ABSTRACT
The small regulatory C-TERMINALLY ENCODED PEPTIDE (CEP) peptide family plays crucial roles in plant growth and stress response. However, little is known about this peptide family in Brassica species. Here, we performed a systematic analysis to identify the putative Brassica rapa L. CEP (BrCEP) gene family. In total, 27 BrCEP genes were identified and they were classified into four subgroups based on the CEP motifs similarity. BrCEP genes displayed distinct expression patterns in response to both developmental and several environmental signals, suggesting their broad roles during Brassica rapa development. Furthuremore, the synthetic BrCEP3 peptide accelerated Brassica rapa primary root growth in a hydrogen peroxide (H2O2) and Ca2+ dependent manner. In summary, our work will provide fundamental insights into the physiological function of CEP peptides during Brassica rapa development.
Introduction
Plant development and stress response are tightly regulated by external and internal signals. Recently, studies demonstrate that hormone-like peptides modulate plant growth in a manner similar to phytohormones.Citation1,Citation2 The Arabidopsis thaliana genome contains more than 7000 small encoding genes, and 200 of them are likely to encode hormone-like peptides.Citation2 The C-TERMINALLY ENCODED PEPTIDE (CEP) peptide family belongs to the post-translationally modified peptides, and they need proteolytic enzymes to cleave into bioactive forms, thus executing their physiological function.Citation3,Citation4 Each CEP protein contains a secretory N-terminal signal sequence, a central variable region, and highly conserved CEP motifs at C-terminus.Citation5–7 CEP peptides have been reported to play pivotal roles in various plant developmental programs.Citation8–14
Members of the CEP family have been uncovered in many plant species.Citation5–7,Citation9,Citation13–18 The CEP motif (15 amino acids in length) is the biological form of CEP peptide and application of synthetic CEP motifs have been shown to arrest both primary and lateral root development in Arabidopsis,Citation8,Citation10,Citation12,Citation13 and nodulation in Medicago truncatula.Citation19,Citation20 In addition, CEP genes expression is significantly induced by various abiotic stress, indicating that they also play critical roles in multiple abiotic stress responses.Citation5,Citation16,Citation21 Indeed, Arabidopsis CEP5 peptide regulates osmotic and drought stress response via interfering with auxin signaling.Citation21
CEP peptides also play vital roles in plant nitrogen (N) uptake and transport.Citation22–24 Under nitrogen starvation conditions, CEP peptides are transported from N rich side to N deficiency side, and are perceived by the receptor C-TERMINALLY ENCODED PEPTIDE RECEPTOR (CEPR) to upregulate nitrate transporter expression, resulted in N acquisition in roots.Citation22 The two identified shoot-derived polypeptides, CEP DOWNSTREAM 1 (CEPD1) and CEPD2, also act as long-distance mobile signals to upregulate the transcription of nitrate transporter genes, thus mediate systemic N-demand signaling downstream of the CEP-CEPR signaling.Citation23 CEPD-LIKE2 (CEPDL2) is a leaf-derived signal to regulate N uptake and transport. When roots are unable to obtain enough N to meet shoots N demand, CEPDL2 expression is significantly upregulated in a CEP-CEPRs dependent manner, CEPDL2 then regulates N uptake and transport via upregulating the transcription of N transporters.Citation24
Despite the essential role of CEP peptides in plant development, nitrogen signaling and stress responses, the physiological roles of CEP peptide in Brassica species, however, is poorly understood. Therefore, we selected Brassica rapa L., as a model plant with high economic impact to explore the potential roles of CEP peptide family. In this study, we performed a genome-wide search of putative CEP genes in Brassica rapa and studied their tissue-specific expression patterns as well as their expression in response to environmental stress. The synthetic BrCEP peptides further demonstrate their critical roles in Brassica rapa root development. Overall, our work will extend our understanding of the pivotal roles of small regulatory peptides in crop plants growth and their adaptions to the adverse environments.
Materials and methods
Identification of the BrCEP gene family
To identify the putative BrCEP genes, the full-length sequences of 15 CEP proteins from ArabidopsisCitation5,Citation6 were used to perform BLASTP against the Brassica rapa genome data base (https://phytozome.jgi.doe.gov/pz/portal.html, Brassica rapa FPsc V1.3). The identified hits were used as a new query to conduct BLASTP against the same database to avoid any missed BrCEP proteins. The BLASTP was performed repeatedly until no additional novel proteins were found. All the identified BrCEP proteins were aligned with the Arabidopsis CEP proteins, and proteins with a similar CEP domain were defined as BrCEP proteins.Citation5–7
BrCEP protein features analysis
SignalP 5.0 (http://www.cbs.dtu.dk/services/SignalP/), the TargetP (http://www.cbs.dtu.dk/services/TargetP), iPSORT (http://ipsort.hgc.jp) and SecretomeP (http://www.cbs.dtu.dk/services/SecretomeP-2.0) were used to predict N-terminal signal peptides of each BrCEP protein. Multiple Expectation Maximization for Motif Elicitation (MEME) was performed to discover the CEP motifs (http://meme.nbcr.net/meme/cgi-bin/meme.cgi).Citation25 Weblogo (http://weblogo.berkeley.edu/logo.cgi) was conducted to determinate BrCEP proteins domain features.Citation26 Average of isoelectric points and molecular weight of BrCEP proteins were analyzed via the ExPASy Proteomics Server tool (https://web.expasy.org/compute_pi/).
Genomic organization and chromosome localization of BrCEPs
The genomic sequences and corresponding coding sequences (CDS) of the BrCEPs were download. The genomic organization of the BrCEP genes were performed via gene structure display server with default settings (http://gsds.cbi.pku.edu.cn).Citation27 The distribution of the BrCEP genes on the chromosome were conducted via the MG2C online software with default settings (http://mg2c.iask.in/mg2c_v2.0/).
Identification of cis-acting regulatory elements
Upstream region (1500 bp) of the BrCEP genes were used to perform the cis-acting regulatory element analysis at PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).Citation28
Alignment and phylogenetic analysis
Multiple alignments were performed using ClustalX,Citation29 then refined and displayed using GeneDoc (http://www.psc.edu/biomed/genedoc). Phylogenetic trees were constructed by MEGA X using the conserved CEP domains or the full length of CEP proteins by the Maximum likelihood method.Citation30 Bootstrap analysis was conducted with 1000 replicates to verify the significance of nodes.
Expression patterns of BrCEPs
4 microarray datasets (GSE73963, GSE43245, GSE123654 and GSE55264) were collected from Gene Expression Omnibus database (GEO) at NCBI website to determine the BrCEP genes expression in a given developmental context or under environmental stress. Transcription abundances based on RNA-Seq data were normalized and calculated as log2 expression value. Heatmaps were generated based on the expression profiles via the online tool (https://www.omicstudio.cn/tool/4).
Synthesis of the BrCEP peptides and chemicals
The 15 amino acids motif of the BrCEP3 (DFRPTTPGHSPGIGH) and BrCEP26 (TFRPTVPGHSPGIGH) were sent for synthesis by DGpeptide company (http://www.dgpeptides.com/) with a purity higher than 90%. All peptides were dissolved in distilled water to a concentration of 10 mM and were aliquoted to avoid repeated freeze-thaw cycles, and were stored at -20°C. Catalase (CAT), diphenylene iodonium (DPI), hydrogen peroxide (H2O2) and lanthanum chloride were obtained from Macklin company (http://www.macklin.cn/).
Plant Material and Growth Conditions
The cultivar Brassica rapa seeds were bought from Beijing Dongsheng company (http://dsseed.fsfseed.com/Index.asp). All seeds were washed with 70% ethanol for 1 minute. Then seeds were sterilized with 0.1% HgCl2 for 8 minutes, afterward seeds were washed with distilled water for 5 times. The sterilized seeds were sown on 1% agar plate containing half-strength Murashige & Skoog medium (½MS). The seedlings were grown vertically at 21°C under a 16 h: 8 h (light: dark photoperiod) with a light intensity 112 μmol m−2 sec−Citation1 in a plant growth chamber.
Pharmacological treatments and root length quantification
After germination (roughly 2 days), the seedlings with a similar root length were transferred to new plates supplied with BrCEP3 (1 μM), BrCEP26 (1 μM), CAT (1000 units), DPI (10 μM) and lanthanum chloride (500 μM), H2O2 (1 mM) or the combination with BrCEP3 peptide, and the seedlings were cultured for another 4 days in the same growth chamber. Plates were imaged via the EPSON V370 scanner. The root length was quantified via ImageJ. At least three independent experiment was performed to yield the similar results. For each treatment, 10–15 roots were quantified.
H2O2 staining
The DAB (3,3′-diaminobenzidine, BIORIGIN company http://www.biorigin.ltd/, BN20341) staining kit was used to quantify H2O2 level in Brassica rapa roots, the staining was performed follow the instructions. Briefly, Brassica rapa seedlings treated with mock or 1 μM BrCEP3 peptide for 4 days were stained with 1× DAB buffer (0.1% DAB in 1× PBS buffer, pH 7.4) for 3–5 minutes at room temperature, then the staining was terminated by washing seedlings with 1× PBS buffer (0.01 M, pH 7.4) for 3 times. The samples were mounted on slides immediately, and were captured with an inverted BDS 400 microscopy (http://www.cnoptec.com/). Three independent biological repeats with 10 seedlings for each treatment were performed to yield a similar result. The H2O2 level was quantified via ImageJ.
Statistical analysis
All statistical analysis was performed using the one-way ANOVA test with a significant difference (* P < .05, ** P < .01, *** P < .001).
Results
Genome-wide search and characterization of BrCEP gene family
We used 15 Arabidopsis CEP (AtCEP) full protein sequences to perform BLASTP against the Brassica rapa genome database.Citation5,Citation6 As a result, 27 putative BrCEP genes were identified, and they displayed similar CEP motifs (, b). The corresponding coding sequence (CDS) of BrCEP genes ranged from 210 base pairs (BrCEP2) to 732 base pairs (BrCEP25) with the protein size ranging from 69 to 243 amino acids in length, respectively. The molecular weight and isoelectric point of BrCEP proteins ranged from 7902.06 Da (BrCEP2) to 26040.83 Da (BrCEP25) and from 4.71 (BrCEP5) to 10.82 (BrCEP14), respectively (Table S1).
Figure 1. Identification of BrCEP gene family. (a) Alignment of the 27 BrCEP CEP motifs. (b) WebLogo representation of the conserved BrCEP and AtCEP motifs. (c) Signal peptide of BrCEP proteins. The red boxes represent signal peptides, and the scissors represent the predicted signal peptide cleavage posotion. (d) WebLogo representation of the signal cleavage sites.
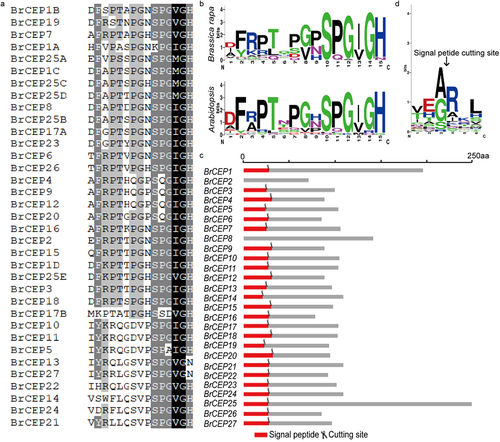
We next performed a detailed characterization of the BrCEP proteins, including CEP motif distribution, N-terminal signal peptide, genomic organization and chromosome localization. Based on the MEME analysis, BrCEP1 and BrCEP25 displayed four and five CEP domains, respectively; BrCEP8 contained two identical CEP domains; BrCEP17 contained two CEP domains (; ; Table S1). We next searched the presence and location of the putative N-terminal signal peptide cleavage sites in each BrCEP protein (; Table S2). Based on the prediction, it is likely that the cleavage site occurred at a conserved arginine site (; Table S2), which also has been shown for apple CEP proteins.Citation15 However, we could not find any cleavage site for BrCEP2 and BrCEP8 proteins, this may due to the limitations of the software.
Figure 2. Genomic organization and chromosome localization of BrCEP genes. (a) Gene structure of BrCEP genes. (b) Distribution of BrCEP genes on Brassica rapa chromosomes.
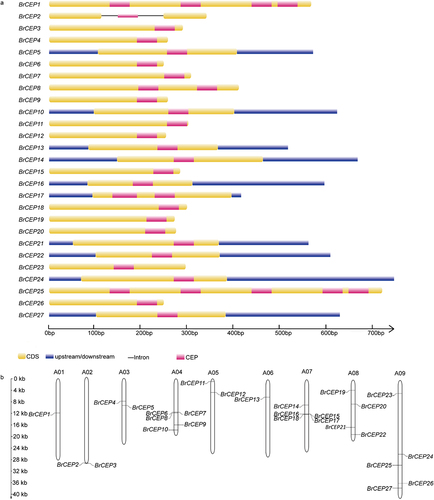
We also analyzed the genomic organization and chromosome localization of BrCEP genes. Only BrCEP2 contained two introns, the rest of the BrCEPs lack introns (). BrCEPs scattered over on 9 chromosomes (). The chromosome A01 and A06 only contained one BrCEP gene although some clustering was observed in other chromosomes (). For example, BrCEP6, BrCEP7 and BrCEP8 were organized sequentially in tandem on chromosome A04. A similar cluster was also observed for BrCEP15, BrCEP16, BrCEP17 and BrCEP18 on chromosome A07. Notably, the clustered BrCEPs showed low sequence similarity, and the CEP motifs were not totally identical, suggesting that these genes might not arise from recent tandem duplication events (; ). Interestingly, some BrCEPs localized on different chromosomes encoded identical CEP domains, for instance, the CEP motifs of the pairs of BrCEP4/BrCEP9/BrCEP12, BrCEP10/BrCEP11 were identical (; Table S1). These results suggested that genome-scale duplication of BrCEP genes occurred in different regions of Brassica rapa chromosomes.
Taken together, our analysis showed that BrCEP proteins displayed several features similar to the known CEP proteins, including C-terminal conserved CEP motifs, N-terminal signal and a central variable region (; Figure S1; Table S1).
Phylogenetic analysis of BrCEP proteins
CEP motif and full-length sequences of BrCEP proteins were used to build phylogenetic trees. Based on the conversed CEP motifs, the BrCEP proteins were divided into four subgroups (). The CEP motifs of the four subgroups were aligned, which resulted in a consensus sequences supporting for classification of the four groups (). Whereas the multiple CEP domains of BrCEP1 and BrCEP25 proteins were not grouped into the same group due to the divergent sequence (). Notably, the phylogenetic relationship based on the CEP motifs was not well supported when the full-length BrCEP proteins were analyzed, due to the divergent amino acid sequences outside the CEP motifs (Figure S2).
Figure 3. The BrCEP proteins are classified into four subgroups. (a) The phylogenetic tree of BrCEP proteins baesd on the CEP motifs. (b) WebLogo representation of CEP motifs in each subgroups. (c) Comparative alignment of the AtCEP and BrCEP proteins with identical or nearly identical CEP motifs. Each clade contains a known function of the AtCEP protein and their closest BrCEP protein derived from the phylogenetic analysis of all AtCEP and BrCEP CEP motifs (Fig. S3).
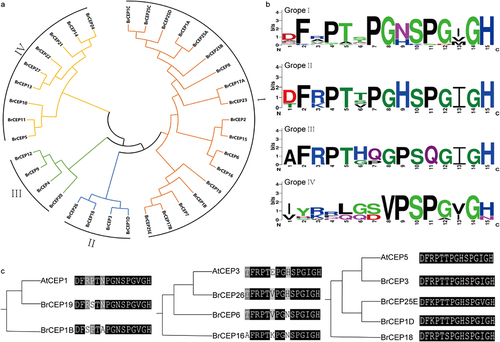
To probe the potential roles of the BrCEP proteins, the phylogenetic relationship between AtCEP proteins and BrCEP proteins were analyzed using either the CEP motifs (Figure S3) or the full-length protein sequences (Figure S4). The BrCEP and AtCEP proteins were grouped into several clades with varying degrees. Notably, some of the BrCEP motif that matched completely or near identical to the known function of the AtCEP peptides (; Figure S5).Citation5,Citation10–12,Citation21 For instance, AtCEP5 and BrCEP3 encoded an identical CEP motif, suggesting that BrCEPs would play essential roles in Brassica rapa development.
BrCEPs respond to developmental and environmental signals
The cis regulatory elements in the promoter region are important for gene expression regulation, we first performed an analysis to identify the cis-acting regulatory elements in BrCEP promoter regions. Numbers of cis-acting regulatory elements were discovered (Figure S6). The stress-related regulatory elements such as TC-rich repeat, MBS (Drought responsive element), LTR (Low temperature responsive element), and ABRE (ABA responsive element) elements were found in many BrCEP genes, further suggesting the potential roles of BrCEPs in stresses-related developmental processes. Furthermore, the identified phytohormone-related elements indicating potential crosstalk between CEP peptides and hormones signalings during Brassica rapa development.
We next addressed BrCEPs expression patterns in Brassica rapa tissues. The BrCEP genes expression levels in 5 tissues, including stem, root, leaf, sillque and flower were visualized (Figure S7a). The expression patterns of BrCEPs were varied in the examined tissues. For example, BrCEP25 showed highest expression in roots; BrCEP22 was highly expressed in sillque; BrCEP16 showed relative high transcription level in flowers. The diverse expression patterns of BrCEP genes in various tissues which indicates distinct functions of BrCEPs during various aspects of Brassica rapa developmental processes.
As many stress-related regulatory elements were found in BrCEP promoters (Figure S6), we then examined whether BrCEPs expressions were induced by environmental stress. Our analysis showed that BrCEPs expression level were differentially regulated by several environmental signals such as drought, circadian rhythm and trace element (Figure S7b, c, d,). The expression levels of BrCEP genes were varied in time points upon drought induction (Figure S7b). For example, BrCEP10 was upregulated after 4 hours and 8 hours drought induction; and BrCEP11 was upregulated and BrCEP25 was downregulated after 4 hours drought induction; BrCEP24 was upregulated after 8 hours drought induction, but it was downregulated after 12 hours drought treatment. The distinct spatio-temporal expression of BrCEPs indicates that BrCEPs would play distinct roles in various stages of drought response.
Plant circadian rhythm coordinates the complicated cellular responses to multiple and often simultaneous environmental signals, resulting in efficient changes of dynamic metabolic, physiological and defense networks to guarantee plant growth.Citation31 A result of the circadian integration of the environmental cues is the activation of the complex cellular response to a given stimulus such as drought response.Citation31 BrCEPs genes were also differentially induced by circadian rhythms (Figure S7c), indicating that circadian rhythms potentially activate the BrCEP expression and stresses-related regulatory networks to coordinate Brassica rapa development under unfavorable conditions.Citation32
Trace element minerals such as iron (Fe) and zinc (Zn) are essential for plant growth. Zinc (Zn) is a crucial micronutrient and involved in many physiological processes; however, excessive Zn content triggers plant chlorosis, photosynthesis inhibition, resulted in crop growth perturbations.Citation33 Our analysis revealed that BrCEPs expression was differentially regulated by Zn and Fe (Figure S7d). Under Zn or Fe deficiency conditions, BrCEP8 and BrCEP11 was up-regulated. Under Zn excessive condtion, BrCEP8 was downregulated, however, BrCEP10 was upregulated. We hence speculated that CEP peptides participate in maintaining Fe and Zn homeostasis during Brassica rapa development.
Cadmium (Cd) is ranked the 7th on the list of the top 275 hazardous materials.Citation34 Brassica species can absorb most of the Cd from the soil, they are considered as potential candidates for Cd phytoremediation.Citation35 Brassica species can tolerate Cd via multiple mechanism, as a result, they can be used to produce biofuel. Notaly, Cd also seriously affect Brassica growth and development. Our analysis showed that BrCEPs were induced by Cd treatment, for example, BrCEP8 and BrCEP19 were upregulated (Figure S7d). It is likely that BrCPEs could help Brassica species to absorb the Cd and improve Brassica tolerance to excess Cd content.
Taken together our analysis obtained from public microarray data suggests that BrCEPs respond differentially to both developmental and several environmental signals, indicating that BrCEPs may play distinct roles in various Brassica rapa developmental processes and adaptations.
BrCEP3 and BrCEP26 peptides promote Brassica rapa primary root growth
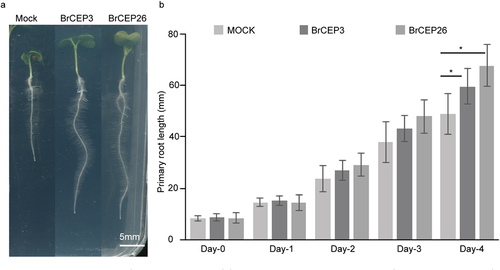
We synthesized two BrCEP peptides, BrCEP3 and BrCEP26, to further explore their physiological effects on Brassica rapa seedling development. After germination, Brassica rapa seedlings with a comparable primary root length were transferred to new agar plates supplied with 1 μM of BrCEP3 and BrCEP26 peptides, respectively. The seedlings were grown for another 4 days, and primary root length was quantified. Under our experimental conditions, we observed that both BrCEP3 and BrCEP26 peptides significantly promoted Brassica rapa primary root growth ().
Figure 4. BrCEP3 and BrCEP26 peptide promote Brassica rapa primary root growth. (a) Representative images showing the Brassica rapa seedlings treated with BrCEP3, BrCEP26 peptide for 4 days. (b) Quantification of 1 μM of BrCEP3 and BrCEP26 peptide treated Brassica rapa seedlings primary root length. N = 10–15 seedlings, Error bars are SD, * P < .05 was determined by One-way ANOVA. Scare bar = 5 mm.
Hydrogen peroxide and Ca2+ is involved in BrCEP3-mediated root growth
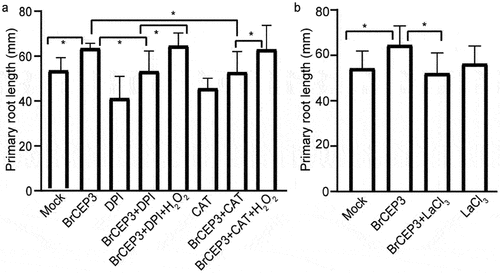
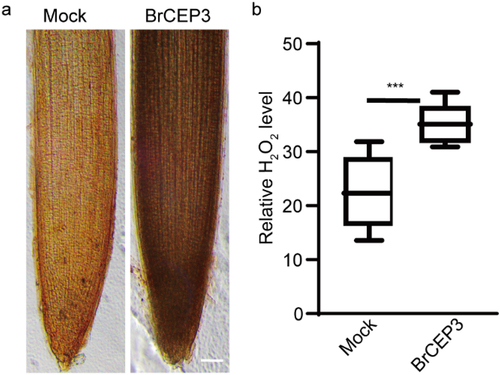
We next addressed the involvement of hydrogen peroxide (H2O2) and Ca2+ in BrCEP3-mediated root growth.Citation36,Citation37 We first tested the impact of BrCEP3 peptide on H2O2 level in Brassica rapa primary root. Our DAB staining result showed that BrCEP3 triggered a prominent accumulation of H2O2 in primary root (). Next, we examined the effects of CAT (a H2O2 scavenger enzyme), and DPI (an inhibitor of NADPH oxidase) on BrCEP3-mediated root growth. DPI prominently inhibited BrCPE3-mediated root growth, whereas CAT slightly repressed BrCEP3 effect on root growth, even the CAT and DPI showed an inhibitory effect on root growth. Notably, exogenous application H2O2 suppressed CAT and DPI effects (). In addition, when Ca2+ channel was blocked by lanthanum chloride (Ca2+ channel blocker), BrCEP3-induced root growth promotion was also abolished (). These collective data showed that H2O2 and Ca2+ signaling are necessary for BrCEP3 peptide to facilitate Brassica rapa primary root elongation.
Figure 5. BrCEP3 peptide promotes H2O2 accumulation in Brassica rapa primry roots. (a) Representative images showing ROS level in Brassica rapa primary roots treated with mock or 1 μM BrCEP3 peptide for 4 days. (b) Quantification of ROS level. N = 10, *** P < .001 was determined by One-way ANOVA test. Data represents mean ± SD. Scare bar = 50 μm.
Discussion
In this study, a systematic analysis of the CEP gene family in Brssica rapa L. was performed. A total of 27 BrCEP genes were uncovered, and they displayed similar features to the CEP members identified in various species. BrCEP genes also exhibited distinct expression patterns in response to both developmental and environmental signals. In addition, H2O2 and Ca2+ were involved in BrCEP3 peptide-mediated Brassica rapa root development.
It has been suggested that post-translational modifications mediated by many enzymes are necessary to produce the functional mature peptides.Citation38–40 However, how functional mature BrCEP peptides are generated remains elusive. On the other hand, how these modification enzymes affect the BrCEP function and signaling transduction are also unknown. The answers to these fundamental questions will greatly help to understand BrCEP peptides function.
Data obtained from public transcriptome datasets showed that BrCEP genes displayed diverse expression patterns in a given tissue or under environmental stress conditions (Fig. S7). Considering only some BrCEP genes can be detected from the public expression database, the transcription levels of other BrCEP genes require further confirmation.
To our surprise, BrCEP3 and BrCEP26 peptide promoted primary root growth (), indicating diverse CEP peptide functions in species. Antagonistic peptide technology has been developed to dissect CLE peptide function,Citation41,Citation42 it is likely BrCEP26 peptide maybe an antagonistic form, but it definitely requires careful examinations in future. However, BrCEP3 encodes an identical CEP motif to Arabidopsis CEP5 (), hence, it is possible that BrCEP3 peptide may target different downstream signaling networks in Brassica rapa to promote primary root growth (). Additionally, conserved serine (at position 10) and glycine (at position 14) in the apple MdCEP1 motif are crucial for its function,Citation15 thus, it is an open question to examine whether these conserved serines and glycine are also essential for BrCEP peptide function. Nevertheless, how BrCEP peptide is perceived by its putative receptors remains elusive, identification of RLKs in Brassica species will provide novel insights into BrCEP signaling transduction.Citation43–45
Reactive oxygen species (ROS) acts as essential signaling molecule to regulate plant root development. When ROS homeostasis is disturbed in root, plants are unable to adjust their growth in the changing environments.Citation46 H2O2 is a kind of ROS, which is generated by the two main NADPH/RBOH oxidases, RBOHD and RBOHF.Citation47–49 Our pharmacological experiment showed that H2O2 is required for BrCEP3-mediated root growth (). How BrCEP3 peptide affects H2O2 level via RBOHD and RBOHF is elusive. On the other hand, we can not exclude the involvement of other ROS forms, such as nitric oxide (NO) in BrCPE3-mediated root growth. Additionally, Ca2+ was also involved in BrCPE3-mediated root growth (). The genetic mutants related to ROS biosynthesis and signaling,Citation36 as well as Ca2+ signalingCitation37 will help to uncover more essential components involved in BrCEP3 peptide signaling pathway.
Environmental stress severely affects crop growth and yield. Therefore, plants have evolved multiple strategies including activation of CEP peptide signaling cascade to adjust their adaptions to the changing environment.Citation5,Citation16,Citation21 The induction of BrCEPs expression by environmental stress further indicates their roles in Brassica rapa adaptions to adverse environments (Figure S7b, c, d). To discover more key participants and the precise mechanisms in BrCEP-mediated stress adaptions is one of fascinating topics in future investigations.
Author contributions
H.H. conceived the project, designed the experiments and wrote the manuscript. Z.Q., K.Z., Y.L. conducted the experiments and analyzed the data. X.G., C.C. assisted with the writing. S.H. provided the facility. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (3.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Tavormina P, De Coninck B, Nikonorova N, De Smet I, Cammue BP. The plant peptidome: an expanding repertoire of structural features and biological functions. Plant Cell. 2015;27(8):2095–10. doi:10.1105/tpc.15.00440.
- Takahashi F, Hanada K, Kondo T, Shinozaki K. Hormone-like peptides and small coding genes in plant stress signaling and development. Curr Opin Plant Biol. 2019;51:88–95. doi:10.1016/j.pbi.2019.05.011.
- Tanco S, Gevaert K, Van Damme P. C‐terminomics: targeted analysis of natural and posttranslationally modified protein and peptide C‐termini. Proteomics. 2015;15(5–6):903–914. doi:10.1002/pmic.201400301.
- Stührwohldt N, Schaller A, Staiger D. Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biol (Stuttg). 2019;1:49–63. doi:10.1111/plb.12881.
- Delay C, Imin N, Djordjevic MA. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. J Exp Bot. 2013;64(17):5383–5394. doi:10.1093/jxb/ert332.
- Roberts I, Smith S, De Rybel B, Van Den Broeke J, Smet W, De Cokere S, Mispelaere, M, De Smet I, Beeckman T. The CEP family in land plants: evolutionary analyses, expression studies, and role in Arabidopsis shoot development. J Exp Bot. 2013;64(17):5371–5381. doi:10.1093/jxb/ert331.
- Ogilvie HA, Imin N, Djordjevic MA. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes. BMC Genomics. 2014;15(1):870. doi:10.1186/1471-2164-15-870.
- Ohyama K, Ogawa M, Matsubayashi Y. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC‐MS‐based structure analysis. Plant J. 2008;55(1):152–160. doi:10.1111/j.1365-313X.2008.03464.x.
- Sui Z, Wang T, Li H, Zhang M, Li Y, Xu R, Xing G, Ni Z, Xin M. Overexpression of peptide-encoding OsCEP6.1 results in pleiotropic effects on growth in Rice (O. sativa). Front Plant Sci. 2016;7:228. doi:10.3389/fpls.2016.00228.
- Roberts I, Smith S, Stes E, De Rybel B, Staes A, Van De Cotte B, Njo MF, Dedeyne L, Demol H, Lavenus J, et al. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. J Exp Bot. 2016;67(16):4889–4899. doi:10.1093/jxb/erw231.
- Chapman K, Taleski M, Ogilvie HA, Imin N, Djordjevic MA. CEP-CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. J Exp Bot. 2019;70(15):3955–3967. doi:10.1093/jxb/erz207.
- Delay C, Chapman K, Taleski M, Wang Y, Tyagi S, Xiong Y, Djordjevic MA. CEP3 levels affect starvation-related growth responses of the primary root. J Exp Bot. 2019;70(18):4763–4774. doi:10.1093/jxb/erz270.
- Zhou Y, Sarker U, Neumann G, Ludewig U. The LaCEP1 peptide modulates cluster root morphology in Lupinus albus. Physiol Plant. 2019;166(2):525–537. doi:10.1111/ppl.12799.
- Liu Y, Zuo T, Qiu Z, Zhuang K, Hu S, Han H. Genome-wide identification reveals the function of CEP peptide in cucumber root development. Plant Physiol Biochem. 2021;169:119–126. doi:10.1016/j.plaphy.2021.11.007.
- Yu Z, Xu Y, Liu L, Guo Y, Yuan X, Man X, Liu C, Yang G, Huang J, Yan K, et al. The importance of conserved serine for C-Terminally Encoded Peptides function exertion in Apple. Int J Mol Sci. 2019;20(3):775. doi:10.3390/ijms20030775.
- Aggarwal S, Kumar A, Jain M, Sudan J, Singh K, Kumari S, Mustafiz A. C-terminally encoded peptides (CEPs) are potential mediators of abiotic stress response in plants. Physiol Mol Biol Plants. 2020;26(10):2019–2033. doi:10.1007/s12298-020-00881-4.
- Xu R, Li Y, Sui Z, Lan T, Song W, Zhang M, Zhang Y, Xing J, . A C-terminal encoded peptide, ZmCEP1, is essential for kernel development in maize. J Exp Bot. 2021;72(15):5390–5406. doi:10.1093/jxb/erab224.
- Zhang L, Ren Y, Xu Q, Wan Y, Zhang S, Yang G, Huang J, Yan K, Zheng C, Wu C. SiCEP3, a C-terminally encoded peptide from Setaria italica, promotes ABA import and signaling. J Exp Bot. 2021;72(18):6260–6273. doi:10.1093/jxb/erab267.
- Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J Exp Bot. 2013;64:5395–5409.
- Mohd-Radzman NA, Laffont C, Ivanovici A, Patel N, Reid D, Stougaard J, Djordjevic MA. Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiol. 2016;171(4):2536–2548. doi:10.1104/pp.16.00113.
- Smith S, Zhu S, Joos L, Roberts I, Nikonorova N, Vu LD, Stes E, Cho H, Larrieu A, Xuan W, et al. The CEP5 peptide promotes abiotic stress tolerance, as revealed by quantitative proteomics, and attenuates the AUX/IAA equilibrium in Arabidopsis. Mol. Cell Proteomics. 2020;19(8):1248–1262. doi:10.1074/mcp.RA119.001826.
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science. 2014;346(6207):343–346. doi:10.1126/science.1257800.
- Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat Plants. 2017;3:17029. doi:10.1038/nplants.2017.29.
- Ota R, Ohkubo Y, Yamashita Y, Ogawa-Ohnishi M, Matsubayashi Y. Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nat Commun. 2020;11(1):641. doi:10.1038/s41467-020-14440-8.
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208.
- Crooks GE, Hon G, Chandonia JM, Brenner SE. Weblogo: a sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi:10.1101/gr.849004.
- Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi:10.1093/bioinformatics/btu817.
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi:10.1093/nar/30.1.325.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi:10.1093/nar/25.24.4876.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K, . MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi:10.1093/molbev/msy096.
- Greenham K, McClung CR. Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet. 2015;16(10):598–610. doi:10.1038/nrg3976.
- Greenham K, Sartor RC, Zorich S, Lou P, Mockler TC, McClung CR. Expansion of the circadian transcriptome in Brassica rapa and genome-wide diversification of paralog expression patterns. Elife. 2020;9:e58993. doi:10.7554/eLife.58993.
- Pan W, You Y, Weng YN, Shentu JL, Lu Q, Xu QR, Liu HJ, Du ST. Zn stress facilitates nitrate transporter 1.1-mediated nitrate uptake aggravating Zn accumulation in Arabidopsis plants. Ecotoxicol Environ Saf. 2020;190:110104. doi:10.1016/j.ecoenv.2019.110104.
- Mench MJ. Cadmium availability to plants in relation to major long-term changes in agronomy systems. Agric Ecosyst Environ. 1998;67:174–187. doi:10.1016/S0167-8809(97)00117-5.
- Rizwan M, Ali S, Zia Ur Rehman M, Rinklebe J, Tsang DCW, Bashir A, Maqbool A, Tack FMG, Ok YS. Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ. 2018;631–632:1175–1191. doi:10.1016/j.scitotenv.2018.03.104.
- Mhamdi A, Van Breusegem F. Reactive oxygen species in plant development. Development. 2018;145(15):dev164376. doi:10.1242/dev.164376.
- Tang RJ, Wang C, Li K, Luan S. The CBL-CIPK calcium signaling network: unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020;25(6):604–617. doi:10.1016/j.tplants.2020.01.009.
- Tabata R, Sawa S. Maturation processes and structures of small secreted peptides in plants. Front Plant Sci. 2014;5:311. doi:10.3389/fpls.2014.00311.
- Taleski M, Imin N, Djordjevic MA. CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J Exp Bot. 2018;69(8):1829–1836. doi:10.1093/jxb/ery037.
- Patel N, Mohd-Radzman NA, Corcilius L, Crossett B, Connolly A, Cordwell SJ, Ivanovici A, Taylor K, Williams J, Binos S, et al. Diverse peptide hormones affecting root growth identified in the Medicago truncatula secreted peptidome. Mol Cell Proteomics. 2018;17(1):160–174. doi:10.1074/mcp.RA117.000168.
- Song XF, Guo P, Ren SC, Xu TT, Liu CM. Antagonistic peptide technology for functional dissection of CLV3/ESR genes in Arabidopsis. Plant Physiol. 2013;161:1076–1085. doi:10.1104/pp.112.211029.
- Czyzewicz N, Wildhagen M, Cattaneo P, Stahl Y, Pinto KG, Aalen RB, Butenko MA, Simon R, Hardtke CS, De Smet I. Antagonistic peptide technology for functional dissection of CLE peptides revisited. J Exp Bot. 2015;66:5367–5374. doi:10.1093/jxb/erv284.
- Rameneni JJ, Lee Y, Dhandapani V, Yu X, Choi SR, Oh MH, Lim YP, . Genomic and post-translational modification analysis of Leucine-Rich-Repeat receptor-like kinases in Brassica rapa. PLoS One. 2015;10(11):e0142255. doi:10.1371/journal.pone.0142255.
- Chen G, Wang J, Wang H, Wang C, Tang X, Li J, Zhang L, Song J, Hou J, Yuan L. Genome-wide analysis of proline-rich extension like receptor protein kinase (PERK) in Brassica rapa and its association with the pollen development. BMC Genomics. 2020;21(1):401. doi:10.1186/s12864-020-06802-9.
- Yang H, Bayer PE, Tirnaz S, Edwards D, Batley J. Genome-Wide identification and evolution of receptor-like kinases (RLKs) and receptor like proteins (RLPs) in Brassica juncea. Biology (Basel). 2020;10(1):17. doi:10.3390/biology10010017.
- Mase K, Tsukagoshi H. Reactive oxygen species link gene regulatory networks during Arabidopsis root development. Front Plant Sci. 2021;12:660274. doi:10.3389/fpls.2021.660274.
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell. 2012;24(1):275–287. doi:10.1105/tpc.111.093039.
- Kadota Y, Shirasu K, Zipfel C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015;56(8):1472–1480. doi:10.1093/pcp/pcv063.
- Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–1928. doi:10.1083/jcb.201708007.