ABSTRACT
The PIEZO protein family was first described in animals where these mechanosensitive calcium channels perform numerous essential functions, including the perception of light touch, shear, and compressive forces. PIEZO homologs are present in most eukaryotic lineages and recently we reported that two PIEZO homologs from moss Physcomitrium patens localize to the vacuolar membrane and modulate its morphology in tip-growing caulonemal cells. Here we show that predicted structures of both PpPIEZO1 and PpPIEZO2 are very similar to that of mouse Piezo2. Furthermore, we show that both moss PIEZO genes are ubiquitously expressed in moss vegetative tissues and that they are not required for normal vacuolar pH or intracellular osmotic potential. These results suggest that moss PIEZO proteins are widely expressed mechanosensory calcium channels that serve a signaling rather than maintenance role in vacuoles.
Introduction
Mechanosensitive ion channels are one of the main mechanisms by which cells perceive mechanical forces. In response to lateral membrane tension, these channels open and release ions down their electrochemical gradients.Citation1–3The PIEZO protein family was first described in animalsCitation4 and has been implicated in the perception of light touch, shear stress, compressive forces, nociception, and other mechanostimuli. Animal PIEZO channels are embedded in the plasma membrane and conduct cations, including calcium.Citation4–6 Cryo-EM structures of two mouse homologs showed that animal PIEZOs form large propeller-shaped complexes comprised of three identical subunits.Citation7–11 Each subunit has 38 transmembrane domains, the last two of which are called the inner and outer helix and form the pore module (). The pore module also includes the cap domain, which connects the inner and outer helix and sits above the pore, the anchor domain, and the C-terminal domain. The first 36 transmembrane domains of each monomer are organized in 9 consecutive PIEZO repeats to form the mechanotransduction module. The beam domain connects the mechanotransduction module to the pore module.Citation7–11
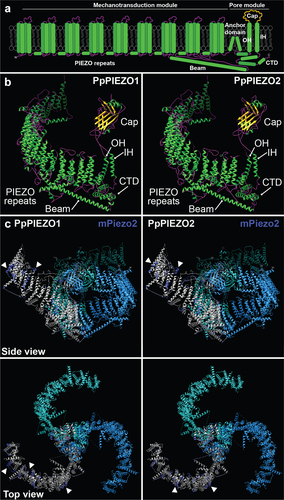
Figure 1. Models of PpPIEZO1 and PpPIEZO2 predicted protein structures. (a) Diagram of a typical PIEZO monomer, based on cryo-EM structures of mPiezo1 and mPiezo2. Not drawn to scale. OH, outer helix; IH, inner helix; CTD, C-terminal domain. (b) Phyre2-generated models of PpPIEZO1 and PpPIEZO2 monomers. Helices, strands/sheets, and coils are depicted in green, yellow, and magenta, respectively. (c) Predicted models of PpPIEZO1 and PpPIEZO2 monomers (gray) superimposed onto one subunit of the mPiezo2 homotrimeric complex (dark blue). The other two subunits of the mPiezo2 complex (light blue and cyan) are shown without the overlay. Models were visualized with UCSF Chimera software.Citation12
While both the structure and functions of animal PIEZOs are well-described, little is known about their homologs in plants. We recently reported that two PIEZO homologs from the moss Physcomitrium patens (PpPIEZO1 and PpPIEZO2) are required for normal growth and cytoplasmic calcium oscillations in the tip-growing moss caulonemal cells.Citation13 Surprisingly, moss PIEZOs localized to the vacuolar membrane, in contrast to their animal counterparts. Plant vacuoles are large aqueous organelles with numerous functions, including storage of ions and metabolites, maintenance of cellular osmotic potential and pH, and degradation.Citation14 Vacuolar morphology is highly cell-type specific and dynamic, often changing in response to external or internal cues.Citation15 Moss PIEZOs may be involved in this process, as the disruption of PpPIEZO1/2 led to a dramatic expansion of tubule-like vacuoles normally seen in WT caulonemal cells. This vacuolar localization and function appear to be conserved among land plant PIEZOs, as we observed a similar vacuolar expansion in pollen tubes from Arabidopsis thaliana lines lacking the single PIEZO homolog AtPIEZO1.Citation13 AtPIEZO1 has also been implicated in the suppression of systemic viral spreadCitation16 and mechanotransduction in the root tip,Citation17,Citation18 and a chimera containing the pore of AtPIEZO1 within the mPIEZO1 sequence has mechanosensitive ion channel activity.Citation19 Here we further explore the predicted structure, tissue-specific expression patterns, and physiological roles of moss PIEZO homologs.
Predicted structures of PpPIEZO1 and PpPIEZO2
We used Phyre219 to predict the structures of PpPIEZO1 and PpPIEZO2 using the cryo-EM structure of mPiezo2 (6KG7 model fromCitation7) as a template. For both proteins, 67% of the sequence was modeled. In the models, the main structures of the pore domainCitation7 () can be identified: cap, beam, inner and outer helix, and C-terminal domain (). Furthermore, the transmembrane domains that comprise the PIEZO repeats within the mechanotransduction module are organized into the characteristic propeller blade shape of other PIEZO channels.Citation7,Citation9
While there is little conservation of primary protein sequence between moss and mouse PIEZO homologs (20% identity and 34% similarity for full-length PpPIEZO1 and mPiezo2 and 20% identity and 33% similarity for full-length PpPIEZO2 and mPiezo2; calculated with EMBOSS NeedleCitation20) (Fig. S1), we wanted to assess their structural similarities. To this end, we superimposed the Phyre2 models of PpPIEZO1/2 monomers onto the mPiezo2 homotrimeric complex cryo-EM structure.Citation7 As shown in , the overall structures of moss and mouse PIEZO homologs are well conserved, with an almost complete overlap in the pore module. In the mechanotransduction module, a substantial overlap can also be observed. However, in a few regions along the blade (, arrowheads) some discrepancies between mouse and moss PIEZOs can be seen.
While using mPiezo2 as a template for modeling of PpPIEZO1/2 likely accounts for some of the similarities between the structures, these models show that moss PIEZO homologs could achieve a very similar organization to the animal ones. To provide further support for this idea, we used the AlphaFold2 program,Citation21,Citation22 to predict the structures of the C-terminal regions of mPiezo2, PpPIEZO1 and PpPIEZO2 (this region corresponds to the pore module and the last PIEZO repeat in mammalian PIEZO homologs). In all three cases, very similar structures with easily identifiable domains characteristic of PIEZO channels were obtained (Fig. S2).
This evidence for structural conservation between plant and animal homologs suggests that plant PIEZOs also function as mechanosensitive calcium channels, analogous to their animal counterparts. This is in line with our previous finding that moss PIEZOs are properly oriented within the vacuolar membrane to release calcium from the vacuolar stores into the cytosol,Citation13 as well as with a recent report from Mousavi et al.Citation18 that a chimeric Arabidopsis/mouse PIEZO channel can conduct calcium in response to mechanical force. We further note that the predicted full-length structures of several flowering plant PIEZO homologs present in the AlphaFold Database show a striking resemblance to animal PIEZO cryo-EM structuresCitation7–11 (alphafold.ebi.ac.uk/search/text/PIEZO).
Expression pattern of PpPIEZO1 and PpPIEZO2 in moss vegetative tissues
In our previous work,Citation13 we focused on the function of PpPIEZOs in caulonemal cells. The publicly available microarray dataset available on the Physcomitrella eFP Browser suggested their ubiquitous expression in all moss tissues. Here we set out to validate such a broad expression pattern by isolating RNA from protonemal cells, caulonemal cells, rhizoids, gametophores, and protoplasts and determining PpPIEZO1 and PpPIEZO2 expression levels using qPCR. As can be seen in , mRNA transcripts for both PpPIEZO1 and PpPIEZO2 were detected in all tissues tested. Levels of PpPIEZO1 transcripts were comparable in all tissues, except in gametophores where higher transcript levels were detected. PpPIEZO2 transcript levels were lower in juvenile tissue (protonema and caulonema) compared to mature tissues (gametophores and rhizoids) and protoplasts. We did not test expression in the reproductive organs.
Figure 2. PpPIEZO1 and PpPIEZO2 are ubiquitously expressed in moss vegetative tissues. Relative expression of PpPIEZO1 (left) and PpPIEZO2 (right) in various moss vegetative tissues. Ct values were normalized to the geometric mean of two housekeeping genes (Pp3c8_16590 and Pp3c14_7550). Datapoints shown are from three biological replicates, three technical replicates each (except for PpPIEZO1 protonema, where one outlier technical triplicate was removed from the second biological replicate). Bars, average values. Statistics, one-way ANOVA with Post-Hoc Tukey’s test (p < .05). Letters denote grouping based on statistical differences.
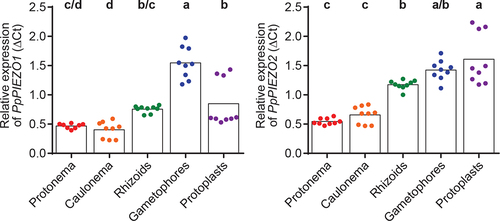
Both PpPIEZO transcripts accumulated to significantly higher levels in gametophore cells than in caulonemal cells, where PpPIEZOs play an important role in the modulation of vacuolar morphology.Citation13 As gametophore cells grow through cell expansion, these results suggest that PpPIEZO channel function is not limited to tip-growing caulonemal cells. Higher PpPIEZO expression could be an adaptation to cell expansion-based growth, as comparted to tip growth, or could be linked to a specific need associated with vacuolar morphology or mechanical signaling in this cell type. Future experiments to examine the localization of PIEZO1 and PIEZO2 in these tissues, and to understand the effect of PIEZO1 and PIEZO2 mutations on vacuolar morphology and on the mechanical responsiveness of gametophytic tissue will help address these questions.
Vacuolar pH and osmotic potential in PpPIEZO knock-out moss lines
Our previous work showed that deletion of moss PIEZO homologs leads to altered growth and dramatic expansion of the vacuoles in the tip region of the apical caulonemal cells.Citation13 We next wondered whether deletion of PpPIEZOs might affect vacuolar function as well as morphology. As mentioned above, vacuoles play a role in the maintenance of pH homeostasis and are characterized by a lower luminal pH than the surrounding cytoplasm.Citation14 We therefore tested whether PpPIEZO1/2 double mutants (ΔPP1/2) have different vacuolar pH than the WT by staining cells with the pH-sensitive dye 2′,7′-bis-(Carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF). We imaged BCECF-stained vacuoles in apical caulonemal cells and found no difference in 488/445 nm ratio values between WT and ΔPP1/2 cells (). This shows that ΔPP1/2 cells are able to maintain normal vacuolar pH, despite striking changes in their morphology. It thus appears that PpPIEZO activity does not affect the systems important for vacuolar pH maintenance.
Figure 3. PpPIEZO1/2 mutants have normal vacuolar pH and intracellular osmotic potential. (a) Ratio of BCECF fluorescence after excitation with 488 and 445 nm light. Datapoints shown are individual cells from six experiments, each normalized to their respective WT average (N = 84, 75, and 61 cells for WT, ΔPP1/2-I, and ΔPP1/2-II, respectively). Statistics, Kruskal-Wallis test with Dunn’s multiple comparisons test (P < .05). (b) Percentage of non-plasmolyzed apical caulonemal calls after 5 min exposure to BCDAT media supplemented with 350 mM mannitol. Datapoints shown are from three independent experiments. Each data point corresponds to the percentage calculated from 50 cells from a single plant. Bars, average values. Statistics, as in (A).
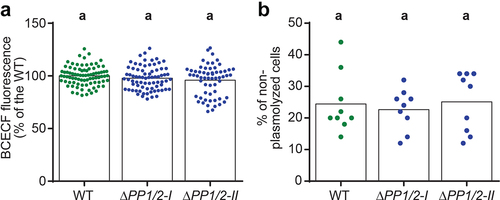
Vacuoles also participate in the maintenance of intracellular osmotic potential, so we tested whether WT and ΔPP1/2 cells differ in their osmotic potential. In cells with osmotic potential lower than that of the environment, the protoplast (cell content) will lose water, shrink, and detach from the cell wall in a process known as plasmolysis. On the other hand, in non-plasmolyzed cells, the osmotic potential is equal to or great than that of the environment. Thus, the likelihood of plasmolysis under hyperosmotic stress is often used as a proxy for relative intracellular osmotic potential.Citation23 We observed no significant difference in the percentage of non-plasmolyzed cells in 350 mM mannitol between WT and ΔPP1/2 caulonemal cells (), suggesting that the altered vacuolar morphology in ΔPP1/2 mutants does not affect their ability to maintain intracellular osmotic potential. These results also indicate that the observed growth defects in ΔPP1/2 mutant caulonemal cellsCitation13 cannot be attributed to differences in osmotic potential. Instead, it may be that the changes in cytosolic Ca2+ oscillatory profiles observed in ΔPP1/213 affect growth processes, or that the expanded vacuoles found in these cells prevent the proper spatial assembly of the cytoskeleton or other cellular elements.
Summary
The findings presented here supplement our recent publication,Citation13 and improve our understanding of PIEZO homologs in moss. PpPIEZOs have similar predicted structures as mPiezo2, providing support for a conserved molecular function as mechanosensitive cation channels. The fact that PpPIEZOs are ubiquitously expressed in vegetive tissues indicates that PIEZOs likely have functions beyond those already described in caulonemal cells, yet to be discovered. Finally, PpPIEZOs modulate the vacuolar morphology in caulonemal cells without affecting pH or osmotic potential. Altogether, these data suggest a model wherein moss PIEZOs function as mechanosensitive calcium channels that serve to modulate vacuolar morphology through signaling, rather than through general maintenance of vacuolar functions.
Methods
Tissue sampling and QPCR
Moss was cultured as previously described.Citation13 Protonemal cell samples were collected from plants cultured on cellophaned BCDAT media for 5 days after grinding. Caulonemal cells were manually cut and separated from dark-grown (described inCitation13) plants. Rhizoids and gametophores were collected from 5-week-old plants cultured on uncellophaned BCD plates (without ammonium-tartrate) under a standard light cycle. Gametophores together with rhizoids were pulled from the media and the two were separated with a scalpel. The middle section connecting the two tissue types was discarded. The excess water in the tissue was removed by squeezing between paper wipes, before freezing in liquid nitrogen. Protoplasts were isolated from 5-day-old protonema tissue as previously described.Citation13 Total RNA from each sample was isolated with a RNeasy Plant Mini Kit and on-column DNase treatment (Qiagen). 100 ng of RNA was then used to synthesize cDNA (Oligo(dT) primer) with Superscript IV (ThermoFisher).
For qPCR reactions, approximately 2 ng of cDNA was mixed with 2x PowerUp SYBR Green Master Mix (Applied Biosystems) and primers (Supplementary Table 1). Reactions were run on the StepOnePlus system from Applied Biosystems (95°C for 10 min; 40 cycles of 95°C for 15 s and 60°C for 1 min; followed by a melt curve). PpPIEZO1 and PpPIEZO2 transcript levels were normalized to the geometric mean of two housekeeping genes (Pp3c8_16590 and Pp3c14_7550;Citation24 Supplementary Table 1) using the ΔCt method.
BCECF staining and imaging
To evaluate vacuolar pH, 6-day-old WT and PpPIEZO1/2 double mutant (ΔPP1/2) plants (started from fragmented protonema, seeCitation13 for details) were stained with BCECF as described inCitation25 using liquid BCDAT media. Plants were mounted onto a coverslip at the bottom of a 35 mm Petri dish as described inCitation13 and imaged with an inverted Olympus FV3000 confocal microscope (objective UPLSAPO 60xW NA1.2). BCECF was excited with 445 and 488 nm, and in both cases, emission was collected in the 500–550 nm range. The 488/445 nm ratio (after background subtraction) is proportional to the luminal pH (higher ratio = higher pH).
Plasmolysis quantification
6-day-old WT and ΔPP1/2 plants (started from fragmented protonema) were mounted onto a coverslip at the bottom of a 35 mm Petri dish (seeCitation13 for details) and covered with 3 mL of BCDAT media supplemented with 350 mM mannitol. After a 5 min incubation, plants were imaged using brightfield and UPLSAPO 60xW NA1.2 objective. For each plant, 50 apical caulonema cells were evaluated for signs of plasmolysis (visible separation of the protoplast from the cell wall at the tip).
Supplemental Material
Download PDF (2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Basu D, Haswell ES. Plant mechanosensitive ion channels: an ocean of possibilities. Curr Opin Plant Biol. 2017;40:43–5. doi:10.1016/j.pbi.2017.07.002.
- Cox CD, Bavi N, Martinac B. Bacterial mechanosensors. Annu Rev Physiol. 2018;80:71–93. doi:10.1146/annurev-physiol-021317-121351.
- Ranaade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi:10.1016/j.neuron.2015.08.032.
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi:10.1126/science.1193270.
- Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem. 2014;289:31673–31681. doi:10.1074/jbc.R114.612697.
- Wu J, Lewis AH, Grandl J. Touch, tension, and transduction – the function and regulation of Piezo ion channels. Trends Biochem Sci. 2017;42:57–71. doi:10.1016/j.tibs.2016.09.004.
- Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, Li Y, Lei J, Li X, Xiao B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573:225–229. doi:10.1038/s41586-019-1505-8.
- Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527:64–69. doi:10.1038/nature15247.
- Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554:481–486. doi:10.1038/nature25453.
- Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017;6:e33660. doi:10.7554/eLife.33660.
- Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487–492. doi:10.1038/nature25743.
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi:10.1002/jcc.20084.
- Radin I, Richardson RA, Coomey JH, Weiner ER, Bascom CS, Li T, Bezanilla M, Haswell ES. Plant PIEZO homologs modulate vacuole morphology during tip growth. Science. 2021;373:586–590. doi:10.1126/science.abe6310.
- Tan X, Li K, Wang Z, Zhu K, Tan X, Cao J. A Review of Plant Vacuoles: formation, Located Proteins, and Functions. Plants. 2019;8:327. doi:10.3390/plants8090327.
- Cui Y, Zhao Q, Hu S, Jiang L. Vacuole biogenesis in plants: how many vacuoles, how many models? Trends Plant Sci. 2020;25:538–548. doi:10.1016/j.tplants.2020.01.008.
- Zhang Z, Tong X, Liu S-Y, Chai L-X, Zhu -F-F, Zhang X-P, Zou J-Z, Wang X-B. Genetic analysis of a Piezo-like protein suppressing systemic movement of plant viruses in Arabidopsis thaliana. Sci Rep. 2019;9:3187. doi:10.1038/s41598-019-39436-3.
- Fang X, Liu B, Shao Q, Huang X, Li J, Luan S, He K. AtPiezo plays an important role in root cap mechanotransduction. Int J Mol Sci. 2021;22:467. doi:10.3390/ijms22010467.
- Mousavi SAR, Dubin AE, Zeng WZ, Coombs AM, Do K, Ghadiri DA, Keenan WT, Ge C, Zhao Y, Patapoutian A. PIEZO ion channel is required for root mechanotransduction in Arabidopsis thaliana. Proc Natl Acad Sci. 2021;118:e2102188118. doi:10.1073/pnas.2102188118.
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi:10.1038/nprot.2015.053.
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi:10.1093/nar/gkz268.
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi:10.1038/s41586-021-03819-2.
- Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold - Making protein folding accessible to all. bioRxiv. 2021. doi:10.1101/2021.08.15.456425.
- Rabillé H, Billoud B, Tesson B, Le Panse S, É R, Charrier B. The brown algal mode of tip growth: keeping stress under control. PLoS Biol. 2019;17:e2005258. doi:10.1371/journal.pbio.2005258.
- Le Bail A, Scholz S, Kost B. Evaluation of reference genes for RT qPCR analyses of structure-specific and hormone regulated gene expression in physcomitrella patens gametophytes. PLoS One. 2013;8:e70998. doi:10.1371/journal.pone.0070998.
- Viotti C, Kruger F, Krebs M, Neubert C, Fink F, Lupanga U, Scheuring D, Boutte Y, Frescatada-Rosa M, Wolfenstetter S, et al. The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in arabidopsis. Plant Cell. 2013;25:3434–3449. doi:10.1105/tpc.113.114827.
