ABSTRACT
The δ-aminolevulinic acid dehydratase (ALAD) enzyme is an intermediate in the biosynthetic pathway of tetrapyrroles. It combines two δ‐aminolevulinic acid (δ‐ALA) molecules to form the pyrrole, porphobilinogen, an important precursor for plant pigments involved in photosynthesis, respiration, light-sensing, and nutrient uptake. Our recent efforts showed that, in citrus, silencing of ALAD gene via Citrus tristeza virus-induced gene silencing, caused yellow spots and necrosis in leaves and in developing new shoots. Silencing of ALAD gene reduced leaf pigments and altered leaf metabolites. Moreover, total phenolic content, H2O2, and reactive oxygen species (ROS) increased, indicating that silencing of ALAD induced severe stress. Herein, we hypothesized that conditions including lower sucrose, elevated ROS, alteration of microRNA involved in RNAi regulatory protein Argonaute 1 (AGO1) and ROS lead to higher deposition of callose in phloem tissues. Using aniline blue staining and gene expression analysis of callose synthases, we showed significant deposition of callose in ALAD-silenced citrus.
Virus-induced gene silencing (VIGS) is a biotechnological approach that has been broadly adopted for studying plant functional genomics.Citation1,Citation2 A mild strain (T36) of the Citrus tristeza virus (CTV) has been developed as a VIGS vector for Citrus spp.Citation3–6 The targeted repoter genes of citrus include those for phytoene desaturase and δ-aminolevulinic acid dehydratase due to their respective roles in carotenoid and chlorophyll biosynthesis and photosynthesis.Citation7,Citation8
Delta (δ)-aminolevulinic acid dehydratase (ALAD), a key enzyme in tetrapyrrole synthesis, joins two δ-aminolevulinic acid (δ-ALA) molecules through a condensation reaction to form the pyrrole molecule, porphobilinogen, an important intermediate for chlorophyll biosynthesis.Citation8 In our previous work, we produced ALAD-silenced citrus using a truncated antisense ALAD in the binary vector pCAMBIA‐1380 containing the infectious cDNA clone of CTV isolate T36 (GenBank accession no. AY170468).Citation9 The plasmid, CTV-tALAD-as was propagated in Nicotiana benthamiana and the purified virions were infiltrated in Citrus macrophylla. The empty plasmid, CTV-wt was inoculated in C. macrophylla to be used as a control in our study.Citation9 C. macrophylla plants inoculated with CTV-tALAD-as virions showed a specific phenotype including yellow spots and necrosis in leaves, stems, and the apical meristem. For leaves, the phenotype starts as a few spots in developing young leaves and the number of spots and intensity increase with maturity, and often connect to each other until they cover most of the leaf surface.Citation9 Inoculation with CTV-wt does not cause any of the described phenotype in ALAD-silenced plants.Citation9
When the ALAD gene was silenced in C. macrophylla using CTV-tALAD-as, several effects were observed: (1) δ-ALA accumulated, (2) the levels of chlorophylls, starch, sucrose, and pigments (trans- and cis-violaxanthin, and α- and β-cryptoxanthin) were reduced, (3) phytohormones including salicylic acid and jasmonic acid levels increased, and (4) emission of some released volatile sesquiterpenes such as (E)-α-bergamotene and (E)-β-farnesene increased.Citation9 These responses indicated the gene silencing-induced stresses. In addition, polar metabolites including L-alanine, gamma-aminobutyric acid, L-threonine, L-glutamic, and L-phenylalanine also were significantly decreased in leaves while L-asparagine, fumaric acid, and succinic acid significantly increased in leaves of ALAD-silenced plants.Citation9 Furthermore, expression profiles of 63 conserved microRNAs (miRNA) implicated in auxin biosynthesis and signaling, axillary shoot meristem formation and leaf morphology, starch metabolism, and oxidative stress were differentially expressed in ALAD-silenced citrus plants.Citation10 Levels of total phenolics, H2O2 and superoxide anion (O2‧−) were increased, further supporting the hypothesis that ALAD silencing-induced stress on citrus plants.Citation10
As a response to both biotic and abiotic stresses, thickening of the cells walls via callose deposition can occur in plants.Citation11,Citation12 Folimonova and AchorCitation13 and Koh, Zhou, Williams, Park, Ding, Duan and KangCitation14 observed mild to excessive deposits of callose material (β-1,3-glucan polysaccharide) in the sieve elements of citrus plants infected with ‘Candidatus. Liberibacter asiaticus’, the bacterial pathogen associated with citrus greening disease. Callose deposition is thought to help restrict the movement of pathogens within the plant, and has been shown to be controlled by multiple callose synthase genes.Citation15,Citation16 In Citrus, nine genes for callose synthase were recently identified, and upregulation of gene expression was correlated with ‘Ca. L. asiaticus’ infection.Citation16 In addition, callose deposition can respond to factors including light intensity, antioxidant supplementation, and sucrose concentration. Callose formation was enhanced in growth media with lower sucrose (1% compared to 5% sucrose) in Arabidopsis.Citation11
Thus, we hypothesized that the combination of increased ROS, altered miRNAs, and reduced photosynthates including chlorophyll, carotenoids, and sucrose from the δ-ALA-deficient citrus model will lead directly to increased callose deposition. To test this hypothesis, we stained the phloem tissue of C. macrophylla inoculated with CTV-wt or CTV-tALAD-as expressing varying degrees of phenotype using aniline blue.Citation17 The plants used for the current study were produced for our previous study.Citation9 We examined the effect of ALAD silencing on the gene expression of citrus callose synthases and the density of callose deposition in citrus leaves expressing a gradient of phenotypes scaled from zero (CTV-wt) and 1 to 5 (CTV-tALAD-as) (). The aniline blue staining revealed significant depositions of callose in phloem tissue when peeled barks were stained. Higher levels of callose deposition were observed in the moderate phenotype (degree 3) in ALAD-silenced plants while it was barely observed in control plants (). In addition to the peeled bark, callose deposition was also observed in the phloem tissue in cross sections of the petiole in ALAD-silenced plants (–h).
Figure 1. Silencing of δ‐aminolevulinic acid dehydratase via Citrus tristeza virus-induced gene silencing (CTV-IGS) causes callose deposition in citrus phloem tissue. Note the increased amount of callose deposits in the phloem tissue as seen in peeled stem bark is correlated with the increase in phenotype, but the highest amount is found in the moderate phenotype. Callose deposits were visualized by staining with aniline blue. Zero: control plants (CTV-wt). 1–5: degrees of phenotype in ALAD-silenced plants (CTV-tALAD-as).
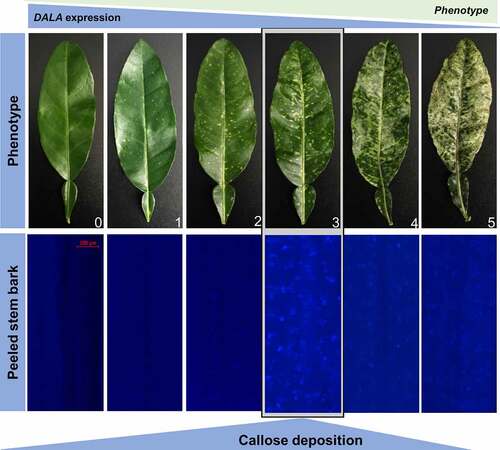
Figure 2. Visualization and quantification of callose deposits in the moderate phenotype of ALAD-silenced plants (CTV-tALAD-as) phloem tissue compared to control plants (CTV-wt). A-H: Visualization of callose deposits by staining with aniline blue. A-D: Control plants. E-H: ALAD-silenced plants. I: Total callose deposits quantified as fluorescence intensity. J: Fold change in callose synthase gene expressions performed with RT-PCR. Arrows indicate callose deposits.
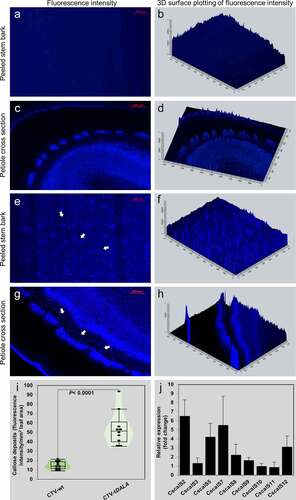
To quantify the density of callose deposition, we used ZEISS ZEN 3.1 blue edition image processing software. The total fluorescence signal density was significantly higher in ALAD-silenced C. macrophylla than the control plants (). Furthermore, the expression of callose synthase genes were upregulated in ALAD-silenced C. macrophylla (). We used the previously published primers to perform the gene expression analysis as described by Granato, Galdeano, D’Alessandre, Breton and Machado.Citation16
In summary, this current work provides evidence that callose deposition occurs in ALAD-silenced C. macrophylla. By integrating the present findings and our published work,Citation9,Citation10 we illustrated a hypothetical model for callose formation in ALAD-silenced plants (). In this proposed model, silencing ALAD causes shifts in the microRNA profile prompting the reactive oxygen species (ROS) and RNAi regulatory protein Argonaute 1 (AGO1).Citation10 The last two promote callose synthesis by activation of callose synthases.Citation18 On the other hand, the reduced photosynthesis caused by the downregulation of ALAD produces lower amounts of sucrose.Citation9 Abscisic acid stimulates the callose deposition in the presence of low sucrose concentrations.Citation18
Figure 3. Proposed model on how silencing of δ‐aminolevulinic acid dehydratase leads to deposition of callose. See main text for details.
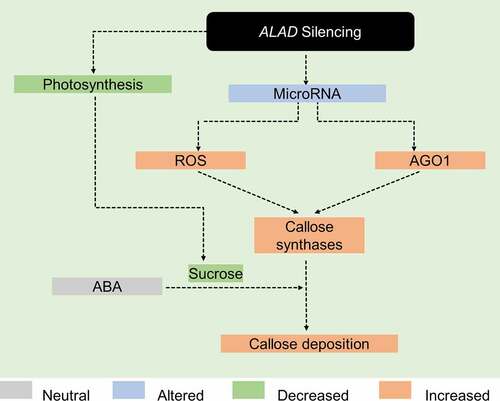
Finally, we assume that the callose deposition reported for ‘Ca. L. asiaticus’-infected citrus may not only be stimulated by the presence of the citrus greening pathogen, but that the reduction of chlorophyll pathway metabolites, increased ROS, altered miRNAs and reduced sucrose levels also contribute to callose formation. Further investigation is required to fully understand the interplay of these mechanisms and to prove the proposed hypotheses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Romero I, Tikunov Y, Bovy A. Virus-induced gene silencing in detached tomatoes and biochemical effects of phytoene desaturase gene silencing. J Plant Physiol. 2011;168(10):1129–5. doi:10.1016/j.jplph.2010.12.020.
- Ruiz MT, Voinnet O, Baulcombe DC. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10(6):937–946. doi:10.1105/tpc.10.6.937.
- Dawson WO, Folimonova SY. Virus-based transient expression vectors for woody crops: a new frontier for vector design and use. Annu Rev Phytopathol. 2013;51(1):321–337. doi:10.1146/annurev-phyto-082712-102329.
- Dawson WO, Bar-Joseph M, Garnsey SM, Moreno P. Citrus tristeza virus: making an ally from an enemy. In: VanAlfen NK. editor. Annual review of phytopathology. 2015;53:137–155. doi:10.1146/annurev-phyto-080614-120012.
- Folimonov AS, Folimonova SY, Bar-Joseph M, Dawson WO. A stable RNA virus-based vector for citrus trees. Virology. 2007;368(1):205–216. doi:10.1016/j.virol.2007.06.038.
- Hajeri S, Killiny N, El-Mohtar C, Dawson WO, Gowda S. Citrus tristeza virus-based RNAi in citrus plants induces gene silencing in Diaphorina citri, a phloem-sap sucking insect vector of citrus greening disease (Huanglongbing). J Biotechnol. 2014;176:42–49. doi:10.1016/j.jbiotec.2014.02.010.
- Qin GJ, Gu HY, Ma LG, Peng YB, Deng XW, Chen ZL, Qu L-J. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 2007;17(5):471–482. doi:10.1038/cr.2007.40.
- Tanaka R, Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol. 2007;58(1):321–346. doi:10.1146/annurev.arplant.57.032905.105448.
- Killiny N, Hijaz F, Nehela Y, Hajeri S, Gowda S. Effects of δ-aminolevulinic acid dehydratase silencing on the primary and secondary metabolisms of citrus. Plant Direct. 2018;2(7):e00072–e. doi:10.1002/pld3.72.
- Killiny N, Nehela Y, Hijaz F, Gonzalez-Blanco P, Hajeri S, Gowda S. Knock-down of delta-aminolevulinic acid dehydratase via virus-induced gene silencing alters the microRNA biogenesis and causes stress-related reactions in citrus plants. Plant Sci. 2020;299:110622.
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact. 2011;24(2):183–193. doi:10.1094/MPMI-07-10-0149.
- Flors V, Ton J, Jakab G, Mauch-Mani B. Abscisic acid and callose: team players in defence against pathogens? J Phytopathol. 2005;153(7–8):377–383. doi:10.1111/j.1439-0434.2005.00987.x.
- Folimonova SY, Achor DS. Early events of citrus greening (huanglongbing) disease development at the ultrastructural level. Phytopathology®. 2010;100(9):949–958. doi:10.1094/PHYTO-100-9-0949.
- Koh E-J, Zhou L, Williams DS, Park J, Ding N, Duan Y-P, Kang B-H. Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with “Candidatus Liberibacter asiaticus”. Protoplasma. 2012;249(3):687–697. doi:10.1007/s00709-011-0312-3.
- Ellinger D, Voigt CA. Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot. 2014;114(6):1349–1358. doi:10.1093/aob/mcu120.
- Granato LM, Galdeano DM, D’Alessandre NDR, Breton MC, Machado MA. Callose synthase family genes plays an important role in the Citrus defense response to Candidatus Liberibacter asiaticus. Eur J Plant Pathol. 2019;155(1):25–38. doi:10.1007/s10658-019-01747-6.
- Stone BA, Evans NA, Bonig I, Clarke AE. The applic ation of Sirofluor, a chemically defined fluoro chrome from aniline blue for the histo chemical detection of callose. Proto plasma. 1984;122(3):191–195. doi:10.1007/BF01281696.
- Wang Y, Li X, Fan B, Zhu C, Chen Z. Regul ation and function of defense-related callose depo sition in pla nts. Int J Mol Sci. 2021;22(5):2393. doi:10.3390/ijms22052393.
