ABSTRACT
Piriformospora indica is a root endophyte having a vast host range in plants. Plant growth promotion is a hallmark of the symbiotic interaction of P. indica with its hosts. As a plant growth-promoting microorganism, it is important to know the mechanisms involved in growth induction. Hitherto, multiple reports have demonstrated various molecular mechanisms of P. indica-mediated growth promotion, including protein kinase-mediated pathway, enhanced nutrient uptake and polyamine-mediated growth phytohormone elevation. Here, we briefly present a discussion on the state-of-the-art molecular mechanisms of P. indica-mediated growth promotion in host plants, in order to obtain a future prospect on utilization of this microorganism for sustainable agriculture.
Introduction
Piriformospora indica (syn. Serendipita indica, Basidiomycota), a root endophytic fungus, exhibits an extensive host range including monocots and dicots.Citation1–3 P. indica colonization is observed mostly in the root epidermis and cortex of most of the host plants, including Arabidopsis, maize, tobacco, barley, rice and poplar.Citation1,Citation4–6 In most of the hosts, it significantly stimulates growth, including biomass and seed production.Citation7–10 Apart from growth promotion, this endophyte increases defense against microbial pathogens by altering microbial defense signaling pathways as well as defense phytohormones levels.Citation11,Citation12 Moreover, P. indica helps plants in tolerance of abiotic stresses, such as salinity, water stress, drought, low temperature and heavy metal toxicity.Citation13–18 Hitherto, the overall impression of P. indica colonization signifies beneficial interactions with the hosts, indicating involvement of general recognition and signaling pathways.Citation9 Furthermore, an elevation of the intracellular calcium concentration in the root cells with this fungus and fungal exudate indicated a trigger of intracellular signaling cascade upon plant–fungal interaction.Citation18,Citation19,Citation20 However, very little is understood about the mechanisms of growth promotion in plants upon P. indica colonization; here, we have concisely compiled and focused on recent major explorations of molecular mechanisms of P. indica-mediated growth induction in plants.
OXI1 kinase pathway mediates Piriformospora indica-induced growth promotion
OXI1 (OXIDATIVE SIGNAL INDUCIBLE1), an oxidative stress responsive serine/threonine protein kinase gene (At3g25250) in plant, plays an important role in pathogen response and is regulated by H2O2 and PDK1 (3-PHOSPHOINOSITIDE-DEPENDENT PROTEIN KINASE1), another serine/threonine kinase in eukaryotes involved in auxin transports and vascular development in plants.Citation19,Citation21,Citation22
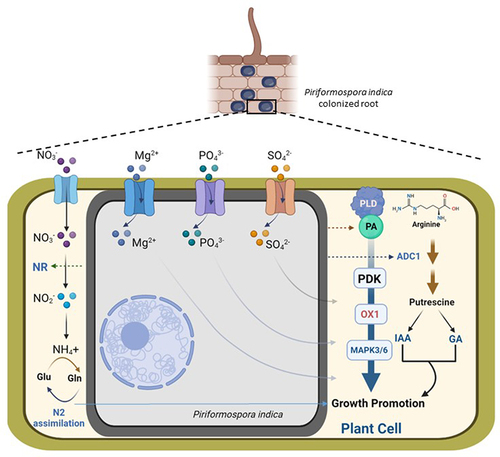
In search of genes responsible for P. indica-induced growth response, Camehl et al., 2011 genetically screened Arabidopsis, which revealed P. indica to show impaired growth promotion in OXI1 and PDK1 loss-of-function mutants,Citation9 and both OXI1 (and an homologue AGC2-2) and PDK1 transcript levels were upregulated upon P. indica colonization in Arabidopsis. They also demonstrated that a phospholipase D (PLD) facilitated a cascade consisting PDK1 and OXI1 (and AGC2-2) where P. indica induces PLD-mediated biosynthesis of phosphatidic acid (PA) and PA activates PDK1, which results in the induction of OXI1 and AGC2-2 triggering MAPK 3/6 (MAP kinases) (). This whole cascade is required for beneficial interaction, especially P. indica-mediated growth promotion in Arabidopsis.
Figure 1. Schematic representation of cumulative molecular mechanisms of P. indica-mediated growth promotion in plants. Nitrates (NO3−) are transported in the P. indica-colonized plant cell and are converted to nitrites (NO2−), which are further used in nitrogen (N2) assimilation. P. indica induces nitrate reductase (NR) activity that enhances N2 assimilation. Nutrient transporters (Mg2+, PO43- and SO42- transporters) induce nutrients uptake in P. indica-colonized root and promotes growth in plants. But how these nutrients are transported to the host cell is not known; therefore, simple arrows are used to indicate their transportations from the P. indica cell to the plant host cell. P. indica mediates the induction of phospholipase D-mediated biosynthesis of phosphatidic acid (PA), which activates cascade of PDK1 (3-PHOSPHOINOSITIDE-DEPENDENT PROTEIN KINASE1), OXI1 (OXIDATIVE SIGNAL INDUCIBLE1) and MAPK3/6 (MAP Kinase 3/6) and results in growth promotion of plants. P. indica induces ADC1 (ARGININE DECARBOXYLASE 1)-mediated biosynthesis of putrescine, which elevates IAA (indole-3-acetic acid) and GAs (gibberellins) in host plant, which promotes growth.
P. indica improves some of the macronutrients transport inside the plant cell and induces growth promotion
A foremost cause for P. indica-induced plant growth promotion is increased nutrient uptake in the host plants.Citation23–29 During colonization in roots, P. indica elevates nutrient uptake in host plants even in a low nutrient condition. The fungi contain a high-affinity phosphate (PO43-) transporter (PiPT), which belongs to the phosphate: H1 symporter (PHS) family within the Major Facilitator Superfamily (MFS) and the crystal structure of this transporter with inorganic phosphate were solved by Pederson et al., 2013.Citation23 They demonstrated a 2.9A° structure of a fungal transporter in an inward-facing occluded state where the phosphate was visible in the membrane-buried binding site. This P. indica transporter improves the phosphate intake of plants (), but P. indica does not induce plant’s own phosphate uptake capabilities.Citation24
Nitrogen is one of the key macronutrients for plant’s growth and development, which is utilized by plants majorly in the form of nitrate (NO3−) or ammonium (NH4+). Sometimes, limited nitrogen source may affect the plant’s growth. In a report, it was demonstrated that P. indica stimulates nitrogen accumulation in the roots of Arabidopsis and tobacco seedlings by inducing nitrate reductase and the starch-degrading enzyme, glucan-water dikinase (SEX1), resulting in growth promotion.Citation25 P. indica was also found to improve nitrogen acquisition during colonization in tomatoes, which helps in host’s growth and development.Citation26 Nitrogen was transported from the culture medium to P.indica, which was further transferred to the host. It was also hypothesized that hosts control the amount of nitrogen transferred from the fungi.Citation26 Nevertheless, further studies are needed to explore the molecular and physiological mechanism of transportation regulation.
Subsequently, P. indica was also found to increase magnesium (Mg2+), an important macronutrient, uptake in Arabidopsis, which results in growth promotion of the plants (). In search of the mechanism involved in increased Mg2+ uptake, a magnesium transporter named PiMgT1 was determined and functionally characterized in P. indica, which belongs to the CorA-like protein family of bacteria.Citation27 PiMgT1 showed complementation in a yeast magnesium transporter-mutant CM66, which confirmed its functionality by restoring the growth of Mg2+ transport mutants, lacking the plasma membrane-localized Mg2+ transporter genes, ALR1 and ALR2.
In a recent report, a sulfur-specific high-affinity transporter, SiSulT, has been identified and characterized in P. indica during interaction with maize.Citation28 This transporter has been demonstrated to be crucial for sulfur uptake in the sulfate form (SO42-) to the host plant and promoting growth and development of the host under low-sulfate conditions (). During transport of the sulfur, P. indica also induces the expression of host’s genes related to the sulfur assimilation pathways, indicating alteration of overall sulfur metabolism in a beneficial way to the host plant upon colonization.Citation29
Carbohydrate or sugar is one of the most important primary metabolite groups for growth and development in plants. Genome sequencing of P. indica identified 19 putative hexose transporter genes, which show 15–91% homology with each other;Citation30 among them, a PiHXT5 hexose transporter was characterized during interaction with maize (). This fungal transporter was 64-fold upregulated during colonization inside the host as compared to the axenic state, which indicates that symbiotic signaling controls the expression of this transporter. Its expression at a low glucose concentration revealed its high-affinity nature and host’s cellular glucose concentration dependency. This transporter belongs to the MFS superfamily with 12 predicted transmembrane helices. However, the relationship between host’s photosynthetic activity and the transcript level of fungal transporter and the correlation of symbiotic signaling and glucose signaling have not been revealed yet.Citation30 It is also not known whether P. indica with its accumulated sugars can help the host in the extreme sugar-deficient environment. Therefore, more research is required to comprehend P. indica’s role in sugar transportation in the host plants and whether it truly takes part in the growth promotion.
Among the macronutrients required for uninterrupted plant growth, potassium (K+) is crucial. A study on P. indica-mediated alteration of K+ transportation in Arabidopsis exhibited the presence of multiple K+ transporters in the P. indica, i.e. SiHAK1, SiTRK1, SiTRK2 and SiTOK1.Citation31 Nonetheless, in the low K+ condition P. indica did not promote the growth of Arabidopsis. Surprisingly, under the condition of K+ deficiency, P. indica reduces overall K+ accumulation in the host plant while improving its own growth by expressing its own K+ transporter gene SiHAK1. Therefore, P. indica does not improve K+ transportation or accumulation inside the host cell for growth promotion.Citation30 Furthermore, it was demonstrated that, under salt stress, P. indica induces K+ concentration in the host, which does not take part in growth promotion but alters Na+/ K+ homeostasis.Citation32
P. indica induces putrescine-mediated elevation of growth phytohormones that promotes plant growth
P. indica alters multiple phytohormonal regulated pathways during colonization, e.g. jasmonate-regulated secondary metabolite, glucosinolate pathway during early stages of interaction,Citation31 rapid increase in auxin levels during early recognition of the host, which is important for reprogramming the root development,Citation33 - elevated level of auxins in Arabidopsis roots,Citation5 and P. indica itself also produces IAA in the liquid culture.Citation34 In a recent study, it has been elucidated that P. indica employs putrescine for plant’s growth promotion.Citation10 P. indica induces putrescine biosynthesis in tomato (Solanum lycopersicum) through the ARGININE DECARBOXYLASE 1 (SlADC1)-mediated pathway, as metabolomics and gene expression data indicated that P. indica induces putrescine content and SlADC1 transcript level in tomato upon colonization. Growth promotion with P. indica was found to be impaired in SlADC1-suppressed or -mutant lines of tomato and/or Arabidopsis, and the growth promotion was restored by complementation with exogenous putrescine supplementation. Exogenous putrescine treatment also elevates auxin (indole-3-acetic acid) and specific gibberellins (GA4 and GA7) in tomato, which suggests that P. indica induces putrescine to elevate growth phytohormone levels, which finally results in plant’s growth promotionCitation10 ().
Conclusion
P. indica-mediated growth promotion in plants is a crucial area in plant biology as it will have a great impact on agriculture to improve crop yield in the future. However, a few major physiological mechanisms of growth induction have been explored, and the complexity and characteristics of this mutualistic interaction of P. indica vary greatly from host to host. Therefore, there are possibilities to have host-specific mechanisms of P. indica-mediated growth induction, which has to be explored. There are major gaps in the information regarding P. indica-mediated nutrient transportation. For example, although P. indica induces the nutrient uptake through nutrient transporters, the mechanism of nutrient transportation to the plant cell from fungal spores or hyphae is not clear yet, which has to be elucidated. Hexose transporter characterized in the P. indica helps the fungi to accumulate sugars in the low sugar condition of the host; however, it is not revealed that whether these accumulated sugars are further transferred to the host’s cell for aiding it to overcome the sugar deficiency. Apart from this, multiple questions have been raised from the current information regarding P. indica-mediated growth promotion, such as how the expressions of nutrient transporters are being regulated upon establishment of P. indica colonization in host?, whether P. indica only induces sugar uptake or does it also induce sugar biosynthesis in the host? How does putrescine induce growth phytohormone levels in the P. indica-colonized plants? All these questions need to be answered to shed more light on this complex mechanism of plant’s growth promotion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Varma A, Verma S, Sahay N, Bütehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol. 1999;65:2741–4. doi:10.1128/AEM.65.6.2741-2744.1999.
- Johnson JM, Sherameti I, Nongbri PL, and Oelmüller R. Standardized conditions to study beneficial and nonbeneficial traits in the Piriformospora indica/Arabidopsis thaliana interaction. In Varma A, Kost G, Oelmüller R, editors. Piriformospora indica: sebacinales and their biotechnological applications. soil biology. Vol. 33. Berlin (Heidelberg):Springer, 325–343; 2013.
- Qiang X, Weiss M, Kogel KH, Schäfer P. Piriformospora indica-a mutualistic basidiomycete with an exceptionally large plant host range. Mol Plant Pathol. 2012;13:508–518. doi:10.1111/j.1364-3703.2011.00764.x.
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA. 2005;102:13386–13391. doi:10.1073/pnas.0504423102.
- Vadassery J, Ritter C, Venus Y, Camehl I, Varma A, Shahollari B, Novák O, Strnad M, Ludwig-Müller J, Oelmüller R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol Plant Microbe Interact. 2008;21:1371–1383. doi:10.1094/MPMI-21-10-1371.
- Jogawat A, Vadassery J, Verma N, Oelmüller R, Dua M, Nevo E, Johri AK. PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Sci Rep. 2016;6:36765. doi:10.1038/srep36765.
- Peskan-Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Kost , G, Varma , A, and Oelmüller, R . Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant-microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant. 2004;122:465–477. doi:10.1111/j.1399-3054.2004.00424.x.
- Oelmüller R, Sherameti I, Tripathi S, Varma A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis. 2009;49:1–17. doi:10.1007/s13199-009-0009-y.
- Camehl I, Sherameti I, Venus Y, Bethke G, Varma A, Lee J, Oelmüller R. Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 2010;185(4):1062–1073. doi:10.1111/j.1469-8137.2009.03149.x.
- Kundu A, Mishra S, Kundu P, Jogawat A, Vadassery J. Piriformospora indica recruits host-derived putrescine for growth promotion in plants. Plant Physiol. 2022;188(4):2289–2307. doi:10.1093/plphys/kiab536.
- Molitor A, Zajic D, Voll LM, Pons-Kühnemann J, Samans B, Kogel KH, Waller F. Barley leaf transcriptome and metabolite analysis reveals new aspects of compatibility and Piriformospora indica–mediated systemic induced resistance to powdery mildew. Mol Plant Microbe Interact. 2011;24:1427–1439. doi:10.1094/MPMI-06-11-0177.
- Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel K-H, Schäfer P, Schwarczinger I. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008;180(2):501–510. doi:10.1111/j.1469-8137.2008.02583.x.
- Sun C, Johnson JM, Cai D, Sherameti I, Oelmuller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid localized CAS protein. J Plant Physiol. 2010;167:1009–1017. doi:10.1016/j.jplph.2010.02.013.
- Husaini AM, Abdin MZ, Khan S, Xu YW, Aquil S, Anis M. Modifying strawberry for better adaptability to adverse impact of climate change. Curr Sci. 2012;102:1660–1673.
- Zarea MJ, Hajinia S, Karimi N, Goltapeh EM, Rejali F, Varma A. Effect of Piriformospora indica and Azospirillum strains from saline or non-saline soil on mitigation of the effects of NaCl. Soil Biol Biochem. 2012;45:139–146. doi:10.1016/j.soilbio.2011.11.006.
- Ansari MW, Bains G, Shukla A, Pant RC, Tuteja N. Low temperature stress ethylene and not Fusarium might be responsible for Mango malformation. Plant Physiol Biochem. 2013;69:34–38. doi:10.1016/j.plaphy.2013.04.019.
- Unnikumar KR, Sowjanya SK, Varma A. Piriformospora indica: a versatile root endophytic symbiont. Symbiosis. 2013;60:107–113. doi:10.1007/s13199-013-0246-y.
- Vadassery J, Ranf S, Drzewiecki C, Mithöfer A, Mazars C, Scheel D, Lee J, Oelmüller R. A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J. 2009;59:193–206. doi:10.1111/j.1365-313X.2009.03867.x.
- Camehl I, Drzewiecki C, Vadassery J, Shahollari B, Sherameti I, Forzani C, Munnik T, Hirt H, Oelmuller R. The OXI1 kinase pathway mediates Piriformospora indica-induced growth promotion in Arabidopsis. PLoS Pathog. 2011;7:e1002051. doi:10.1371/journal.ppat.1002051.
- Jogawat A, Meena MK, Kundu A, Varma M, Vadassery J. Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J Exp Bot. 2020;71:2752–2768. doi:10.1093/jxb/eraa028.
- Petersen LN, Ingle RA, Knight MR, Denby KJ. OXI1 protein kinase is required for plant immunity against Pseudomonas syringae in Arabidopsis. J Exp Bot. 2009;60:3727–3735. doi:10.1093/jxb/erp219.
- Xiao Y, Offringa R. PDK1 regulates auxin transport and Arabidopsis vascular development through AGC1 kinase PAX. Nat Plants. 2020;6:544–555. doi:10.1038/s41477-020-0650-2.
- Pedersen BP, Kumar H, Waight AB, Risenmay AJ, Roe-Zurz Z, Chau BH, Schlessinger A, Bonomi M, Harries W, Sali A, et al. Crystal structure of a eukaryotic phosphate transporter. Nature. 2013;496(7446):533–536. doi:10.1038/nature12042.
- Bakshi M, Sherameti I, Meichsner D, Thürich J, Varma A, Johri AK, Yeh KW, Oelmüller R. Piriformospora indica reprograms gene expression in Arabidopsis phosphate metabolism mutants but does not compensate for phosphate limitation. Front Microbiol. 2017;8:1262. doi:10.3389/fmicb.2017.01262.
- Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem. 2005;280(28):26241–26247. doi:10.1074/jbc.M500447200.
- Cruz C, Fegghi Z, Martins-Loução MA, Varma A. Plant nitrogen use efficiency may be improved through symbiosis with Piriformospora indica. In: Varma A, Kost G, Oelmüller R editors. Piriformospora indica: sebacinales and their biotechnological applications; soil biology. Vol. 33. Berlin (Heidelberg): Springer; 2013. p. 285–293.
- Prasad D, Verma N, Bakshi M, Narayan OP, Singh AK, Dua M, Johri AK. Functional characterization of a magnesium transporter of root endophytic fungus Piriformospora indica. Front Microbiol. 2019;9:3231. doi:10.3389/fmicb.2018.03231.
- Narayan OP, Verma N, Jogawat A, Dua M, Johri AK. Sulfur transfer from the endophytic fungus Serendipita indica improves maize growth and requires the sulfate transporter SiSulT. Plant Cell. 2021;33:1268–1285. doi:10.1093/plcell/koab006.
- Zuccaro A, Lahrmann U, Guldener U, Langen G, Pfiffi S, Biedenkopf D, Wong P, Samans B, Grimm C, Basiewicz M, et al. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 2011;7(10):e1002290. doi:10.1371/journal.ppat.1002290.
- Rani M, Raj S, Dayaman V, Kumar M, Dua M, Johri AK. Functional characterization of a hexose transporter from root endophyte Piriformospora indica. Front Microbiol. 2016;7:1083. doi:10.3389/fmicb.2016.01083.
- Conchillo LB, Haro R, Benito B. K+ nutrition exchange in the serendipita-Arabidopsis symbiosis: study of the fungal K+ transporters involved front. Ecol Evol. 2021;9:876.
- Lahrmann U, Strehmel N, Langen G, Frerigmann H, Leson L, Ding Y, Scheel D, Herklotz S, Hilbert M, Zuccaro A. Mutualistic root endophytism is not associated with the reduction of saprotrophic traits and requires a non-compromised plant innate immunity. New Phytol. 2015;207:841–857. doi:10.1111/nph.13411.
- Meents AK, Furch ACU, Almeida-Trapp M, Özyürek S, Scholz SS, Kirbis A, Lenser T, Theißen G, Grabe V, Hansson B, et al. Beneficial and pathogenic Arabidopsis root-interacting fungi differently affect auxin levels and responsive genes during early infection. Front Microbiol. 2019;10:380.
- Sirrenberg A, Göbel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, Santos P, Feussner I, Pawlowski K. Piriformospora indica affects plant growth by auxin production. Physiol Plant. 2007;131:581–589. doi:10.1111/j.1399-3054.2007.00983.x.
