 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Plant-plant interactions like competition or facilitation between seedlings can have profound implications on their establishment and posterior development. These interactions are variable and depend upon the presence of neighbouring plants and environmental factors. In this work, we studied the effects of the interaction by the roots of Eucalyptus urophylla seedlings from a population under various environmental stressful conditions: water deficit, nutrient deficit, low light, low temperature, and high temperature. To evaluate it, we measured some growth and morphological parameters. We demonstrated that shoot parameters such as the number of leaves, leaf area, and dry weight of the leaves were the most affected parameters due to the belowground plant-plant interaction under various environmental conditions. We did not find evidence for competition among the plants, especially under restrictive conditions. Therefore, the study corroborates the stress-gradient hypothesis, which states that plants’ differences under stressful conditions lead to facilitative interactions. It has implications for plant ecology and forestry techniques.
1 Introduction
In natural environments, resource availability can vary widely through space and time.Citation1,Citation2 Since most plants live in social groups, where neighbours interact both above and belowground, resource limiting is traditionally considered the primary mechanism influencing underground interactions.Citation3,Citation4 In a group of plants where environmental resources are shared, the individuals who uptake more resources through time are generally considered the most well-succeeded and potentially the ones that will have the best fitness.Citation5,Citation6 Therefore, competition has been recognised as an essential factor influencing individuals’ fate and species distribution.Citation7
Competition is often held as the only interaction present in all plant communities.Citation7,Citation8 Nevertheless, many studies have demonstrated that positive interactions such as facilitation are as ubiquitous as negative interactions. Furthermore, its influence is equally essential for regulating communities’ composition and diversity.Citation9–11 Facilitation can be defined as the positive interactions that benefit development despite stressing biotic or abiotic interactions.Citation10,Citation12,Citation13 On the other hand, competition has been regarded as a negative interaction – the struggle for available resources in a given environment which reduces plant growth and fitness.Citation5,Citation14,Citation15
Through the last decades, some studies have demonstrated that the plants’ strategy in acquiring resources from the soil might depend on the identity of their neighbours.Citation4,Citation14,Citation16–18 Plants that grow together in the same soil volume depend on the same resources to develop and may organise their root systems to have better access to the limited resources considering the presence of competitors.Citation19–22 There is much evidence suggesting that the root system develops differently in the presence of neighbouring roots. These responses change according to the species, degree of kinship, genotype, and self/nonself discrimination by the roots.Citation18,Citation23–26 The last one means that plants can distinguish self (roots from the plant itself) from nonself (roots from other plants), consequently altering its growth.Citation25,Citation27
Several studies have demonstrated that the roots of many species change their behaviour and spatial distribution depending on the presence or absence of other roots, and also the species and genetic relatedness of the interacting plants.Citation27–31 Studies like these demonstrated that not all species respond in the same way to the presence of neighbouring plants.Citation25 However, there are certain trends like the facilitative interaction some plants engage when they recognise their neighbours as kin. In this case, in a general fashion, kin recognition has been characterised by reducing root proliferation and biomass allocation to the root system.Citation16,Citation17,Citation32
Conversely, there is a perspective in evolutionary ecology that suggests that a group of related plants (i.e., with low genetic variability) may be more prone to engage in competitive interactions. Consequently, they have less reproductive success than a more genetically varied group, which is likely more successful in sharing resources.Citation14,Citation33,Citation34 Presumably, increased genetic kinship implies reduced variability in individuals’ size and a more symmetric competition between them.Citation33,Citation35 On the other hand, individuals with similar genetic material may share a ‘common interest’ in the groups’ productivity. In this case, facilitation may be favoured because positive interactions spend fewer resources than competitive ones (i.e., more biomass allocation in roots or leaves). Besides, it indirectly promotes the reproductive success of relatives.Citation36,Citation14
Furthermore, the interactions between seedlings may influence their establishment and subsequent success in forestry management. Pickles et al.Citation37 have demonstrated that sibling plants possibly collaborate more with each other than with not related seedlings. They showed that Douglas-fir (Pseudotsuga menziesii var. glauca (Beissn.) Franco) seedlings transfer more labeled 13C through ectomycorrhizal fungi to sibling plants than to unrelated seedlings. Although the amount of carbon transferred was very small, according to the authors, “the timing and transfer of micronutrients or signaling compounds may have a substantial ecological impact”,Citation37 which can determine the success and establishment of these seedlings.
Bertoli et al.Citation28 demonstrated that the capacity of discriminating self from nonself in seedlings of Eucalyptus urophylla S.T. Blake is related to the genetic similarity of the interacting plants. When compared with ramets interacting, half-sibling seedlings (obtained from the same mother-plant and open pollination) and seedlings from other populations (different mother-plants) recognised their neighbours as nonself and competed with them. It led to more competitive morphologies and reduced growth parameters and dry weight in the populational and half-sibling groups than the clonal group.
However, it is unknown how E. urophylla from a populational group, genetically dissimilar, would interact when facing stressful environments. It is widely known that the environment can trigger different physiological and behavioural responses and change foraging and competitive strategies [e.g.,Citation20,Citation38,Citation39]. Understanding how neighbours’ presence alters the plants’ behaviour when facing abiotic stress is essential due to the ecological relevance of these interactions with both other plants and the environment and when planning reforestation in forestry management. E. urophylla is native to the Lesser Sunda Islands and is widely used for timber production in forestry plantations in Brazil and Southwestern Asia because of the relative quality of its wood, the facility to grow in other tropical regions and the resistance to the canker disease (Cryphonectria cubensis (Bruner) Hodges).Citation40,Citation41
In this work, we hypothesised that adverse environmental conditions such as limited resources or restrictive conditions would trigger more competitive behaviours in a population of E. urophylla seedlings than favourable conditions. The differences in the behaviour of plants would be elicited by the interaction amongst themselves. The seedlings’ interaction would lead to competitive morphologies (e.g., depending on the conditions, more biomass allocation to the roots or shoot, increased leaf asymmetry, etc.). Since it seems that interactions by the roots are essential for triggering competitive behaviours,Citation16,Citation17,Citation21 we further hypothesised that plants that were not interacting with others by the roots would not present such behaviuor.Citation1
2 Material and methods
2.1 Plant material
Seeds of different populations of E. urophylla were obtained from the Forest Science and Research Institute (IPEF, Piracicaba, SP, Brazil). The seeds came from a commercial population from Seed Production Area T10 B71 (22°42ʹ24.63”S, 47°36ʹ25.83”W), composed of E. urophylla from 24 different progeny lines. They were sowed in tubes with 55.0 cm3 of commercial Plantmax® substrate and kept in nursery conditions with 50% shading. After germination (approx. 7–10 days) and thinning, we left only one plant per tube. The seedlings received ten-minute irrigation by a microsprayer every three hours from 6:00 a.m. to 6:00 p.m. Besides, Hoagland and Arnon solutionCitation42 was sprinkled at alternate days for ensuring adequate nutrition.Citation43 When the plants grew up to 7 cm and had 2 fully developed leaves, around 150 days after sowing, they were used in the experiment.
2.2 Experimental setup
For testing our hypothesis, we divided the plants into two treatments: interacting and solo plant (control treatment). Each treatment consisted of eight replicates of a triplet of plants () that did not share (solo) or shared pots with the roots of other plants (interacting).
Figure 1. Schematic representation of both treatments. A. Solo treatment: the plants had their roots split but did not share the pot with other plant’s root. B. Interacting treatment: the plants had their roots split and shared the pots with neighbouring plants’ roots. C. Representation of the symmetry measures made from the medial vein in each side of three portions of the leaves
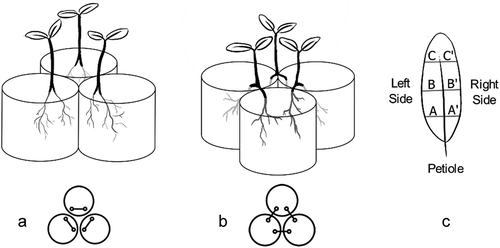
For doing so, we employed the split-root technique. Plants with the same height (approx. 7 cm) had their roots pruned to obtain two primary roots 10.0 cm long. They were replanted in polystyrene pots filled with 400 mL of sand with 3 mm grain size to minimise root adherence to the substrate’s grains. In the solo treatment, plants grew in a single pot, one plant per pot ()). In the interacting treatment, plants grew in two pots, one root in each of them, thus they shared the pot with two neighbouring plants ()). The distance of the roots between them and between the pot walls was approximately the same in both treatments. Furthermore, regardless of the root treatment (interacting/solo), all the shoots were equidistant amongst themselves.
All the assays were conducted in a growing chamber (Phytotron, Eletrolab, São Paulo, SP, Brazil). For the control treatment, all the triplets were daily irrigated with distilled water until total pot capacity (soil water capacity), plus 5.0 mL of Hoagland and Arnon nutrient solution in each pot (15 mL per triplet). The active photosynthetic irradiance was fixed in 600 μmol photons m−2 s−1 in a photoperiod of 14 h daylight, and air humidity was controlled at 60%. Day/night temperatures switched from 28°C to 22°C, respectively.Citation44
Unless differently stated, the plants were kept under these conditions in all the treatments. All the assays lasted 30 days from the transplanting day.
2.3 Test conditions
Control condition: The triplets were kept under the conditions described above for 30 days.
Water deficit: The seedlings were kept at 30% of pot water capacity. We used the gravimetric method for water reposition, with the triplets being weighed daily in both the control and water deficit treatment. All the pots that composed the triplets were equally watered and received the daily 5 mL of Hoagland and Arnon solution.
Nutrient deficit: Each triplet was daily irrigated with 15 mL of Hoagland and Arnon solution, just like the control, but using a concentration of 25%.Citation45 Pots were kept at 100% of water retention capacity by adding distilled water.
Low irradiance: All the triplets were kept under 150 μmol photons m−2 s−1 throughout the assay.
High temperature: Plants were kept under 34°C during the day and 22°C during the night.
Low temperature: Plants were kept under constant 22°C during the whole essay.
2.4 Experimental design
The experimental design was completely randomised. For each environmental treatment, two interaction treatments (interacting and solo/control) with eight repetitions were performed. Only high temperature treatment had six repetitions.
2.5 Morphological and growth analyses
We used leaf symmetry deviation (LSD) to evaluate the stability of leaf development as an indication of the effect of environmental disturbances.Citation46,Citation47,Citation48,Citation49 The stability of development refers to the full expression of a genetically determined phenotype. Environmental disturbances can destabilise this expression, leading to an altered phenotype, which is called fluctuating asymmetry when the alterations are random, non-directed.Citation46,Citation47 Therefore, asymmetry measures are an indication of stressful conditions in plants.Citation48
For inferring LSD, we measured with a digital calliper the width of the left and right side, in relation to the central vein of a fully expanded leaf of each plant. The measurements were made on the leaf’s basal, central, and apical segments ()). LSD was calculated as LSD , where x is the left side and x’, the right side ()) This method removes errors related to differences in size scales. To normalise asymmetric data, we used Box-Cox transformation as LSD*
).Citation49
For inferring shoot and roots growth, we measured the height of the plants and calculated its mean value (HP), the mean number of leaves (LN), the average total leaf area of all the plants (LA), total dry weight (DWT), the dry weight of all the leaves for each treatment and condition (DWL), the same for the shoot (DWS), and roots (DWR). We also measured the total root area (RA), the volume of the roots (RV), and total root length (RL) for each treatment (solo and interacting) under each of the conditions (control, water deficit, nutrient deficit, etc.). Besides, we measured the specific root area (SRA ), specific leaf area (SLA
), root area:leaf area (RA/LA), and DWR:DWS ratio (R/S).
We measured the plant height with a ruler from the basis of the shoot to the apical meristem. The leaves were sectioned in the petiole basis and measured with a portable leaf area meter (LI-3000A, LI-COR, Lincoln, NE, USA). For the root measures, we stained the roots with methylene blue (1% solution) for 15 s, placed them on a lightbox (Ultra Slim LED, Biotron, Santa Rita do Sapucaí, MG, Brazil), and photographed them with a digital camera at a distance of 30 cm. The images were then analysed with the software Safira© (Embrapa, Stonway, São Carlos, SP, Brazil).Citation50 Afterward, all the roots and shoots were dried in a ventilation oven at 70°C.
2.6 Statistical analyses
Each treatment (solo and interacting) under each environmental condition (water deficit, nutrient deficit, low irradiance, etc.) was analysed independently with one way ANOVA (p ≤ 0.05) and the means compared with the Tukey test (p < 0.05) to reach specific effects of each treatment.
3 Results
The results obtained for the growth and biomass parameters in each treatment are shown in :
Table 1. Mean values for growth and biomass parameters analysed for the solo and interacting (Int.) treatments under all the conditions. LA: leaf area; LN: number of leaves; HP: plant height; DWL: dry weight of leaves; DWS: dry weight of shoot; DWR: dry weight of roots; DWT: total dry weight; SLA: specific leaf area; R/S: root/shoot ratio; RA/LA: root area/leaf area ratio; SRA: specific root area; RL: root length; RA: root area; RV: root volume; A: leaf symmetry deviation on leaf segment A; B: leaf symmetry on leaf segment B; C: leaf symmetry on segment C. Numbers in bold indicate significant (p < 0.05) difference between treatments according to Tukey test
For most of the parameters analysed, there was no significant (p < 0.05) difference between the solo and interacting treatments in all the environmental conditions. Leaf area, however, was a noticeable exception, and there were significant differences between solo and interacting treatment in all the conditions except low irradiance and high temperature. The interaction also impacted other parameters related to the leaves (LN and DWL).
However, in the control condition, the interaction with neighbouring roots led to a reduction in the values of 7 of the 17 parameters analysed, such as most of the parameters related to the shoot (e.g., LA, LN, DML, DMS), total dry weight and root volume ().
4 Discussion
Many studies have demonstrated that plant behaviour is affected when the plant contacts neighbouring roots.Citation21,Citation28,Citation29,Citation51,Citation52,Citation53. It occurs regardless of soil resources and volume.Citation54,Citation55,Citation56 However, until now, the performance of individuals of the same species genetically unrelated under more than two different environmental conditions was not studied.
The results show that the control condition (presumably, the most favourable one) led to most of the significant (p < 0.05) differences between solo and interacting treatments for the parameters analysed (). This result agrees with what was found by 4. They demonstrated that seedlings of E. urophylla from a clonal population, under an interaction treatment and grown in the same conditions as the control treatment described here (i.e., not limiting), had reduced growth below and aboveground when compared with the solo treatment.
It seems that limiting conditions buffered the growth and morphological alterations when the plants were interacting by the roots because few parameters had significant (p < 0.05) differences between the treatments. The exceptions were nutrient deficit and low temperature. They presented differences between the treatments in some parameters, which contrasts with the seedlings’ general behaviour. Under nutrient deficit, the plants that shared the pot with their neighbours had decreased LN, LA, DWL, consequently increasing the R/S ratio. However, under this condition, DWT was not altered, which do not suggest a competitive behaviour. For the low temperature condition, the interaction treatment only influenced the leaves’ mass and area, but DWT too, which perhaps is an indication of competition because it was lower in the interacting treatment when compared with the solo treatment.
The parameters related to the shoot, particularly leaf area [LA), seemed to be the most sensitive variables to observe, aboveground, an effect of belowground interactions regardless of the environmental conditions experienced by the seedlings. This result is similar to what was found by Murphy and Dudley (2009) and Bertoli et al. (2020).Citation17,Citation28 The roots’ interaction was necessary to trigger alterations in the aboveground organs such as shoots and leaves because, in the absence of this interaction, the alterations were not observed.Citation17,Citation28 We suggest, therefore, LN, DWL, and, especially, LA as a promising variable to observe the effect of plant-plant interaction by the roots in E. urophylla and, conceivably, other plants. However, except for the plants under water deficit, belowground interaction was not a stressful factor since leaf symmetry was not affected (), indicating maintenance of developmental stability.Citation48
The DWT was smaller in the interacting treatment under control and low temperature conditions. This could be interpreted as the result of competition because of the lost energy and resources in competitive interactions. Therefore, in a general fashion, all the other restrictive conditions indicated a facilitative or at least neutral interaction among the seedlings. This behaviour was particularly noticeable aboveground, where its impact was greater (i.e., more significant differences between the treatments). There is another possible interpretation to the smaller DWT under these conditions: because the plants were sharing resources in a limited space, they uptook fewer nutrients individually, which led to smaller morphology, even though they did not present any sign of water or nutrient deficit. Similar to what was found by Bertoli et al.,Citation28 the seedlings would be competing with each other by other means not measurable by any of the techniques presented here.
The apparent uniformity in the seedlings’ development under the solo and interacting treatments in all the restrictive conditions, and also in the control condition, seems aligned with the stress-gradient hypothesis,Citation12 which is receiving increased support over the last decades.Citation57–60 According to this hypothesis, competition among plants is higher under favourable conditions, but it may turn into facilitation as the environment becomes abiotically more stressful.Citation12 Hence, it may explain why the seedlings of E. urophylla did not show competition in the interacting treatment under restrictive conditions. Besides, despite the restrictions of resources and limiting conditions, it seems that it was not enough to alter the leaves’ symmetry, which is an indication that the plants were not sufficiently stressed, possibly due to facilitative interactions.
Facilitation between plants can be experimentally inferred when they reduce their competitivity in the presence of neighbours and enhance their neighbours’ fitness.Citation14,Citation17,Citation61 Here, since it is not possible to observe the consequences of this interaction on the reproduction of both groups, we cannot say that the interaction between the plants under nutrient and water deficit conditions was negative. There was no difference in the total dry weight in both cases, which suggests that the interaction with other plants’ roots did not cause any loss in biomass.
The results obtained with this experiment with different environmental conditions are consistent with earlier studies, indicating that resource restriction leads to a similarity in the plants’ ability to compete.Citation62,Citation63 Rewald and LeuschnerCitation62 suggested that the individuals’ competitive capacity may decrease continuously as the environment becomes more stressful and environmental resource reduction becomes similar for all individuals involved.
Concluding, our results partially support our hypothesis. Restrictive environmental conditions did not trigger competitive interactions in most of the cases analysed, allowing plants to keep their developmental stability. We propose leaf number, leaf area, and dry mass of leaves as a potential method to observe belowground interactions in this species. Furthermore, we have not observed sound evidence for competition in any tested conditions due to the seedlings’ interaction by the roots except, maybe, under the control and low temperature conditions. Therefore, it is likely that the results corroborate the stress-gradient hypothesis because, under restrictive conditions, competition among the seedlings of an E. urophylla population was not observed. It has potential implications on forestry management because it seems that, under stressful situations, diversity may favour facilitation.
Acknowledgments
This work was supported by the São Paulo Research Foundation (FAPESP; process 2011/21591-1) and the National Council for Scientific and Technological Development (CNPq; process 2053), and financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The IPEF kindly provided the seeds of E. urophylla. We thank Dr Carlos Henrique Britto de Assis Prado for the critical review and appraisal of the manuscript, as well as the anonymous reviewers that contributed to its perfecting.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Caldwell M, Pearcy R, eds. Exploitation of environmental heterogeneity by plants: ecophysiological process above- and belowground. London (UK): Academic Press; 1994.
- Luo D, Qian Y-Q, Han L, Liu J-X, Sun Z-Y. Phenotypic responses of a stoloniferous clonal plant Buchloe dactyloides to scale-dependent nutrient heterogeneity. PLOS One. 2013;8(6):1. doi:10.1371/journal.pone.0067396.
- Rajaniemi TK. Root foraging traits and competitive ability in heterogeneous soils. Oecologia. 2007;153(1):145–7. doi:10.1007/s00442-007-0706-2.
- Semchenko M, Zobel K, Hutchings MJ. To compete or not to compete: an experimental study of interactions between plant species with contrasting root behavior. Evolutionary Ecology. 2010;24(6):1433–1445. doi:10.1007/s10682-010-9401-6.
- Bazzaz FA. Plants in changing environments: linking physiological, population, and community ecology. UK: Cambridge University Press; 1996.
- Mooney HA. Some contributions of physiological ecology to plant population biology. Systematic Botany. 1976;1(3):269–283. doi:10.2307/2418720.
- Novoplansky A. Picking battles wisely: plant behaviour under competition. Plant Cell Environ. 2009;32(6):726–741. doi:10.1111/j.1365-3040.2009.01979.x.
- Connell JH. On the prevalence and relative importance of interespecific competition: evidence from field experiments. Am Nat. 1983;122(5):661–696. doi:10.1086/284165.
- Callaway RM. Positive interactions and interdependence in plant communities. Berlin (Germany): Springer; 2007.
- Gagliano M, Renton M. Love thy neighbour: facilitation through an alternative signalling modality in plants. BMC Ecol. 2013;13(1):1–6. doi:10.1186/1472-6785-13-19.
- Holmgren M, Scheffer M, Huston M. The interplay of facilitation and competition in plant communities. Ecology. 1997;78(7):1966–1975. doi:10.1890/0012-9658(1997)078[1966:TIOFAC]2.0.CO;2.
- Bertness MD, Callaway R. Positive interactions in communities. Trends Ecol. Evol. 1994;9(5):191–193. doi:10.1016/0169-5347(94)90088-4.
- Cavieres LA, Brooker RW, Butterfield BJ, Cook BJ, Kikvidze Z, Lortie CJ, Michalet R, Pugnaire FI, Schöb C, Xiao S, et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol Lett. 2014;17(2):193–202. doi:10.1111/ele.12217.
- Biernaskie JM. Evidence for competition and cooperation among climbing plants. Proceedings of the Royal Society B. 2011;278(1714):1989–1996. doi:10.1098/rspb.2010.1771.
- Conell JH. Apparent versus “real” competition in plants. In: Grace JB, Tilman D, editors. Perspectives on plant competition. San Diego (US): Academic Press; 1990. p. 9–26.
- Dudley SA, File AL. Kin recognition in an annual plant. Biol Lett. 2007;3(4):435–438. doi:10.1098/rsbl.2007.0232.
- Murphy GP, Dudley SA. Kin recognition: competition and cooperation in Impatiens (Balsaminaceae). Am J Bot. 2009;96(11):1990–1996. doi:10.3732/ajb.0900006.
- Novoplansky A. What plant root knows? Semin Cell Dev Biol. 2019;92(126):126–133. doi:10.1016/j.semcdb.2019.03.009.
- Cabal C, Martínez-García R, Aguilar AC, Valladares F, Pacala SW. The exploitative segregation of plant roots. Science. 2020;370(6521):1197–1199. doi:10.1126/science.aba9877.
- Cahill JJF, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St Clair CC. Plants integrate information about nutrients and neighbors. Science. 2010;328(5986):1657. doi:10.1126/science.1189736.
- Jacob CE, Tozzi E, Willenborg CJ. Neighbour presence, not identity, influences root and shoot allocation in pea. PLoS ONE. 2017;12(3):e0173758. doi:10.1371/journal.pone.0173758.
- Robinson D. The responses of plants to non-uniform supplies of nutrients. New Phytologist. 1994;127(4):635–674. doi:10.1111/j.1469-8137.1994.tb02969.x.
- Biedrzycki ML, Bais HP. Kin recognition: another biological function for root secretions. Plant Signal Behav. 2010a;5(4):401–402. doi:10.4161/psb.5.4.10795.
- Chen BJW, During HJ, Anten NPR. Detect thy neighbor: identity recognition at the root level in plant. Plant Science. 2012;195:157–167. doi:10.1016/j.plantsci.2012.07.006.
- Depuydt S. Arguments for and against self and nonself root recognition in plants. Front Plant Sci. 2014;5(614):1–7. doi:10.3389/fpls.2014.00614.
- Schenk HJ, Callaway RM, Mahall BE. Spatial root segregation. Are Plants Territorial? Advances in Ecological Research. 1999;28:145–180. doi:10.1016/S0065-2504(08)60032-X.
- Falik O, de Kroon H, Novoplansky A. Physiologically mediated self/nonself root discrimination in Trifolium repens has mixed effects on plant performance. Plant Signal Behav. 2006;1(3):116–121. doi:10.4161/psb.1.3.2639.
- Bertoli SC, Neris DM, Sala HR, Vieira WL, Souza GM. The level of relatedness affects self/nonself discrimination in Eucalyptus urophylla seedlings. Canadian Journal of Forest Research. 2020;50(5):500–509. doi:10.1139/cjfr-2019-0376.
- de Kroon H, Mommer L, Nishiwaki A. Root competition: towards a mechanistic understanding. In: de Kroon H, Visser EJW, editors. Root ecology: ecological studies (analysis and synthesis). Vol. 168. Berlin (Heidelberg): Springer; 2003. p. 215–234. doi:10.1007/978-3-662-09784-7_9.
- Falik O, Reides P, Gersani M, Novoplansky A. Self/nonself discrimination in roots. Journal of Ecology. 2003;91(4):525–531. doi:10.1046/j.1365-2745.2003.00795.x.
- Mahall BE, Callaway RM. Root communication among desert shrubs. Proceedings of the National Academy of Sciences. 1991;88(3):847–876. doi:10.1073/pnas.88.3.874.
- Biedrzycki ML, Bais HP. Kin recognition in plants: a mysterious behavior unsolved. J Exp Bot. 2010b;61(15):4123–4128. doi:10.1093/jxb/erq250.
- Cheplick G, Kane K. Genetic relatedness and competition in Triplasis purpurea (Poaceae): resource partitioning or kin selection? Int J Plant Sci. 2004;165(4):623–630. doi:10.1086/386556.
- Ellstrand N, Antonovics J. Experimental studies of the evolutionary significance of sexual reproduction II. A test of the density-dependent selection hypothesis. Evolution. 1985;39(3):657–666. doi:10.1111/j.1558-5646.1985.tb00402.x.
- Aikio S, Pakkasmaa S. Relatedness and competitive asymmetry: implications for growth and population dynamics. Oikos. 2003;100(2):283–290. doi:10.1034/j.1600-0706.2003.11814.x.
- Hamilton WD, Amala G. The genetical evolution of social behavior I. J Theor Biol. 1964a;7(1):1–16. doi:10.1016/0022-5193(64)90038-4
- Pickles BJ, Wilhelm R, Asay AK, Hahn AS, Simard SW, Mohn WW. Transfer of 13C between paired Douglas-fir seedlings reveals plant kinship effects and uptake of exudates by ectomycorrhizas. New Phytologist. 2017;214(1):400–411. doi:10.1111/nph.14325.
- de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ. A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant Cell Environ. 2009;32(6):704–712. doi:10.1111/j.1365-3040.2009.01936.x.
- Shemesh H, Arbiv A, Gersani M, Ovadia O, Novoplansky A. The effects of nutrient dynamics on root patch choice. PLOS One. 2010;5(5):e10824. doi:10.1371/journal.pone.0010824.
- Scanavaca JL 2001. Caracterização silvicultural, botânica e tecnológica do Eucalyptus urophylla S.T. Blake e de seu potencial para utilização em serraria. Mater’s thesis. Piracicaba (Brazil): Escola Superior de Agricultura Luiz de Queiroz. p.108. doi: 10.11606/D.11.2002.tde-22052002-154220
- Sein CC, Mitlöhner R. Eucalyptus urophylla S.T. Blake: ecology and silviculture. Bogor (Indonesia): CIFOR; 2011.
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn. 1950;347:32
- Rosa LS, Wendling I, Grossi F, Reissmann CB. Efeito da dose de nitrogênio e de formulações de substratos na miniestaquia de Eucalyptus dunnii maiden. Revista Árvore. 2009;33(6):1025–1035. doi:10.1590/S0100-67622009000600005.
- O’Brien EE, Gersani M, Brown JS. Root proliferation and seed yield in response to spatial heterogeneity of belowground competition. New Phytologist. 2005;168(2):401–412. doi:10.1111/j.1469-8137.2005.01520.x.
- Cooper MA. Maximização do potencial de enraizamento de estacas de Eucalyptus dunnii Maiden. Master’s thesis (Master’s in Forestry Science) – Universidade Federal do Paraná, Curitiba. 1990.
- Møller AP, Swaddle JP. Asymmetry, developmental stability, and evolution. Inc. New York: Oxford University Press; 1997.
- Palmer AR, Strobeck C. Fluctuating asymmetry: measurement, analysis, patterns. Annual Review of Ecology and Systematics. 1986;17(1):391–421. doi:10.1146/annurev.es.17.110186.002135.
- Freeman DC, Graham JH, Emlen JM. Developmental stability in plants: symmetries, stress and epigenesis. Genetica. 1993;89(1–3):97–119. doi:10.1007/BF02424508.
- Souza GM, Aidar ST, de Oliveira RF. Developmental stability and network connectance in Phaseolus vulgaris L. genotypes under water deficit. Isr. J. Plant Sci. 2004;52(3): 205–212. doi:10.1560/UCEB-7X6Y-6N99-6GWV
- Costa MCG, Cunha IML, Jorge LAC, Araújo ICS. Public-domain software for root image analysis. Revista Brasileira De Ciência Do Solo. 2014;38(5):1359–1366. doi:10.1590/S0100-06832014000500001.
- Hess L, de Kroon H. 2007. Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. J. Ecol. 2007;95(2):241–251. doi:10.1111/j.1365-2745.2006.01204.x
- Meier IC, Angert A, Falik O, Shelef O, Rachmilevitch S. Increased root oxygen uptake in pea plants responding to non-self neighbors. Planta 2013;238:577–586. doi:10.1007/s00425-013-1910-4
- Yang L, Callaway RM, Atwater DZ. Root contact responses and the positive relationship between intraspecific diversity and ecosystem productivity. AoB Plants 2015;7:plv053. doi:10.1093/aobpla/plv053
- Holzapfel C, Alpert P. Root cooperation in a clonal plant: connected strawberries segregate roots. Oecologia. 2003;134(1):72–77. doi:10.1007/s00442-002-1062-x.
- Mahall BE, Callaway RM. Effects of regional origin and genotype on intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae). Am J Bot. 1996;83(1):93–98. doi:10.1002/j.1537-2197.1996.tb13879.x.
- Maina GG, Brown JS, Gersani M. Intra-plant versus inter-plant root competition in beans: avoidance, resource matching or tragedy of the commons. Plant Ecol. 2002;160:pages235–247. doi:10.1023/A:1015822003011
- Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417(6891):844–848. doi:10.1038/nature00812.
- Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress‐gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology. 2009;97(2):199–205. doi:10.1111/j.1365-2745.2008.01476.x.
- Malkinson D, Tielbörger K. What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos. 2010;119(10):1546–1552. doi:10.1111/j.1600-0706.2010.18375.x.
- Michalet R, Le Bagouse-pinget Y, Maalouf J-P, Lortie CJ. Two alternatives to the stress-gradient hypothesis at the edge of life: the collapse of facilitation and the switch from facilitation to competition. Journal of Vegetation Science. 2014;25(2):609–613. doi:10.1111/jvs.12123.
- Milla R, Forero DM, Escudero A, Iriondo JM. Growing with siblings: a common ground for cooperation or for fiercer competition among plants? Proceedings of the Royal Society B. 2009;276(1667):2531–2540. doi:10.1098/rspb.2009.0369.
- Rewald B, Leuschner C. Does root competition asymmetry increase with water availability? Plant Ecol Divers. 2009;2(3):255–264. doi:10.1080/17550870903022865.
- Wichmann L. Annual variations in competition symmetry in even-aged sitka spruce. Ann Bot. 2001;88(1):145–151. doi:10.1006/anbo.2001.1445.
