ABSTRACT
Sugars Will Eventually be Exported Transporter (SWEET) is a newly characterized family of sugar transporters, which plays critical roles in plant–pathogen interactions. However, the function of SWEET in tobacco and its interaction with Fusarium oxysporum, a causal agent of root rot, remain unclear. This study aimed to dissect the function of NtSWEETs in tobacco root rot using stem bases from tobacco plants inoculated with F. oxysporum. RNA-sequencing (RNA-Seq) analysis was performed, and the results indicated that Sucrose Transporter 4 (NtSUC4), Sugar Transporter 12 (NtSTP12), Hexose Transporter 6 (NtHEX6), NtSWEET1, NtSWEET3b, and NtSWEET12 were downregulated by infection with F. oxysporum. The expression of NtSWEET1, but not of NtSUC4, NtSTP12, NtHEX6, NtSWEET3b, or NtSWEET12, was suppressed at all the time points tested after inoculation with F. oxysporum. The NtSWEET1-green fluorescent protein was localized on the plasma membrane and possessed the ability to transport glucose, fructose and galactose. Compared with the wild-type plants, NtSWEET1 RNAi plants were more susceptible to root rot, indicating that NtSWEET1 positively regulated the defense of tobacco against root rot. This study identified the role of SWEETs in tobacco and their interaction with F. oxysporum. The results might be useful in protecting tobacco plants from root rot.
Plant–pathogen interactions are complex traits determined by several factors at different times and spaces. Pathogens secrete transcription activator–like effectors into host cells during the interaction between bacterial pathogens and plants, where they act as transcriptional activators on plant target genes, such as Sugars Will Eventually be Exported Transporters (SWEETs), to benefit the pathogen or interfere with plant basic immunity.Citation1,Citation2 Sugar transporters, for example, SWEETs and sugar transport proteins (STPs) have been known to play a significant role in plant disease resistance.Citation3 IbSWEET10, a sucrose transporter, was reported to regulate sweet potato resistance to F. oxysporum.Citation4 However, the molecular mechanism underlying sugar transporter SWEET-mediated resistance in plant–pathogen interaction remains largely unknown. Tobacco root rot is a soil-borne disease caused by FusariumCitation5–7 that severely affects the production of tobacco. However, the mechanism of how tobacco plants defend themselves against Fusarium remains unclear; fungicide treatments are still the main approach used to protect plants from root rot.Citation8
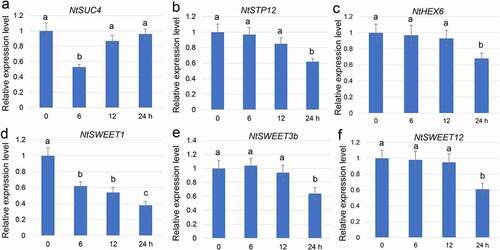
F. oxysporum was inoculated on Nicotiana tabacum plants to analyze the function of SWEET in resisting tobacco root rot. The base of the stem where the most severe symptom appeared was isolated to extract the RNA. An RNA-sequencing (RNA-Seq) analysis was performed on the tobacco samples after 0, 6, 12, and 24 h of inoculation with F. oxysporum. The RNA-Seq results showed that the expression of NtSUC4, NtSTP12, NtHEX6, NtSWEET1, NtSWEET3b, and NtSWEET12 was suppressed by infection with F. oxysporum. Among these, NtSWEET1 was suppressed from 6, 12, and 24 h of inoculation. NtSUC4 was suppressed from 6 h of inoculation, while NtSTP12, NtHEX6, NtSWEET3b, and NtSWEET12 were suppressed from 24 h of inoculation (), indicating that the infection with F. oxysporum suppressed the expression of sugar transporter genes.
Figure 1. Fusarium oxysporum infection–mediated gene expression analysis. The base of a N. tabacum seedling was sampled 0, 6, 12, and 24 h after inoculation with F. oxysporum. F. oxysporum infection–mediated NtSUC4 (a), NtSTP12 (b), NtHEX6 (c), NtSWEET1 (d), NtSWEET3b (e), and NtSWEET12 (d) patterns of expression were analyzed using qRT-PCR. The mRNA levels in the samples were normalized against those of Actin mRNA. Data are the means ± SE (n = 3), and the experiments were repeated at least three times. Different letters indicate significant differences between groups (P < 0.05). qRT-PCR, Quantitative real-time reverse transcriptase–PCR; SE, standard error
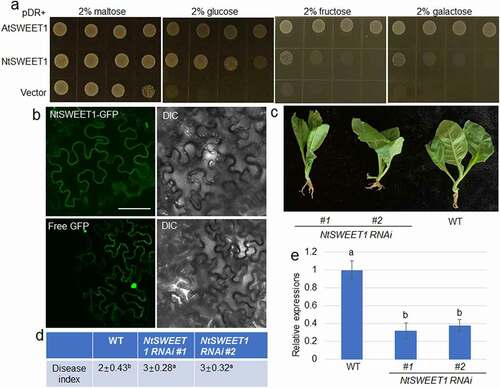
The expression of NtSWEET1 was suppressed at all the time points after inoculation with F. oxysporum. Hence, the function of NtSWEET1 in tobacco and its interaction with the fungus were examined. First, the sugar transport activity of NtSWEET1 was analyzed using the hexose transport–deficient EBY4000 strain of yeast. The yeast growth assay showed that the transformation with NtSWEET1 and the positive control AtSWEET1 successfully rescued the ability of EBY4000 yeast cells to transport glucose, fructose, and galactose, while the empty vector transformed with EBY4000 failed to grow on media with glucose, fructose, and galactose as the sole sugar source. Compared with AtSWEET1, NtSWEET1 showed similar glucose transport activity, but the fructose and galactose transport activities of NtSWEET1 was much lower than those of AtSWEET1 (), indicating that NtSWEET1 was a hexose transporter. Next, the localization of NtSWEET1-green fluorescent protein (GFP) indicated that it was present in the plasma membrane, while free GFP was detected in the cytosol and nucleus in tobacco leaves (). In addition, we generated NtSWEET1 RNAi plants (#1 and #2) and inoculated them with F. oxysporum to investigate the function of NtSWEET1 in resisting tobacco to root rot. The results indicated that NtSWEET1 RNAi plants (#1 and #2) were more susceptible to root rot compared with the wild-type plants (). In addition, the results of a quantitative real-time reverse transcriptase–polymerase chain reaction (qRT-PCR) analysis of the expression level of NtSWEET1indicated that it was significantly suppressed in NtSWEET1 RNAi plants (#1 and #2) than in the wild-type plants. These results suggested that the expression level of NtSWEET1 was positively associated with the resistance of tobacco to root rot disease. These data indicated that the infection with F. oxysporum suppressed the expression of NtSWEET1, NtSWEET3b, NtSWEET12, NtSUC4, NtSTP12, and NtHEX6. The RNA-Seq assay did not detect F. oxysporum–induced sugar transporter genes, suggesting that F. oxysporum infection transcriptionally inhibited the sugar transporter gene, and the suppression of the sugar transporter gene NtSWEET1 inhibited the resistance of tobacco to root rot. F. oxysporum infected plants and moved through the vasculature. Also, SWEET is a uniporter that bidirectionally transported sugar, implying that NtSWEET1 might inhibit sugar loading to the vasculature or the uptake of sugar from the vasculature to phloem cells during the interaction with F. oxysporum. Our findings suggested that the transport and translocation of sugar mediated by NtSWEET1 could be important for the defense of tobacco to root rot, and F. oxysporum could suppress the expression of NtSWEET1 so that it could successfully infect tobacco.
Figure 2. NtSWEET1 function and its role in defense of tobacco against root rot. (a) Hexose transport activity of NtSWEET1 was analyzed in the EBY4000 yeast strain. NtSWEET1 -transformed cells were grown on YNB media that contained 2% glucose, fructose, galactose, or maltose. Empty (pDRf1) vector and AtSWEET1 were used as the negative and positive controls, respectively. (b) Localization of NtSWEET1-GFP or free GFP was analyzed in tobacco leaves. The left and right channels indicate GFP and light image, respectively. (c) Wild-type (WT) and NtSWEET1 RNAi (#1 and #2) plants were inoculated with F. oxysporum. (d) Disease index of plants shown in (c) was calculated (n > 15). (e) Expression level of NtSWEET1 in the WT and NtSWEET1 RNAi plants (#1 and #2). Different letters indicate significant differences between groups (P < 0.05). DIC, Differential interference contrast; GFP, green fluorescent protein; WT, wild-type; YNB, yeast nitrogen base media
Acknowledgments
We appreciate very much for the undergraduate students in help with planting.
Disclosure statement
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Asai Y, Kobayashi Y, Kobayashi I. Increased expression of the tomato SISWEET15 gene during grey moldinfection and the possible involvement of the sugar efflux to apoplasm inthe disease susceptibility. J Plant Pathol Microbiol. 2016;7:1. doi:https://doi.org/10.4172/2157-7471.1000329.
- Tadege B, Stahli S, Dupuis K. Activation of plant defense responses and sugar efflux by expression of pyruvate decarboxylase in potato leaves. Plant J. 1998;16(6):661–3. doi:https://doi.org/10.1046/j.1365-313x.1998.00329.x.
- Devanna BN, Jaswal R, Singh PK, Kapoor R, Jain P, Kumar G, Sharma Y, Samantaray S, Sharma TR. Role of transporters in plant disease resistance. Physiol Plant. 2021;171(4):849–867. doi:https://doi.org/10.1111/ppl.13377.
- Li Y, Wang Y, Zhang H, Zhang Q, Zhai H, Liu Q, He S. The plasma membrane-localized sucrose transporter IbSWEET10 contributes to the resistance of sweet potato to Fusarium oxysporum. Front Plant Sci. 2017;8:197. doi:https://doi.org/10.3389/fpls.2017.00197.
- Jie Z, Jing W, Li N, Kong F, Zhang C, Chao F, Yan X. A study on the pathogenic crude toxin of the tobacco root rot extracted from Fusarium sp. Plant Prot. 2013;39:61–66.
- Qiu R, Li J, Zheng W, Su X, Xing G, Li S, Zhang Z, Li C, Wang J, Chen Y, et al. First report of root rot of tobacco caused by Fusarium brachygibbosum in China. Plant Dis. 2021a. doi:https://doi.org/10.1094/pdis-01-21-0077-pdn.
- Qiu R, Li Q, Li J, Dong N, Li S, Guan W, Zhang Y, Li X, Liu C, Li Y. First report of fusarium root rot of tobacco caused by fusarium sinensis in Henan Province China. Plant Dis 2021b. doi:https://doi.org/10.1094/pdis-11-20-2466-pdn.
- Wang JJ, Wen GS, Ding JL, Li YZ. The research progress of the residues of pesticides of tobacco and the discussion of reduce the residues of pesticides of tobacco leaf. J Yunnan Agric Univ. 2006;21:329–332.
