 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Phosphorus (P), which is taken up by plants as inorganic phosphate (Pi), is one of the most important macronutrients for plant growth and development. Meanwhile, it determines plant architecture in several ways, including leaf inclination. However, the molecular basis underlying the crosstalk between the signaling pathways of plant P homeostasis and architecture maintenance remains elusive. We recently characterized a WRKY transcription factor, OsWRKY108, in rice (Oryza sativa). It functions redundantly with OsWRKY21 to promote Pi uptake in response to Pi supply. Overexpression of either OsWRKY108 or OsWRKY21 led to up-regulation of Pi transporter genes and thus enhanced Pi accumulation. By contrast, transgenic rice plants expressing OsWRKY21-SRDX (a fusion protein transforming OsWRKY21 from an activator into a dominant repressor) but not the OsWRKY108-SRDX fusion showed decreased Pi accumulation under Pi-replete conditions. Here, we report that OsWRKY108 acts as a positive regulator of leaf inclination. OsWRKY108 overexpressors showed increased leaf inclination and OsWRKY108-SRDX plants showed an erect-leaf phenotype, irrespective of the Pi regimes. Nevertheless, the response of leaf inclination to Pi starvation was largely impaired upon OsWRKY108 overexpression. Moreover, in both OsWRKY108-SRDX plants and OsWRKY108 overexpressors, the ‘percentage of leaf angle alteration relative to wild-type’ under Pi-starvation condition was more significant than that under Pi-replete condition. These results suggest that the regulation of OsWRKY108 on leaf inclination is in part dependent on Pi availability. Altogether, our findings demonstrate that OsWRKY108 is an integrative regulator of P homeostasis and leaf inclination, serving as a link between plant nutrient signaling and developmental cues.
Phosphorus (P) is an indispensable mineral element for an array of biological processes of plants.Citation1,Citation2 Plants suffering shortage of this nutrient often display visible alterations in the architecture of both root and shoot, such as an erect-leaf phenotype (decreased leaf inclination) in graminaceous crops.Citation3,Citation4 Leaf inclination, an important agronomic trait determining plant yield, refers to the angle between the adaxial side of leaf blade and the vertical axis (e.g. stem/culm or leaf sheath). It is largely determined by the connective structure between leaf blade and leaf sheath, namely leaf collar or lamina joint. As for a single plant, large leaf angle results in a more spread shoot architecture to receive more light, whereas in a population, small leaf angle avoids mutual shading and enables dense planting.Citation5–7 The mechanisms for controlling leaf inclination have been intensively studied especially in monocotyledonous crops such as maize (Zea mays) and rice (Oryza sativa).Citation8–11 Ruan et al.Citation12 showed that a pair of SPX (Syg1/Pho81/XPR1) domain-containing proteins (OsSPX1 and OsSPX2), which negatively regulate the activity of the central transcription factor (TF) of Pi starvation signaling, OsPHR2 (Phosphate Starvation Response 2), redundantly inhibit leaf inclination via interacting with another MYB TF, OsRLI1 (Regulator of Leaf Inclination 1). Nevertheless, the molecular modules linking leaf inclination and P homeostasis are widely unexplored.
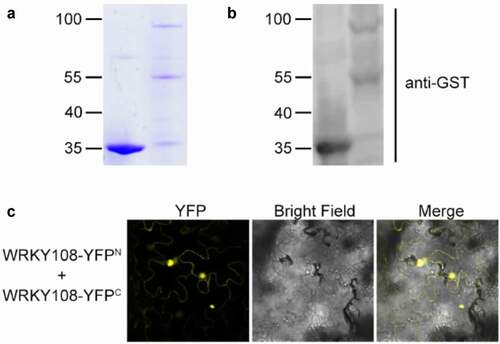
In one of our recent work, we provided evidence that two WRKY TFs in rice, OsWRKY21 and OsWRKY108, interact with each other to maintain the basal/constitutive expression level of a Pi transporter gene, OsPHT1;1, under Pi-sufficient conditions.Citation13 The transgenic rice plants expressing OsWRKY21-SRDX (a chimeric protein converting OsWRKY21 from activator into suppressor) showed lowered Pi accumulation under high Pi (HP: 300 μM) and control (Ctrl: 90 μM) conditions. Since the wrky21 wrky108 double mutant showed lowered Pi accumulation only under extremely high Pi condition (1 mM) but not HP or Ctrl condition, we concluded that OsWRKY21, OsWRKY108 and other unidentified WRKY TF(s) coordinately activate OsPHT1;1 expression.Citation13 Interestingly, unlike that found in Pro35S:OsWRKY21-SRDX plants, rice plants expressing OsWRKY108-SRDX did not show any alteration in Pi accumulation or OsPHT1;1 expression.Citation13 In the Figure 5c of Zhang et al.,Citation13 an unexpected band with a molecular weight approaching 100 kDa is detected. This band, which is almost double the size of the GST-OsWRKY108 monomer (c. 55 kDa), was also detected when the recombinant proteins purified from Escherichia coli extracts were separated in the SDS-PAGE gel () or analyzed by immunoblot analysis (). These results suggest that OsWRKY108 can probably form a homodimer. In this study, we validated this possibility by using bimolecular fluorescent complementation assay (). On this basis, we reasoned that a protein complex comprised of endogenous OsWRKY108 and transgenic OsWRKY108-SRDX is formed, and that endogenous OsWRKY108 (activator) might counteract the effect of OsWRKY108-SRDX (suppressor) on OsPHT1;1 expression. This hypothesis seems to perfectly explain the lack of phenotype in the Pro35S:OsWRKY108-SRDX plants regarding OsPHT1;1 expression and Pi accumulation; however, the Pro35S:OsWRKY108-SRDX plants showed some unexpected phenotype, challenging this hypothesis.
Figure 1. WRKY108 forms a homodimer. (A) SDS-PAGE analysis of GST (left) and GST-WRKY108 (right) after IPTG induction and purified from E. coli BL21 (DE3). (B) Western blot assay indicate WRKY108 may form homodimer. Western blot analysis of GST (left) and GST-WRKY108 (right), GST and GST-WRKY108 were detected by anti-GST antibody. (C) BiFC analysis for the interaction within WRKY108. N- and C- terminal of YFP were fused to WRKY108 for BiFC assay
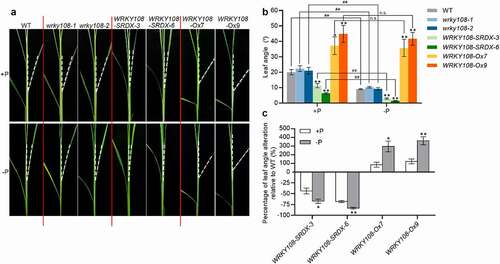
No visible phenotype was observed in oswrky108 mutants. Interestingly, the transgenic rice plants expressing OsWRKY108-SRDX but not that expressing OsWRKY21-SRDX showed a dramatic decrease in leaf inclination; conversely, OsWRKY108 overexpression plants displayed significantly increased leaf angle (). In addition, OsWRKY21 overexpressors also showed enlarged leaf angle (Supplemental Figure S1). Notably, it seems that the regulation of leaf inclination by OsWRKY108 and OsWRKY21 occurs in a Pi-independent manner, since OsWRKY108-SRDX plants and OsWRKY108/21 overexpressors showed altered leaf inclination under both Pi-replete and Pi-starvation conditions (). However, two lines of evidence suggest that OsWRKY108 but not OsWRKY21 exerts its role in leaf inclination via a Pi-dependent manner: (1) the response of leaf inclination to Pi starvation was absent in OsWRKY108 overexpressors (); (2) In both OsWRKY108-SRDX plants and OsWRKY108 overexpressors, the ‘percentage of leaf angle alteration relative to wild-type (WT)’ under Pi-starvation condition was more significant than that under Pi-replete condition (). The partial overlapped and diverged roles of OsWRKY108 and OsWRKY21 suggest that they may recruit distinct counterparts (other unknown WRKY TFs) to maintain P homeostasis and/or leaf inclination. These results together with our reported findingsCitation13 indicate that OsWRKY108 is a positive regulator of both Pi uptake and leaf inclination. By contrast, OsSPX1 and OsSPX2 act as negative regulators of Pi uptake and leaf inclination.Citation12,Citation14,Citation15 Large leaf angle means a relatively high demand for Pi due to enhanced incoming light and photosynthesis which is Pi-consuming, whereas erect leaf may attenuate incoming light and photosynthesis, lowering the demand for Pi. Thus, in a biological view, it makes perfect sense that negative regulators of Pi uptake suppress leaf inclination (e.g. OsSPX1/2), while positive regulators of Pi uptake tend to promote leaf inclination (e.g. OsWRKY108; ).
Figure 2. WRKY108 positively regulates leaf inclination. (A) Phenotype of wild type (WT), wrky108 mutants, WRKY108-SRDX and WRKY108 overexpression plants under +P and -P conditions. The 10-d-old seedlings (the third leaves were fully expanded) were transferred to +P (90 μM) and -P (0 μM) 1/2 Kimura B nutrient solution until the sixth leaves were fully expanded. The fourth leaves were used for observation. (B) Measurement of leaf angle of WT, wrky108 mutants, WRKY108-SRDX and WRKY108 overexpression plants under +P and -P conditions. The fourth leaves were used for measurement. Error bars mean SE (n = 10). Data significantly different from the corresponding controls are indicated (WRKY108-SRDX plants and WRKY108 overexpressors versus WT: *P<.05, **P<.01; Student’s t-test. -P plants versus +P plants: ##P< .01, n.s., not significant; Student’s t-test.). (C) Quantification of the leaf angle alteration of WRKY108-SRDX plants and WRKY108 overexpressors under +P and – P conditions. ×100%. A negative value means a decrease of leaf angle, and a positive value means an increase of leaf angle. Error bars mean SE (n = 10). Data significantly different from the corresponding controls are indicated (*P <.05, **P <.01; Student’s t-test)
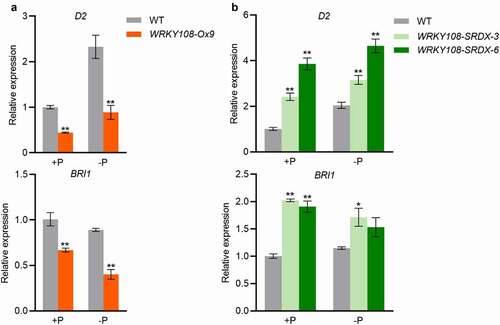
Brassinosteroid (BR) has been demonstrated to be the major phytohormone determining leaf angle.Citation8,Citation16 To investigate the potential molecular mechanisms underlying OsWRKY108 regulation of leaf inclination, we examined the expression of a suite of genes involved in BR biosynthesis and signaling. Given that OsWRKY108-SRDX plants and OsWRKY108 overexpressors showed decreased and increased leaf angles, respectively (), the genes whose expression show opposite alteration trends (compared with WT) between OsWRKY108-SRDX plants and OsWRKY108 overexpressors could be reasonable candidates of OsWRKY108 direct targets. The expression of two genes (D2 and Brassinosteroid Insensitive 1 [BRI1]) showed such opposite alteration trends. D2 and BRI1 were down-regulated in OsWRKY108-SRDX plants, whereas up-regulated in OsWRKY108 overexpressors (). However, D2 and BRI1 both play a positive role in leaf inclination,Citation17,Citation18 thus the shift in their expression is probably an indirect effect and cannot explain the phenotypes of OsWRKY108-SRDX plants and OsWRKY108 overexpressors.
Figure 3. WRKY108 affects BR biosynthesis and signaling. The 10-d-old seedlings (the third leaves were fully expanded) were transferred to +P (90 μM) and -P (0 μM) 1/2 Kimura B nutrient solution until the sixth leaves were fully expanded. All leaf collars were harvested for RNA extraction and RT-qPCR. D2 and BRI1 were detected in WRKY108 overexpressors (A) and WRKY108-SRDX plants (B). The relative expression in the WT under +P condition was set as 1. Error bars mean SE (n = 4). Data significantly different from the corresponding controls are indicated (*P<.05, **P<.01; Student’s t-test)
Despite of the aforementioned findings, it would be of interest and significance to address the following open questions in future work
(1) Several cytological changes in leaf collar can affect leaf inclination, such as altered cell length of the adaxial and/or abaxial cells,Citation12,Citation19 and altered proliferation of abaxial sclerenchyma cells and expansion of adaxial cells.Citation20 The effect of OsWRKY108 on leaf collar development at the cytological level needs to be investigated.
(2) OsWRKY108-SRDX did not show changed Pi accumulation under Pi-replete conditions.Citation13 We reasoned that the endogenous OsWRKY108 (activator) might form a homodimer with OsWRKY108-SRDX fusion protein (repressor), thus counteracting the effect of OsWRKY108-SRDX. However, this cannot explain why OsWRKY108-SRDX plants showed an effect on leaf inclination. It is likely that the regulations of Pi uptake and leaf angle by OsWRKY108 may occur in a dose-dependent manner, namely more OsWRKY108-SRDX proteins are required to counteract the endogenous OsWRKY108 in the P signaling pathway than that in the cascade controlling leaf angle. Nonetheless, the potential distinct regulatory mechanisms of OsWRKY108 underlying the maintenance P uptake and leaf angle need to be verified.
(3) In addition to BR, several other phytohormones (e.g. auxin) have also been found to be implicated in leaf inclination, and show extensive crosstalk.Citation8,Citation10,Citation16 The downstream targets of OsWRKY108 involved in leaf inclination and the biosynthesis/signaling of phytohormone(s) await to be identified.
(4) OsWRKY21-OsWRKY108-OsPHT1;1 is independent of the central Pi signaling pathway characterized by the SPX-PHR module.Citation13,Citation15,Citation21 That whether the involvement of OsWRKY108 in regulating leaf inclination is coupled to the functioning of the OsSPX1/2-OsRLI1 module is also worth studying.Citation12
In conclusion, our findings provide novel molecular evidence that plant Pi status positively correlates with leaf inclination, with OsWRKY108 serving as a linking node of the networks of Pi signaling and hormone signaling. Given that nutrient signaling occurred earlier than hormone signaling during plant evolution and has evolved to interact with the more recent hormone signaling,Citation22 further dissection of the OsWRKY108-implicated processes might provide new insights into the origin and evolution of these signaling cascades as well.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Raghothama KG. Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:1–4. doi:https://doi.org/10.1146/annurev.arplant.50.1.665.
- Wang Y, Chen YF, Wu WH. Potassium and phosphorus transport and signaling in plants. J Integr Plant Biol. 2021;63:34–52. doi:https://doi.org/10.1111/jipb.13053.
- Elliott DE, Reuter DJ, Reddy GD. Phosphorus nutrition of spring wheat (Triticum aestivum L.). 1. Effects of phosphorus supply on plant symptoms, yield, components of yield, and plant phosphorus uptake. Aust J Agric Res. 1997;48:855–868. doi:https://doi.org/10.1071/A96159.
- Mghase JJ, Shiwachi H, Takahashi H, Irie K. Nutrition deficiencies and their symptoms in upland rice. J ISSAAS. 2011;17:59–67.
- Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006;141:924–931. doi:https://doi.org/10.1104/pp.106.077081.
- Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, Mizutani M, Sakata K, Takatsuto S, Yoshida S, et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol. 2006;24:105–109. doi:https://doi.org/10.1038/nbt1173.
- Mantilla-Perez MB, Salas Fernandez MG. Differential manipulation of leaf angle throughout the canopy: current status and prospects. J Exp Bot. 2017;68:5699–5717. doi:https://doi.org/10.1093/jxb/erx378.
- Zhou LJ, Xiao LT, Xue HW. Dynamic cytology and transcriptional regulation of rice lamina joint development. Plant Physiol. 2017;174:1728–1746. doi:https://doi.org/10.1104/pp.17.00413.
- Tian JG, Wang CL, Xia JL, Wu LS, Xu GH, Wu WH, Li D, Qin WC, Chen QY, Jin WW, et al. Teosinte ligule allele narrows plant architecture and enhances high-density maize yields. Science. 2019;365:658–664. doi:https://doi.org/10.1126/science.aax5482.
- Li X, Wu PF, Lu Y, Guo SY, Zhong ZJ, Shen RX, Xie QJ. Synergistic interaction of phytohormones in determining leaf angle in crops. Int J Mol Sci. 2020;21:5052. doi:https://doi.org/10.3390/ijms21145052.
- Guo JF, Li W, Shang LG, Wang YG, Yan P, Bai YH, Da XW, Wang K, Guo QQ, Jiang RR, et al. OsbHLH98 regulates leaf angle in rice through transcriptional repression of OsBUL1. New Phytol. 2021;230:1953–1966. doi:https://doi.org/10.1111/nph.17303.
- Ruan WY, Guo MN, Xu L, Wang XQ, Zhao HY, Wang JM, Yi KK. An SPX-RLI1 module regulates leaf inclination in response to phosphate availability in rice. Plant Cell. 2018;30:853–870. doi:https://doi.org/10.1105/tpc.17.00738.
- Zhang J, Gu M, Liang RSH, Shi XY, Chen LL, Hu X, Wang SC, Dai XL, Qu HY, Li HH, et al. OsWRKY21 and OsWRKY108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1; 1 under phosphate-replete conditions. New Phytol. 2021;229:1598–1614. doi:https://doi.org/10.1111/nph.16931.
- Liu F, Wang ZY, Ren HY, Shen C, Li Y, Ling HQ, Wu CY, Lian XM, Wu P. OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J. 2010;62:508–517. doi:https://doi.org/10.1111/j.1365-313X.2010.04170.x.
- Wang ZY, Ruan WY, Shi J, Zhang L, Xiang D, Yang C, Li CY, Wu ZC, Liu Y, Yu YN, et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc Natl Acad Sci USA. 2014;111:14953–14958. doi:https://doi.org/10.1073/pnas.1404680111.
- Tong HN, Xiao YH, Liu DP, Gao SP, Liu LC, Yin YH, Jin Y, Qian Q, Chu CC. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell. 2014;26:4376–4393. doi:https://doi.org/10.1105/tpc.114.132092.
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell. 2003;15:2900–2910. doi:https://doi.org/10.1105/tpc.014712.
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell. 2000;12:1591–1605. doi:https://doi.org/10.1105/tpc.12.9.1591.
- Zhao SQ, Xiang JJ, Xue HW. Studies on the rice LEAF INCLINATION1 (LC1), an IAA-amido synthetase, reveal the effects of auxin in leaf inclination control. Mol Plant. 2013;6:174–187. doi:https://doi.org/10.1093/mp/sss064.
- Sun SY, Chen DH, Li XM, Qiao SL, Shi CN, Li CX, Shen HY, Wang XL. Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Dev Cell. 2015;34:220–228. doi:https://doi.org/10.1016/j.devcel.2015.05.019.
- Wang F, Deng MJ, Xu JM, Zhu XL, Mao CZ. Molecular mechanisms of phosphate transport and signaling in higher plants. Semin Cell Dev Biol. 2018;74:114–122. doi:https://doi.org/10.1016/j.semcdb.2017.06.013.
- Fichtner F, Dissanayake IM, Lacombe B, Barbier F. Sugar and nitrate sensing: a multi-billion-year story. Trends Plant Sci. 2021;26(4):352–374. doi:https://doi.org/10.1016/j.tplants.2020.11.006.
