ABSTRACT
Nutrient antagonism typically refers to the fact that too high a concentration of one nutrient inhibits the absorption of another nutrient. In plants, Ca2+ (Calcium) and Mg2+ (Magnesium) are the two most abundant divalent cations, which are known to have antagonistic interactions. Hence, maintaining their homeostasis is crucial for plant growth and development. In this study, we showed that MTP10 (Metal Tolerance Protein 10) is an important regulator for maintaining homeostasis of Mg and Ca in Arabidopsis. The mtp10 mutant displayed severe growth retardation in the presence of excess Mg2+, to which the addition of Ca2+ was able to rescue the phenotype of mtp10 mutant. Additionally, the deficiency of Ca2+ in the culture medium accelerated the high-Mg sensitivity of the mtp10 mutant. The yeast complementation assay suggested that AtMTP10 had no Ca2+ transport activity. And the ICP-MS data further confirmed the antagonistic relationship between Ca2+ and Mg2+, with the addition of Ca2+ reducing the excessive accumulation of Mg2+ and high-Mg inhibiting the uptake of Ca2+. We conclude that the Arabidopsis MTP10 is essential for the regulation of Mg and Ca homeostasis.
Introduction
Magnesium (Mg2+) is essential for plant growth, while its over-accumulation in cells can be toxic. As a nutrient and signaling molecule, calcium (Ca2+) levels in plant cells are strictly controlled by channels, carriers, and transporters, providing a mechanistic basis for Ca2+ homeostasis and Ca2+ signaling. Both Ca2+ and Mg2+ are absorbed by plants from the soil and then translocated from the roots to the shoots through the xylem.Citation1 Typically, serpentine soils show low Ca2+ but high Mg2+ concentrations, which often cause Mg2+ toxicity, leading to reduced crop yields.Citation2 Ca2+ and Mg2+ are the two most abundant divalent cations, which appear to have antagonistic interactions in the plant cell, and the homeostasis balance between them is crucial for plant growth and development.Citation1 It is widely believed that Ca2+ and Mg2+ can compete for the same enzymes or channels, transporters, etc. Notwithstanding their distinct physiological and biochemical roles, Ca2+ and Mg2+ homeostases in plants appear to be closely linked and may be regulated, at least partially, by a common signaling network. There are several ion channels and transporters that have been identified, and they are localized to the plasma membrane and various intracellular membrane systems to mediate the transport of Ca2+ or Mg2+. For example, the Ca2+/H+ exchange activity of the vacuole in the cax1 mutant is inhibited, thereby causing Ca2+ to be poorly sequestered into the vacuole and leading to elevated Ca2+ concentrations in the cytoplasm. Consequently, the Arabidopsis cax1 mutant is more resistant to high-Mg toxicity compared to the wild-type plants.Citation3 Consistently, it has also been shown that the Mg deficiency phenotypes upon multiple knockouts of Arabidopsis thaliana MRS2 clade B genes, AtMRS2-1 and AtMRS2-5, can be ameliorated by concomitantly reduced Ca2+ supply.Citation4 A Na+-K+ transporter family member OsHKT2;4 in rice is permeable to both Ca2+ and Mg2+ when heterogeneously expressed in both bacterial and oocyte systems. Moreover, the overexpression of OsHKT2;4 in the Arabidopsis mgt6 mutant results in the seedlings being more sensitive to high-Mg stress,Citation5,Citation6 implying an interaction between Ca2+ and Mg2+. A study on Vicia faba guard cells suggested that Mg2+ can prevent continuous Ca2+-leakage through the channels including CGNCs, thereby assigning Mg2+ an important role in Ca homeostasis and Ca2+-dependent downstream signaling.Citation7 A further example of understanding Ca2+-Mg2+ interactions come from the findings that Ca2+ signaling networks also function in the regulation of Mg homeostasis. In yeast, Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling.Citation8 It has been suggested that high-Mg stress is also capable of activating relevant ion channels, leading to a rapid elevation of Ca2+ concentration in the cytoplasm and stimulating specific Ca2+ oscillation.Citation1 In Arabidopsis, two tonoplast-localized calcineurin B-like (CBL) proteins, CBL2 and CBL3, can perceive the Ca2+ signal and activate a subset of CBL-interacting protein kinases (CIPKs), CIPK3, 9, 23, 26, which in turn phosphorylate the as yet unknown Mg2+ transporters on the tonoplast, ultimately regulating Mg2+ sequestration into the vacuole to reduce the Mg2+ toxicity in the cytoplasm.Citation9
In Arabidopsis, the MTPs (Metal tolerance proteins) belong to the CDF (cation diffusion facilitator) family, and these proteins have been identified as proton/divalent cation transporters, responsible for the efflux of cations from the cytoplasm to the extracellular space or the transport of cations from the cytoplasm to subcellular organelles, consequently making this type of transporters essential to metal tolerance.Citation10 Hitherto, most of the functionally well-defined MTP transporters are capable of transporting divalent metal ions, such as Zn2+, Mn2+, Co2+, and Ni2+.Citation10 Previous studies have shown that MTP10 is capable of transporting Mn2+ in Arabidopsis.Citation11 However, our very recent study showed that plasma membrane-localized MTP10 can transport Mg2+ in the bacterial strain MM281 system, and MTP10 loss-of-function mutant exhibits sensitivity to high-Mg stress.Citation12 In this study, we further provided evidence that MTP10 is an important contributor to the regulation of Ca and Mg homeostasis. The high-Mg-sensitive phenotype of the mtp10 mutant was effectively alleviated when the concentration of Ca2+ was appropriately increased in the medium. Under Ca2+ deficiency conditions, it is instead in a position to exacerbate the high-Mg-sensitive phenotype of the mtp10 mutant. Thus, our study revealed that MTP10 is involved in regulating not only Mg homeostasis but also Ca homeostasis in Arabidopsis.
Material and Methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Col-0 was used in this study. The T-DNA insertion mutant mtp10 (SALK_121470) was obtained from Arabidopsis Biological Resource Center (http://abrc.osu.edu). The Arabidopsis seeds were sterilized using 75% ethanol, and plated on 1/2 strength Murashige and Skoog (MS) medium containing 1% (w/v) sucrose. The pH of the medium was adjusted to 5.7 using KOH and solidified using 1% (w/v) agar. After 2 days of stratification at 4°C, the seedlings were then grown in a growth chamber under 150 µmol/m2/s light intensity with a 16-h light/8-h dark photoperiod at 22°C. For the phenotypic assay, the seedlings were grown in 1/2 strength MS medium for 3 days, and then transferred to a 1/6 MS containing different concentrations of MgCl2 or CaCl2 for a designed time. For the hydroponic culture, seeds of A. thaliana (Col-0 and mtp10) were grown on 1/2 strength MS for 7 days, then transferred to 1/6 strength MS solution and grown for the indicated time period.
Quantitative RT–PCR
Seedlings of wild-type Col-0 and mtp10 mutant were grown in 1/2 MS agar medium for 7 days and then transferred to 1/6 MS liquid medium for another 7 days. The seedlings were then treated with high-Mg (1/6 MS+10 mM MgCl2) for 10 h. Total RNA was isolated using Trizol reagent (Invitrogen), treated with DNase I (Invitrogen), and used as a template to synthesize first-strand cDNA with M-MLV Reverse Transcriptase (Promega) and an oligo dT primer. The qRT-PCR was performed on a Light Cycler 96 (Roche Diagnostics) with SYBR Green I Master mix (Roche Diagnostics) according to the manufacturer’s instructions. ACTIN2 (At3g18730) was used as an internal standard. The primers are listed in Table S1.
Yeast two-hybrid assays (Y2H)
The non-transmembrane C terminal region sequence of MTP10 was cloned into the vector pGADT7, and the CDS sequence of CIPKs was cloned into the vector pGBKT7, respectively. The fusion constructs were transformed into yeast strain AH109 using the lithium acetate transformation method as previously reported.Citation9 Transformants were then grown in a synthetic dropout medium lacking tryptophan and leucine, and a synthetic dropout medium lacking tryptophan, leucine, histidine, and adenine at different dilutions (10−1, 10−2, and 10−3) cells/ml at 30°C.
Bimolecular fluorescence complementation (BiFC) Assay
To generate BiFC constructs, the coding sequence of CIPK7 and CIPK26 without the stop codon was in-frame cloned into the pSPYNE(R)173-CAMBIA1300 vector, and the coding sequence of MTP10 was sub-cloned into the pSPYCE(M)-CAMBIA1300 vector.Citation13 After transfection, N. benthamiana were cultured for 48 h, YFP signals were imaged by the LSM710 META confocal laser scanning microscope (Carl Zeiss). The excitation wavelength for YFP was 514 nm, and the emission wavelength was between 525 and 575 nm.
Heterologous expression of MTP10 in yeast Δgdt1
For complementation of the yeast mutant, the full-length CDS of MTP10 was cloned into the pYES2 vector. The empty pYES2 vector and the recombinant MTP10-pYES2 plasmid were then introduced to the yeast strain Δgdt1, respectively. The wild-type yeast strain BY4741 was set as control. The method of the transformation of the plasmids into the yeast is described in the yeast protocols handbook (Clontech Laboratories). The cells transformed with the indicated constructs were grown on the synthetic medium containing amino acids without uracil (SC-U) and in the presence of 0, 100, 200, or 300 mM Ca2+.
Results
External Ca2+ can rescue the high-Mg sensitive phenotype of mtp10
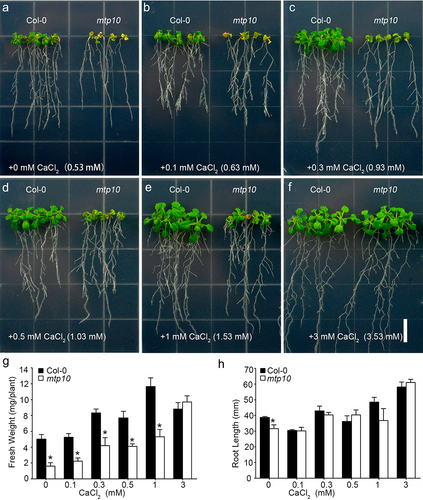
We previously found a mtp10 mutant and characterized its hypersensitive phenotype under high Mg2+ conditions.Citation12 Based on the antagonistic relationship between Ca2+ and Mg2+, we further conducted a detailed assessment of Ca2+ rescue ability of mtp10 under high Mg2+ conditions. The 1/6 MS mediumCitation9 was supplemented with 10 mM MgCl2 and a broad range of Ca2+ concentrations were used for growth assays. In the 1/6 MS medium, it had already contained 0.53 mM Ca2+. On the 1/6 MS medium supplemented with 10 mM MgCl2, the growth of mtp10 mutant was significantly inhibited (). After adding 0.1 mM extra Ca2+ to the medium, the growth of both wild-type plants and mtp10 mutant were recovered slightly (). After adding 0.3 mM, 0.5 mM, or 1 mM extra Ca2+ into the medium, the growth of mtp10 mutant was significantly recovered (). Further increasing the total of Ca2+ to 3.53 mM in the medium can fully rescue the growth of mtp10 mutant (). Quantification of seedling fresh weight () and primary root length () further confirmed that supplementation of external Ca2+ can rescue high-Mg sensitive phenotype of mtp10 mutant in a dosage-dependent manner. The results further suggested a mutually antagonistic relationship between Ca2+ and Mg2+, and also indicated that Arabidopsis MTP10 plays an important role in the regulation of plant Mg and Ca homeostasis.
Figure 1. External supplementation of Ca2+ rescues high-Mg sensitivity of mtp10 mutant in a dose dependent manner (a) The phenotype of Col-0 and mtp10 mutant grown in 1/6 MS medium supplemented with 10 mM MgCl2 and different concentrations of Ca2+ conditions. Seedlings were grown in 1/2 MS medium for 3 days, and then transferred to 1/6 MS medium supplemented with 10 mM MgCl2 and external different concentrations of CaCl2 (0, 0.1, 0.3, 0.5, 1, and 3 mM). Bar = 1 cm. (b,c) Quantification of average primary root length (b) and fresh weight (c) of wild-type Col-0 and mtp10 mutants. Data represent means ± SD of five replicate experiments. Asterisks indicate significant difference between the wild-type Col-0 and mtp10 mutant (Student’s t-test, *P < .05).
The mtp10 mutant is hypersensitive to high Mg2+ conditions in the deficiency of Ca2+
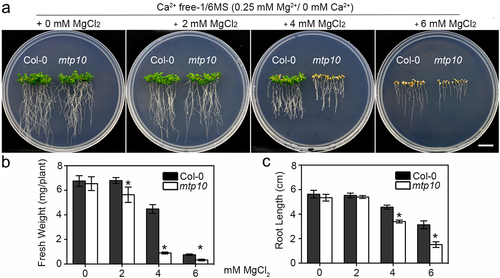
To verify the antagonistic relationship between Mg2+ and Ca2+ in plants, we performed growth assays using Ca2+-free 1/6 MS medium supplemented with different concentrations of Mg2+, in which the Ca2+ was removed as much as possible. After growing on Ca2+-free 1/6 MS medium for 2 weeks, the growth of mtp10 mutant plants was comparable to that of wide-type plants (). After adding extra 2 mM Mg2+ to the Ca2+-free 1/6-MS medium, the fresh weight of mtp10 mutant was slightly lower than that in the wild-type Col-0, and the leaves of mtp10 mutant were significantly yellower than those in the wild-type Col-0 (,b). The mtp10 mutant wilted away directly under the Ca2+-free 1/6-MS medium supplemented with extra 4 mM MgCl2 (). The growth of mtp10 mutant was much more severe than the plants grown in 1/6 MS containing 10 mM MgCl2 (). In the presence of 10 mM MgCl2 supplementation in the Ca2+-free 1/6-MS medium, both the wild-type Col-0 and mtp10 mutant were withered (). Compared to our previous data, the mtp10 mutant only showed hypersensitive to 8–12 mM MgCl2 when in the 1/6 MS medium containing 0.53 mM Ca2+,Citation12 we suggested that the deficiency of Ca2+ can aggravate the hypersensitivity of mtp10 to high-Mg stress.
Figure 2. Ca2+ deficiency can increase the sensitivity of mtp10 to high-Mg stress (a) The phenotype of Col-0 and mtp10 mutant grown in Ca2+ free 1/6 MS medium supplemented with different concentrations of Mg2+. 3-day-old wild-type Col-0 and mtp10 mutant seedlings were planted on 1/2 MS medium and then transferred to the 1/6-MS (without Ca2+) supplemented with different concentrations of MgCl2 (2, 4 and 6 mM) for another two weeks. Bar = 1 cm. (b,c) Quantification of average primary root length and fresh weight of wild-type Col-0 and mtp10 mutants. Data represent means ± SD of five replicate experiments. Asterisks indicate significant difference between the wild-type Col-0 and mtp10 mutant (Student’s t-test, *P < .05).
MTP10 affect the accumulation of Ca2+ in Arabidopsis
In rice, one of the high-affinity K+ transport (HKT) family proteins, OsHKT2;4, was characterized as a calcium-permeable cation channel that conducts current carried by a wide range of monovalent and divalent cations.Citation5 Further work also revealed that OsHKT2;4 could rescue the growth of MM281 in Mg2+-deficient conditions, suggesting that OsHKT2;4 can transport both Mg2+ and Ca2+.Citation6 Considering that the loss-of-function of MTP10 results in seedlings being sensitive to both high-Mg and high-Ca stresses, we wondered whether MTP10 can also transport Ca2+. We then introduced the MTP10 in the yeast strain ∆gdt1, which had been well used to detect the Ca2+ transport activity of heterogeneously expressed proteins.Citation14 Under 0, 100, and 200 mM Ca2+ conditions, the growth of wild-type BY4741 yeast strain, the yeast mutant ∆gdt1 expressing the pYES2 empty vector, and ∆gdt1 expressing the pYES2-MTP10 showed no difference. Under 300 mM Ca2+ conditions, BY4741 yeast showed resistance to high-Ca stress, suggesting that BY4721 yeast is capable of transporting extra Ca2+ out of the cell, thus avoiding the toxicity of high-Ca. Both the ∆gdt1 expressing the pYES2 and pYES2-MTP10 showed growth retardation under 300 mM Ca2+ condition. The growth of ∆gdt1 strains expressing pYES2 was not significantly distinguished from that of ∆gdt1 strains expressing pYES2-MTP10, indicating that MTP10 had no Ca2+ transport activity (Fig. S1).
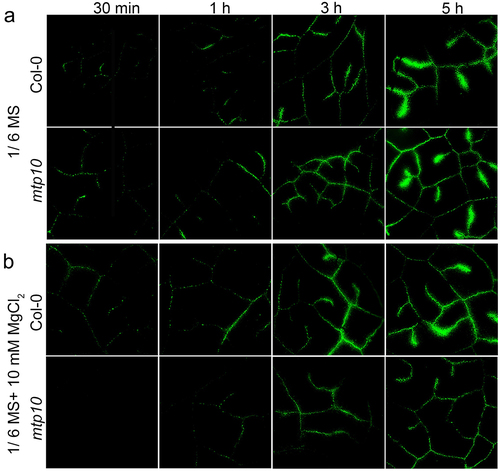
The Oregon Green BAPTA 488 5N (OGB-5N) is a well-established membrane impermeable low-affinity Ca2+ fluorescent dye that has been used to track extracellular Ca2+ transport and distribution in the leaf.Citation15 We first grew the wild-type Col-0 and mtp10 mutant in the 1/2 MS agar medium for 7 days, then the detached mature leaves were cultured in 1/6 MS liquid medium or 1/6 MS containing 10 mM MgCl2. Meanwhile, the 200 μM OGB-5 N was applied in the medium. The fluorescence signals at the time point 30 min, 1 h, 3 h, and 5 h were monitored, respectively. We found a gradually increased fluorescence signal in the vascular system over time (). Under controlled conditions, there was no significant difference between wild-type Col-0 and mtp10 mutant (). However, in the presence of 10 mM MgCl2 supplementation, the mtp10 mutant showed a weaker fluorescence signal compared to the wild-type Col-0 (). Our results further suggest that MTP10 is involved in the homeostasis balance between Mg and Ca.
Figure 3. Fluorescence microscopy of extracellular Ca2+ in leaves of Col-0 and mtp10 mutant. Col-0 and mtp10 seedlings were grown on 1/2 MS solid plates for 7 days and then cultured hydroponically in 1/6 MS medium (a) or 1/6 MS containing 10 mM MgCl2 (b) for another 3 days. Fully expanded leaves were detached and inserted into 200-μL tubes with their corresponding growth medium in the presence of 200-μM fluorescent dye OGB-5 N. Images of the same leaf was taken at different time points (30 min, 1 h, 3 h, and 5 h) of the OGB-5 N treatment.
MTP10 loss-of-function mutant showed defects in the regulation of Ca and Mg homeostasis in Arabidopsis
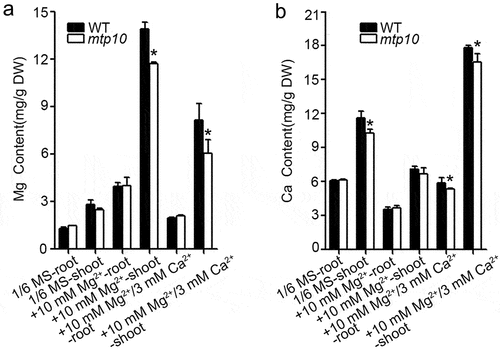
To investigate the causes of Ca2+ being able to rescue the high-Mg sensitive phenotype of mtp10, we measured the major cation contents in wild-type Col-0 and mtp10 mutant under different treatment conditions by using the ICP-MS. The 7-day-old seedlings grown on 1/2 MS medium were transferred to 1/6 MS liquid medium, 1/6 MS supplemented with 6 mM MgCl2, or 1/6 MS supplemented with 6 mM MgCl2 and 3 mM CaCl2 for another 10 days. We separately sampled the roots and shoots of seedlings. Compared with the control group (1/6 MS), we found that both roots and shoots of the wild-type Col-0 and mtp10 mutant seedlings accumulated a large amount of Mg after high-Mg treatment (1/6 MS+10 mM MgCl2), while the shoots accumulated more Mg than that in the roots. When plants were grown in 1/6 MS medium supplemented with 6 mM MgCl2 and 3 mM CaCl2, the seedlings accumulated a relatively lower amount of Mg compared to the treatment with only 10 mM extra MgCl2 (). Meanwhile, the shoot of the mtp10 mutant accumulated a relatively lower amount of Mg compared to that in the wild-type Col-0 when plants were grown in 1/6 MS supplemented with 10 mM MgCl2 or 1/6 MS supplemented with 10 mM MgCl2 and 3 mM CaCl2 (). These results further suggested that high-Mg stress could lead to excessive accumulation of Mg in plants and that the addition of Ca2+ can suppress the over-accumulation of Mg. We also measured the Ca contents in each treatment. Under controlled conditions, the Ca content in the shoot of mtp10 mutant was slightly lower than that in the wild-type Col-0. We also found that high-Mg treatment (1/6 MS+ 10 mM MgCl2) inhibited the Ca accumulation in both the roots and shoots of wild-type Col-0 and mtp10 mutant. When plants were grown on 1/6 MS medium supplemented with 6 mM MgCl2 and 3 mM CaCl2, the shoots of wild-type Col-0 and mtp10 mutant accumulated much higher Ca compared to other treatment groups. Moreover, the Ca contents in the root and shoot of the mtp10 mutant were slightly lower than that in the wild-type Col-0 (). Together with these results, we further provide compelling evidence that a mutually antagonistic relationship exists between Ca and Mg, and that MTP10 plays an essential role in maintaining Ca and Mg homeostasis.
Figure 4. Metal ions contents in wild-type Col-0 and mtp10 mutant. 7-day-old seedlings grown on 1/2 MS medium were transferred to 1/6 MS liquid medium, 1/6 MS supplemented with 6 mM MgCl2, or 1/6 MS supplemented with 6 mM MgCl2 and 3 mM CaCl2. Samples were collected after being treated for another 10 days. The Mg (a) and Ca (b) contents were measured by ICP-MS. Data are means ± SD. n = 4. Asterisks indicate significant difference between the wild-type Col-0 and mtp10 mutant (Student’s t-test, *P < .05).
We also detected the expression of Mg2+ transport-related proteins in wild-type Col-0 and mtp10 mutant under control conditions, high-Mg, and Mg-deficient conditions, we cultured wild-type Col-0 and mtp10 mutant in 1/6 MS hydroponic solution for 1 week, and then transferred them to control (1/6 MS), high-Mg (1/6 MS+ 10 mM MgCl2), or Mg-deficient (1/6 MS-Mg2+) hydroponic solutions. The total RNA of each group of samples was extracted after 10 hours of treatment in the medium, and then the expression of the target genes was detected. CIPK3/9/23/26, CBL2/3, MGT2, and MHX were mentioned previously as transporters associated with Mg2+ transport on vesicles. MGT9 was associated with reproductive development in Arabidopsis, MGT10 was localized in chloroplasts, and MGT6 was associated with Mg2+ uptake in roots under low-Mg environment and resistance to high-Mg stress.Citation9 The expression of these genes was indeed up- or down-regulated in the mtp10 mutant compared to the wild-type Col-0, indicating the disruption of Mg homeostasis in the mtp10 mutant (Figure S1). Under control conditions (1/6 MS), the expression of all our selected genes showed upregulation in the mtp10 mutant, except for the expression of a downregulated MGT9. Among these genes, the CIPK26 and MGT10 exhibited at least a two-fold up-regulated expression in the mtp10 mutant. When seedlings were treated with high-Mg (1/6 MS+ 10 mM MgCl2), all of these genes showed up-regulated expression in the wild -type Col-0 seedlings compared to the control. However, in the mtp10 mutant, the expression patterns of these genes were differentiated. The expression of CIPK3 and CBL2 showed no difference between wild-type Col-0 and mtp10 mutant. The expression of CIPK9, CIPK23, CBL3, and MGT9 showed higher expression in the mtp10 mutant compared to that in the wild-type Col-0 seedlings. While the expression of CIPK26, MGT2, MHX, MGT6, and MGT10 was compromised in the mtp10 mutant (Fig S2). Our qRT-PCR results indicated at the molecular level that Ca2+ signaling is involved in regulating plant responses to high-Mg stress and that MTP10 plays a role in Ca2+ signaling regulated adaptation to high-Mg stress.
Discussion
Ca and Mg are secondary phytonutrients compared to nitrogen (N), phosphorus (P), and potassium (K), but are equally essential for plant growth, although the requirements of those elements are comparatively negligible to that of macronutrients. In plant cells, Ca2+ plays irreplaceable roles in increasing cell wall strength, neutralizing the anion potential in the vacuole, and counteracting external stresses. Ca in the form of calcium pectinate forms an intermediate gelatinous layer of the plant cell wall, enabling cells and cells to join together to form tissues and giving the plant organ or individuals a certain mechanical strength.Citation16 Mg2+ is a component of the chlorophyll molecule, among other functions in plants, and therefore essential for photosynthesis. Ca and Mg exhibit similar chemical properties, and both are doubly positively charged in the soil-water phase and at the soil cation exchange sites.Citation17 The importance of Mg and Ca lies not only in the role they play in plant growth but also in their interaction with each other. In Citrus sinensis (L.) Osbeck seedlings, Mg-deficiency induced leaf vein lignification, enlargement, and cracking.Citation18 These findings imply that Ca2+ and Mg2+ may have mutual antagonism in the cell wall, affecting plant growth, development, and coping with environmental stresses. During our study, we found that the mutation of MTP10 resulted in the plants could not efficiently facilitate Mg2+ diffusion from the xylem to shoots.Citation12 The leaf veins in the mtp10 mgt 6 double mutant also showed abnormal morphology, suggesting that MTP10 is involved in vascular development.Citation12 Under high Mg2+ conditions, we found that the Ca2+ accumulation in the vascular region of the leaf of mtp10 mutant was inhibited (). The ICP-MS data also suggested that high-Mg stress inhibited the accumulation of Ca in the whole plant (). We speculated that one of the possible reasons is that high-Mg stress affects Ca2+ transport in plants, especially in vascular tissues, which in turn affects the cross-linking of plant cell walls. The damage to the cell wall eventually induced high-Mg toxicity in plants, which led to the mtp10 mutant being more sensitive to high-Mg toxicity. In conditions of Ca2+ deficiency, the high-Mg stress further deteriorated Ca2+ transport and absorption by plants, and thus the mtp10 mutant growth was even more inhibited under low-Ca and high-Mg superposition conditions. Applying the appropriate amount of Ca2+ in the medium can effectively alleviate the excessive accumulation of Mg2+ in plants (), and thus the phenotype of mtp10 mutant can be restored (). The absence of MTP10 prevented plants from effectively regulating the balance of Ca and Mg.
In Arabidopsis, there are total of 12 MTP genes which had been identified and clustered in the CDF family, and several of which have been found function in metal ions transport and tolerance.Citation19 However, only MTP10 was found to be localized to the plasma membrane of parenchyma cells in vascular bundles and function in unloading of xylem Mg2+ to confer tolerance to high-Mg stress.Citation12 Among these MTP proteins, MTP1 was discovered to be involved in the response to high-Zn stress.Citation19 The MTP11 was found to transport Mn2+ in a proton-antiport manner and thus conferring high-Mn tolerance.Citation20 The tonoplast localized MTP8 was capable of coordinating Mn homeostasis and Fe reallocation.Citation21 Further study suggested that calcium-dependent protein kinases, CPK4/5/6/11, can interact with and function upstream of MTP8, indicating the presence of tonoplast-associated calcium signaling cascade that orchestrates Mn homeostasis.Citation22 Many studies have shown that Ca2+ signaling plays important role in plant response to high-Mg stress. By performing the Y2H and BiFC assay, we found that CIPK7 and CIPK26 can interact with MTP10 (Fig. S3). In Arabidopsis, its genome contains 34 CPKs, which are essential to growth and development, and function in response diverse to biotic and abiotic stresses.Citation23 It is likely that certain CBL-CIPK complex and CPK kinases interact with MTP10 and act as upstream elements to mediate phosphorylation of MTP10 and act as a calcium signaling network in response to high-Mg stress. Besides MTP10, the CorA-type plasma membrane localized Mg2+ transporter MGT6 was also found to play an essential role in high-Mg tolerance.Citation24 It is also possible that both the CBL-CIPK and CPKs Ca2+ signaling network regulate the activity of MGT6 and thus MTP10 and MGT6 synergistically regulate Mg2+ transport and homeostasis in Arabidopsis.
Apart from the interaction between Ca2+ and Mg2+, Ca2+ or Mg2+ can also affect the homeostasis of other ions, such K+, and Na+. The mechanisms of interaction between these ions are also intriguing to explore. For example, high-Mg stress can disrupt the K+ homeostasis, and the loss-of-function of two K+ transporters, AKT1 and HAK5, results in the seedlings hypersensitive to high-Mg stress.Citation25 High-Na stress can interfere with K+ metabolism, and the presence of Ca2+ or Mg2+ reduces this interference.Citation26 And if the K+ concentration in the cultured medium gets too high, the Ca2+ and Mg2+ uptake can be inhibited. Therefore, combining these studies and our research, we believe that understanding the relationship between ion interactions and the molecular mechanisms that mediate this relationship is a fundamental effort to achieve efficient nutrient uptake by plants and enhance crop yields.
Supplemental Material
Download MS Word (757.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Tang RJ, Luan S. Regulation of calcium and magnesium homeostasis in plants: from transporters to signaling network. Curr Opin Plant Biol. 2017;39:97–8. doi:10.1016/j.pbi.2017.06.009.
- Brady KU, Kruckeberg AR Bradshaw HD Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu Rev Ecol Evol S. 2005;36(1):243–266. doi:10.1146/annurev.ecolsys.35.021103.105730.
- Bradshaw HD Jr. Mutations in CAX1 produce phenotypes characteristic of plants tolerant to serpentine soils. New Phytol. 2005;167(1):81–88. doi:10.1111/j.1469-8137.2005.01408.x.
- Lenz H, Dombinov V, Dreistein J, Reinhard MR, Gebert M, Knoop V. Magnesium deficiency phenotypes upon multiple knockout of Arabidopsis thaliana MRS2 clade B genes can be ameliorated by concomitantly reduced calcium supply. Plant Cell Physiol. 2013;54(7):1118–1131. doi:10.1093/pcp/pct062.
- Lan WZ, Wang W, Wang SM, Li LG, Buchanan BB, Lin HX, Gao JP, Luan S . A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc Natl Acad Sci U S A. 2010;107(15):7089–7094. doi:10.1073/pnas.1000698107.
- Zhang C, Li H, Wang J, Zhang B, Wang W, Lin H, Luan S, Gao J, Lan W . The rice high-affinity K+ transporter OsHKT2;4 mediates Mg 2+ homeostasis under high-Mg 2+ conditions in transgenic Arabidopsis. Front Plant Sci. 2017;8:1823. eCollection 2017. doi:10.3389/fpls.2017.01823.
- Lemtiri-Chlieh F, Arold ST, Gehring C. Mg2+ is a missing link in plant cell Ca2+ signalling and homeostasis—a study on Vicia faba guard cells. Inter J Mol Sci. 2020;21(11):3771. doi:10.3390/ijms21113771.
- Wiesenberger G, Steinleitner K, Malli R, Raier WF, Vormann J, Schweyen RJ, Stadler JA . Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6(4):592–599. doi:10.1128/ec.00382-06.
- Tang RJ, Zhao FG, Garcia VJ, Kleist TJ, Yang L, Zhang HX, Luan S . Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc Natl Acad Sci U S A. 2015;112(10):3134–3139. doi:10.1073/pnas.1420944112.
- Ricachenevsky FK, Menguer PK, Sperotto RA, Williams LE, Fett JP. Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front Plant Sci. 2013;4:144. doi:10.3389/fpls.2013.00144.
- Chu HH, Car S, Socha AL, Hindt MN, Punshon T, Guerinot ML. The Arabidopsis MTP8 transporter determines the localization of manganese and iron in seeds. Sci Rep. 2017;7(1):11024. doi:10.1038/s41598-017-11250-9.
- Ge H, Wang Y, Chen J, Zhang B, Chen R, Lan W, Luan S, Yang L. An Arabidopsis vasculature distributed metal tolerance protein facilitates xylem magnesium diffusion to shoots under high-magnesium environments. J Integr Plant Biol. 2021n/a(n/a. https://doi.org/10.1111/jipb.13187.
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008;56(3):505–516. doi:10.1111/j.1365-313X.2008.03612.x.
- Colinet AS, Sengottaiyan P, Deschamps A, Marie LC, Louise T, Didier D, Marie CD, François F, Pascal H, Pierre M. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci Rep. 2016;6(1):24282. doi:10.1038/srep24282.
- Wang Y, Kang Y, Ma C, Miao R, Wu C, Long Y, Ge T, Wu Z, Hou XY, Zhang JX, et al. CNGC2 is a Ca2+influx channel that prevents accumulation of apoplastic Ca2+in the leaf. Plant Physiol. 2017;173(2):1342–1354. doi:10.1104/pp.16.01222.
- Munarin F, Tanzi MC, Petrini P. Advances in biomedical applications of pectin gels. Inter J Biol Macromol. 2012;51(4):681–689. doi:10.1016/j.ijbiomac.2012.07.002.
- Jalali M, Arian TM, Ranjbar F. Selectivity coefficients of K, Na, Ca, and Mg in binary exchange systems in some calcareous soils. Environ Monit Assess. 2020;192(2):80. doi:10.1007/s10661-019-8022-y.
- Ye X, Huang HY, Wu FL, Cai LY, Lai NW, Deng CL, Guo JX, Yang LT, Chen LS. Molecular mechanisms for magnesium-deficiency-induced leaf vein lignification, enlargement and cracking in Citrus sinensis revealed by RNA-Seq. Tree Physiol. 2021;41(2):280–301. doi:10.1093/treephys/tpaa128.
- Kobae Y, Uemura T, Sato MH, Ohnishi M, Mimura T, Nakagawa T, Maeshima M. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol. 2004;45(12):1749–1758. doi:10.1093/pcp/pci015.
- Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J. 2007;51(2):198–210. doi:10.1111/j.1365-313X.2007.03138.x.
- Eroglu S, Giehl RFH, Meier B, Takahashi M, Terada Y, Ignatyev K, Andresen E, Küpper H, Peiter E, Von Wirén N. Metal tolerance protein 8 mediates manganese homeostasis and iron reallocation during seed development and germination. Plant Physiol. 2017;174(3):1633–1647. doi:10.1104/pp.16.01646.
- Zhang Z, Fu D, Sun Z, Ju C, Miao C, Wang Z, Xie D, Ma L, Gong Z, Wang C. Tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Mol Plant. 2021;14(5):805–819. doi:10.1016/j.molp.2021.03.003.
- Schulze S, Dubeaux G, Ceciliato PHO, Munemasa S, Nuhkat M, Yarmolinsky DA, Jaimee D, Renee AS, Tamar S, Leonie O, et al. A role for calcium-dependent protein kinases in differential CO2- and ABA-controlled stomatal closing and low CO2-induced stomatal opening in Arabidopsis. New Phytol. 2021;229(5):2765–2779. doi:10.1111/nph.17079.
- Yan YW, Mao DD, Yang L, Qi JL, Zhang XX, Tang QL, Li YP, Tang RJ, Luan S. Magnesium transporter MGT6 plays an essential role in maintaining magnesium homeostasis and regulating high magnesium tolerance in Arabidopsis. Front Plant Sci. 2018;9:274. doi:10.3389/fpls.2018.00274.
- Kocourková D, Krčková Z, Pejchar P, Kroumanová K, Podmanická T, Daněk M, Martinec J. Phospholipase Dα1 mediates the high-Mg2+ stress response partially through regulation of K+ homeostasis. Plant Cell Environ. 2020;43(10):2460–2475. doi:10.1111/pce.13831.
- Kopittke PM. Interactions between Ca, Mg, Na and K: alleviation of toxicity in saline solutions. Plant and Soil. 2012;352(1–2):353–362. doi:10.1007/s11104-011-1001-x.
