis K, Holtham L, Roessner U, et al. The response of the maize nitrate transport system to nitrogen demand and supply across the lifecycle. New Phytol. 2013;198:82–94. doi:10.1111/nph.12166.
- Plett D, Baumann U, Schreiber AW, Holtham L, Kalashyan E, Toubia J, Nau J, Beatty M, Rafalski A, Dhugga KS, et al. Maize maintains growth in response to decreased nitrate supply through a highly dynamic and developmental stage-specific transcriptional response. Plant Biotechnol J. 2016;14:342–353. doi:10.1111/pbi.12388.
- Xuan HM, Wang YH, Wei LT, Yang YY, Wang LN, Kang GZ, Guo TC. Transcription analysis of the gene encoding nitrate transporter NRT1 and NRT2 families in response to nitrogen starvation in wheat seedlings leaves. J Triticeae Crops. 2014;34:1019–1028. [In Chinese].
- Yin LP, Li P, Wen B, Wen B, Taylor D, Berry JO. Characterization and expression of a high-affinity nitrate system transporter gene (TaNRT2.1) from wheat roots, and its evolutionary relationship to other NTR2 genes. Plant Sci. 2007;172:621–631. doi:10.1016/j.plantsci.2006.11.014.
- Guo TC, Xuan HM, Yang YY, Wang LN, Wei LT, Wang YH, Kang GZ. Transcription analysis of genes encoding the wheat root transporter NRT1 and NRT2 families during nitrogen starvation. J Plant Growth Regul. 2014;33:837–848. doi:10.1007/s00344-014-9435-z.
- Wang M, Zhang PL, Liu Q, Li GJ, Di DW, Xia GM, Kronzucker HJ, Fang S, Chu JF, Shi WM. TaANR1-TaBG1 and TaWabi5-TaNRT2s/NARs link ABA metabolism and nitrate acquisition in wheat roots. Plant Physiol. 2020;182:1440–1453. doi:10.1104/pp.19.01482.
- Liu XQ, Huang DM, Tao JY, Miller AJ, Fan XR, Xu GH. Identification and functional assay of the interaction motifs in the partner protein OsNAR2.1 of the two‐component system for high-affinity nitrate transport. New Phytol. 2014;204:74–80. doi:10.1111/nph.12986.
- Fan XR, Tang Z, Tan YW, Zhang Y, Luo BB, Yang M, Lian XM, Shen QR, Miller AJ, Xu GH. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc Natl Acad Sci USA. 2016;113:7118–7123. doi:10.1073/pnas.1525184113.
- Xin W, Zhang L, Zhang WZ, Gao JP, Yi J, Zhen XX, Li Z, Zhao Y, Peng CC, Zhao C. An integrated analysis of the rice transcriptome and metabolome reveals differential regulation of carbon and nitrogen metabolism in response to nitrogen availability. Int J Mol Sci. 2019;20:2349. doi:10.3390/ijms20092349.
- Touchette BW, Burkholder JAM. Review of nitrogen and phosphorus metabolism in seagrasses. J Exp Mar Biol Ecol. 2000;250:133–167. doi:10.1016/S0022-0981(00)00195-7.
- Guo JR, Li YD, Han GL, Song J, Wang BS. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct Plant Biol. 2018;45:350–361. doi:10.1071/FP17181.
- Guo JR, Suo SS, Wang BS. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci Res. 2015;25:335–344. doi:10.1017/S0960258515000239.
- Song J, Zhou JC, Zhao WW, Xu HL, Wang FX, Xu YG, Tian CY. Effects of salinity and nitrate on production and germination of dimorphic seeds both applied through the mother plant and exogenously during germination in Suaeda salsa. Plant Spec Biol. 2016;31:19–28. doi:10.1111/1442-1984.12071.
- Wang FX, Xu YG, Wang S, Shi WW, Liu RR, Feng G, Song J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol Bioch. 2015;95:41–48. doi:10.1016/j.plaphy.2015.07.005.
- Zhou JC, Fu TT, Sui N, Guo JR, Feng G, Fan JL, Song J. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. Plant Biosyst. 2016;150:83–90. doi:10.1080/11263504.2014.976294.
- Song J, Shi WW, Liu RR, Xu YG, Sui N, Zhou JC, Feng G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Spec Biol. 2017;32:107–114. doi:10.1111/1442-1984.12132.
- Chen TS, Yuan F, Song J, Wang BS. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Funct Plant Biol. 2016;43:244–253. doi:10.1071/FP15120.
- Song J, Shi GW, Gao B, Fan H, Wang BS. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol Plant. 2011;141:343–351. doi:10.1111/j.1399-3054.2011.01445.
- Sui N, Tian SS, Wang WQ, Wang ML, Fan H. Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in Arabidopsis. Front Plant Sci. 2017;8:1337. doi:10.3389/fpls.2017.01337.
- Song J, Wang BS. Using euhalophytes to understand salt tolerance and to develop saline agriculture: suaeda salsa as a promising model. Ann Bot. 2015;115:541–553. doi:10.1093/aob/mcu194.
- Xu YG, Liu RR, Sui N, Shi WW, Wang L, Tian CY, Song J. Changes in endogenous hormones and seed coat phenolics during seed storage of two Suaeda salsa populations. Aust J Bot. 2016;64:325–332. doi:10.1071/BT16014.
- Liu QQ, Liu RR, Ma YC, Song J. Physiological and molecular evidence for Na+ and Cl− exclusion in the roots of two Suaeda salsa populations. Aquat Bot. 2018;146:1–7. doi:10.1016/j.aquabot.2018.01.001.
- Wang FX, Yin CH, Song YP, Li Q, Tian CY, Song J. Reproductive allocation and fruit-set pattern in the euhalophyte Suaeda salsa in controlled and field conditions. Plant Biosyst. 2018;152:749–758. doi:10.1080/11263504.2017.1330776.
- Ruffel S, Chaput V, Przybyla-Toscano J, Fayos I, Ibarra C, Moyano TC. Genome-wide analysis in response to N and C identifies new regulators for root AtNRT2 transporters. bioRxiv. 2019:822197. doi:10.1101/822197.
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell. 2009;21:3567–3584. doi:10.1105/tpc.109.067041.
- Medici A, Krouk G. The primary nitrate response: a multifaceted signalling pathway. J Exp Bot. 2014;65:5567–5576. doi:10.1093/jxb/eru245.
- He X, Qu BY, Li WJ, Zhao XQ, Teng W, Ma WY, Ren YZ, Li B, Li ZS, Tong YP. The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiol. 2015;169:1991–2005. doi:10.1104/pp.15.00568.
