ABSTRACT
The phytohormones jasmonates (JAs) regulate diverse aspects of plant growth and defense responses. The JA-ZIM domain (JAZ) family of repressors are targeted by the JA receptor Coronatine Insensitive 1 for ubiquitination and subsequent degradation via the 26S proteasome. We previously investigated the functions of JAZs in JA responses by analyzing jaz mutants of the phylogenetic group I (jaz1/2/5/6), group II/III (jaz10/11/12), group IV/V (jaz3/4/7/9 and jaz3/4/7/8/9), and their high-order mutant jaz1/2/3/4/5/6/7/9/10/11/12. Here, we examined JA-regulated root growth, apical hook curvature, flowering time, and defense against the insect Spodoptera exigua in the intermediate jaz mutants jaz1/2/5/6/10/11/12, jaz1/2/3/4/5/6/7/9, and jaz3/4/7/8/9/10/11/12. This study shows that these jaz mutants differentially affect JA responses, suggesting the complexity of JA pathway in these multiple jaz mutants.
The phytohormones jasmonates (JAs) control plant growth and development, such as root growth, apical hook curvature, flowering, and stamen development, and affect plant resistance and defense responses against insect attacks.Citation1–5 The JA-ZIM (JAZ) domain proteins function as core repressors of JA signaling and responses.Citation6–8 In response to JA signals, the JAZ repressors are recruited and ubiquitinated by the JA receptor Coronatine Insensitive 1 (COI1), and are subsequently degraded by the 26S proteasome, leading to activation of JAZs-repressed transcriptional responses and JA responses.Citation6,Citation7,Citation9,Citation10
The thirteen Arabidopsis JAZ repressors are classed into phylogenetic group I (JAZ1/2/5/6), II (JAZ10), III (JAZ11/12), IV (JAZ3/4/9), and V (JAZ7/8/13).Citation6,Citation7,Citation11 –13 The jaz1–1/2-2/5-1/6-5 mutant of group I is partially insensitive to JA in root growth inhibition.Citation13 The jaz10–1/11–1/12–1 mutant of group II/III is hypersensitive to JA in root growth inhibition.Citation13 jaz1/2/5/6 and jaz10/11/12 are more susceptible to the Spodoptera exigua larvae than wild-type.Citation13 The jaz3-5/4-1/7-1/9-1 and jaz3-5/4-1/7-1/8-1/9-1 mutants of group IV/V flower later than wild-type.Citation13 The high-order jaz mutants targeting most of JAZs, including jaz1-1/2-2/3-5/4-1/5-1/6-5/7-1/9-1/10–1/11–1/12–1,Citation13 jaz1-2/2–3/3-4/4-1/5–1/6–4/7–1/9–4/10–1/13–1 (jazD),Citation12 and jaz1-2/2–3/3–4/4–1/5– 1/6–4/7–1/8–VCitation2/9–4/10–1/13–1 (jazU),Citation12 are hypersensitive to JA-inhibitory root growth and apical hook curvature, hyposensitive to ethylene (ET)-enhanced apical hook curvature, more resistant to insect herbivores, and flower later than wild-type.Citation12,Citation13
To further understand JAZ family in JA responses, we performed genetic crosses with our previously characterized jaz mutants,Citation13 and constructed intermediate jaz mutants, including jaz1-1/2-2/5-1/6-5/10–1/11–1/12–1 of group I/II/III, jaz1-1/2-2/3-5/4-1/5-1/6-5/7-1/9-1 targeting group I/IV/V(JAZ7), jaz3-5/4-1/7-1/8-1/9-1/10-1/11-1/12-1 for group II/III/IV/V(JAZ7/8), and analyzed their JA responses.
The primary root growth of wild-type was inhibited by methyl-jasmonate (MeJA), and the undecuple jaz1/2/3/4/5/6/7/9/10/11/12 mutant control exhibited shorter roots under either mock or MeJA treatment ().Citation13 The primary root growth of jaz1/2/5/6/10/11/12 and jaz1/2/3/4/5/6/7/9 was similar to that of wild-type, while the root of jaz3/4/7/8/9/10/11/12 was shorter than that of jaz1/2/3/4/5/6/7/9/10/11/12 under mock or MeJA treatment ().
Figure 1. JA-regulated root growth and apical hook curvature in the multiple jaz mutants. (a) Primary root length of 11-day-old seedlings of the Col-0 wild-type, jaz1/2/5/6/10/11/12, jaz1/2/3/4/5/6/7/9, jaz3/4/7/8/9/10/11/12, and the jaz1/2/3/4/5/6/7/9/10/11/12 control grown on MS medium containing 0, 5 or 20 μM MeJA under a 16-h (20–22°C)/8-h (18–20°C) light/dark photoperiod. Error bars represent SE (n = 20). (b, c) Apical hook curvatures (b) and hook phenotypes (c) of 4-day-old dark grown etiolated seedlings of the Col-0 wild-type, jaz1/2/5/6/10/11/12, jaz1/2/3/4/5/6/7/9, jaz3/4/7/8/9/10/11/12, and the jaz1/2/3/4/5/6/7/9/10/11/12 control on MS medium supplied with mock, 5 μM MeJA, 10 μM ACC (1-aminocyclopropane-1-carboxylic acid, the ethylene biosynthesis precursor), or 5 μM MeJA plus 10 μM ACC. Data are means ±SE (n = 15). Letters indicate significant differences by Tukey’s HSD test (P < .05).
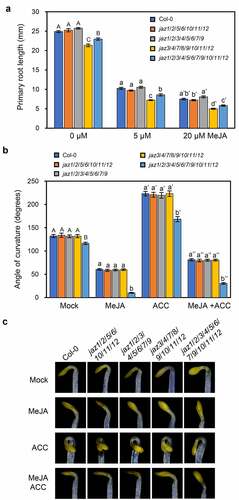
JA represses the apical hook curvature of etiolated Arabidopsis wild-type seedlings, while ET functions oppositely and antagonizes the JA action.Citation14 The jaz1/2/3/4/5/6/7/9/10/11/12 control displayed reduced hook curvature, was hypersensitive to JA-inhibited hook curvature and partially insensitive to ACC (ET precursor)-strengthened hook curvature (,).Citation13 The jaz1/2/5/6/10/11/12, jaz1/2/3/4/5/6/7/9 and jaz3/4/7/8/9/10/11/12 mutants were indistinguishable from wild-type in JA/ET-regulated apical hook curvature (,), indicating the high redundancy of JAZ members in JA/ET-regulated hook curvature.
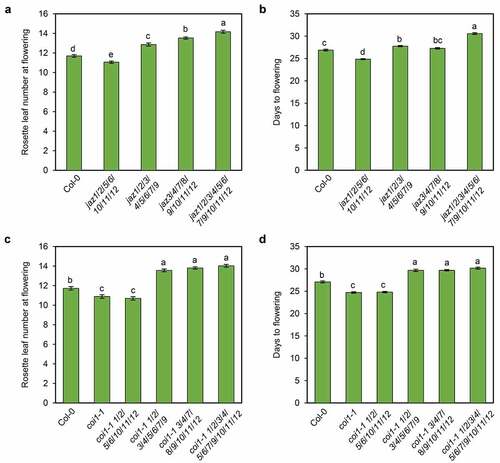
jaz1/2/3/4/5/6/7/9/10/11/12 flowers later than wild-type, while coi1-1 flowers earlier (,).Citation13 Considering rosette leaf numbers at flowering or days to flowering (the appearance of a 1 cm-long boltCitation15), jaz1/2/5/6/10/11/12 flowered earlier than wild-type (,). On the other hand, jaz1/2/3/4/5/6/7/9 and jaz3/4/7/8/9/10/11/12 flowered later than wild-type, but earlier than jaz1/2/3/4/5/6/7/9/10/11/12 (,). jaz1/2/3/4/5/6/7/9 and jaz3/4/7/8/9/10/11/12 suppressed the early flowering phenotype of coi1-1, to the degree of coi1-1 jaz1/2/3/4/5/6/7/9/10/11/12, while jaz1/2/5/6/10/11/12 did not obviously affect the flowering time of coi1-1 (,).
Figure 2. Flowering time in the multiple jaz mutants. Rosette leaf number at flowering (a, c) and days to flowering (b, d) of the Col-0 wild-type, jaz1/2/3/4/5/6/7/9, jaz3/4/7/8/9/10/11/12, and the jaz1/2/3/4/5/6/7/9/10/11/12 control (a, b), or Col-0, coi1-1, and the indicated high-order jaz mutants in coi1-1 background (c, d) grown under a 16-h (20–22°C)/8-h (18–20°C) light/dark photoperiod. Data are means ±SE (n = 36). Letters indicate significant differences by one-way ANOVA analysis with Tukey’s HSD post hoc test (P < .05).
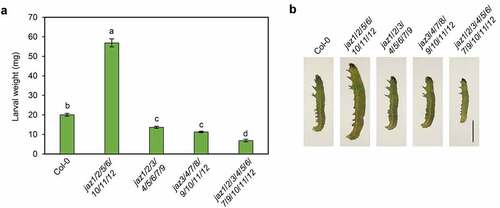
jaz1/2/3/4/5/6/7/9/10/11/12 is more resistant than wild-type in defense against the pest Spodoptera exigua (,).Citation13The S. exigua larvae fed with jaz1/2/3/4/5/6/7/9 and jaz3/4/7/8/9/10/11/12 gained less weight than those with wild-type, but more weight than those with jaz1/2/3/4/5/6/7/9/10/11/12 (,). On the other hand, S. exigua larvae reared with jaz1/2/5/6/10/11/12 gained more weight than those with wild-type (,), suggesting that jaz1/2/5/6/10/11/12 is more susceptible to S. exigua than wild-type.
Figure 3. Multiple JAZs mutations differentially affect defense against insect attack. (a) and (b) Larval weights (a) and representative S. exigua larvae (b) after feeding for 14 days (a) or 7 days (b) on Col-0, jaz1/2/3/4/5/6/7/9, jaz3/4/7/8/9/10/11/12, and the jaz1/2/3/4/5/6/7/9/10/11/12 control. 7-day-old seedlings grown under a 16-h (20–22°C)/8-h (18–20°C) light/dark photoperiod were transplanted into soil and grown under a 10-h (20–22°C)/14-h (18–20°C) light/dark photoperiod. The newly hatched S. exigua larvae were reared on 6-week-old plants. Error bars represent SE (n = 15). Scale bar = 0.2 cm. Letters indicate significant differences by one-way ANOVA analysis with Tukey’s HSD post hoc test (P < .05).
In conclusion, these results showed that the three high-order jaz mutants exhibited different effects on JA responses, including root growth, apical hook curvature, flowering time, and defense against insects, and indicate the redundancy of JAZ members and complexity of JA pathway in high-order jaz mutants.
Acknowledgments
We thank Junqiao Song and Shihai Pang for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wasternack C, Feussner I. The oxylipin pathways: biochemistry and function. Annu Rev Plant Biol. 2018;69:363–12. doi:10.1146/annurev-arplant-042817-040440.
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. doi:10.1146/annurev.arplant.043008.092007.
- Howe GA, Major IT, Koo AJ. Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol. 2018;69:387–415. doi:10.1146/annurev-arplant-042817-040047.
- Huang H, Liu B, Liu L, Song S. Jasmonate action in plant growth and development. J Exp Bot. 2017;68:1349–1359. doi:10.1093/jxb/erw495.
- Sun JQ, Jiang HL, Li CY. Systemin/Jasmonate-mediated systemic defense signaling in tomato. Mol Plant. 2011;4:607–615. doi:10.1093/mp/ssr008.
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi:10.1038/nature06006.
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi:10.1038/nature05960.
- Yan Y, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi:10.1105/tpc.107.050708.
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi:10.1126/science.280.5366.1091.
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, et al. The arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009;21:2220–2236. doi:10.1105/tpc.109.065730.
- Thireault C, Shyu C, Yoshida Y, St Aubin B, Campos ML, Howe GA. Repression of jasmonate signaling by a non-TIFY JAZ protein in arabidopsis. Plant J. 2015;82:669–679. doi:10.1111/tpj.12841.
- Guo Q, Yoshida Y, Major IT, Wang K, Sugimoto K, Kapali G, Havko NE, Benning C, Howe GA. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc Natl Acad Sci U S A. 2018;115:E10768–E77. doi:10.1073/pnas.1811828115.
- Liu B, Seong K, Pang S, Song J, Gao H, Wang C, Zhai J, Zhang Y, Gao S, Li X, et al. Functional specificity, diversity, and redundancy of Arabidopsis JAZ family repressors in jasmonate and COI1-regulated growth, development, and defense. New Phytol. 2021;231:1525–1545. doi:10.1111/nph.17477.
- Song S, Huang H, Gao H, Wang J, Wu D, Liu X, Yang S, Zhai Q, Li C, Qi T, et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in arabidopsis. Plant Cell. 2014;26:263–279. doi:10.1105/tpc.113.120394.
- Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc Natl Acad Sci U S A. 2000;97:3753–3758. doi:10.1073/pnas.97.7.3753.
