ABSTRACT
Root hairs are filamentous extensions from epidermis of plant roots with growth limited to the apical dome. Cell expansion undergoes tightly regulated processes, including the coordination between cell wall loosening and cell wall crosslinking, to form the final shape and size. Tip-focused gradients and oscillations of reactive oxygen species (ROS) together with calcium ions (Ca2+) as indispensable regulated mechanisms control rapid and polarized elongation of root hair cells. ROS homeostasis mediated by plasma membrane-localized NADPH oxidases, known as respiratory burst oxidase homologues (RBOHs), and class III cell wall peroxidases (PRXs), modulates cell wall properties during cell expansion. The expression levels of RBOHC, an NADPH oxidase that produces ROS, and class III PRXs are directly upregulated by ROOT HAIR DEFECTIVE SIX-LIKE 4 (RSL4), encoding a basic-helix-loop-helix (bHLH) transcription factor, to modulate root hair elongation. Cyclic nucleotide-gated channels (CNGCs), as central regulators of Ca2+ oscillations, also regulate root hair extension. Here, we review how the gradients and oscillations of Ca2+ and ROS interact to promote the expansion of root hair cells.
1. Introduction
Root hairs are unicellular extension from root epidermal cells, aiding plants in nutrient acquisition and anchorage as well as interaction with microbe in soil. The expansion rate of root hairs is determined by a complex process including the driving force from vacuolar turgor pressure, the maintenance from the exocytosis of new cell wall materials and local loosening of the existing cell wall, and exceeds 1 µm/min in ArabidopsisCitation1. Arabidopsis root hairs can reach 1 mm or longer in length and approximately 10 µm in diameter.Citation1 The root maturation zone is characterized by certain epidermal cells becoming root hairs. Immature epidermal cells becoming root hair or non-hair cells can be controlled by a position-dependent mechanism.Citation2–5 The cortex of Arabidopsis primary root consists of a ring of eight cells (). At an early stage, the epidermal cells at the junction of two underlying cortical cells (“H” position) will develop root hairs (trichoblasts), whereas non-hair cells (atrichoblasts) contact only one cortical cell (“N” position) (). Therefore, eight immature root-hair cells in Arabidopsis are separated by either one or two immature non-hair cellsCitation4,Citation5,Citation11 (). Additionally, cell-cell communication is important for establishing cell identity in the root epidermis. The Myb-like protein CAPRICE (CPC) translocates from atrichoblasts to trichoblasts and represses the expression of the negative regulator GLABRA2 (GL2), thereby establishing hair cell identity.Citation12,Citation13 ROOT HAIR DEFECTIVE6 (AtRHD6) encodes a basic-helix-loop-helix (bHLH) transcription factor and positively regulates the development of H cells.Citation14 GL2, as an HD-Zip transcription factor, inhibits RHD6 expression in atrichoblasts, thereby preventing differentiation into hair cells.Citation15–17 The expression of AtRHD6 was not observed in the cpc mutant.Citation18,Citation19 Epidermal cell differentiation can be acquired at an early stage prior to the initiation of cell elongation.Citation5 The morphology of trichoblasts is distinguished from atrichoblasts prior to hair initiation.Citation5 Trichoblasts in the meristematic zone display a high rate of cell division,Citation15 reduced cell length before root hair initiation, relatively dense cytoplasm,Citation4,Citation5 and less vacuolated.Citation5
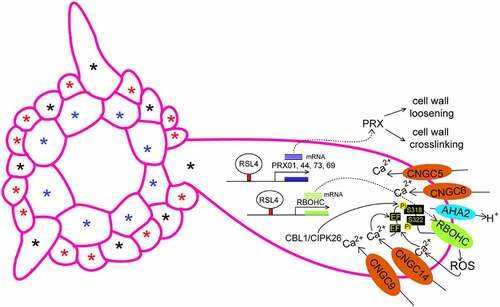
Figure 1. Regulation of tip growth of root hairs by ROS and Ca2+. This model is based on the previous works.Citation1,Citation6–10Apoplastic ROS generated by PRXs and RBOHC (RHD2) control cell wall expansion or crosslinking in the apical zone of root hairs. Ca2+ oscillations focused in root hair tip are modulated by CNGC5, CNGC6, CNGC9 and CNGC14. The CBL1/CIPK26 complex phosphorylates and activates RBOHC to generate ROS. RSL4 directly regulates the expression of genes encoding RBOHC and PRX01 (At1g05240), PRX44 (At4g26010), PRX73 (At5g67400), and PRX69 (AT5G64100). Blue asterisks indicate cortex. Black asterisks indicate H cell position. Red asterisks indicate N cell position.
ROOT HAIR DEFECTIVE SIX-LIKE 4 (RSL4) encodes a bHLH transcription factor that promotes root hair cell elongation.Citation19 The Rho-of-plant (ROP) guanosine triphosphatases (GTPases) are specifically localized to future sites of root hair emergence, and overexpression of ROP2 results in additional and misplaced root hairs.Citation20–22 Plant RAPID ALKALINIZATION FACTORS (RALFs) are secreted peptides that bind to Catharanthus roseus RECEPTOR-LIKE KINASE 1-like (CrRLK1L) family members such as FERONIA (FER), regulating plant cell size and shape.Citation23–26 The RALF1-FER complex promotes the translation of RSL4 and ROP2 mRNAs.Citation27,Citation28 In turn, high-level accumulation of RSL4 protein suppresses RALF1 expression via directly binding to the promoter of RALF1 gene,Citation27,Citation28 fine tuning root hair elongation.
2. Regulation of root hair elongation by reactive oxygen species (ROS)
The cell wall, as a dynamic structure, determines the shape of root hairs. The cell wall not only provides support for expansion at the tip but also functions in resisting against the turgor pressure.Citation29 Cell wall integrity is maintained through the coordination between cell wall loosening and restriction of growth via cell wall stiffening. In root hairs, cell walls are primarily composed of cellulose, xyloglucans, pectins, and hydroxyproline-rich glycoproteins that include extensins and arabinogalactan-proteins.Citation30,Citation31 Following root hair initiation, the apical zone is characterized by apoplastic ROS and cytoplasmic calcium ion (Ca2+) gradients. Hair cell elongation requires a balance between cell wall loosening (ROS activity) and stiffening (crosslinking of polymers). The crosslink between calcium ions and demethylated pectins forms egg-box structures, increasing cell wall stiffness.Citation32 ROS can be generated in the apoplast, and have different forms with different functions, such as, dioxygen (O2), singlet oxygen (1O2), superoxide radical (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•)Citation33 An increase in apoplastic H2O2 concentration affects the degree of cell wall crosslinking by oxidizing cell wall compounds, thus decreasing cell expansion.Citation34–36 OH• mediates scission of long-chain polysaccharides, such as pectin and xyloglucan, leading to non-enzymatic loosening of cell walls.Citation37,Citation38 ROS accumulate at the apex of root hairs during elongation. In contrast, when root hairs stop growing, ROS accumulation at the apex decreases.Citation39,Citation40 Thus, abnormal accumulation of ROS in root hair cells brings about exacerbated growth or root hair burst.Citation6 Respiratory burst homologs (RBOHs) and peroxidases (PRXs) are the main generators of ROS at the apoplast in plants.Citation41–44 The ROS primarily generated by RBOH oxidase are required for normal root hair elongation.Citation34,Citation40,Citation45,Citation46 In plants, RBOHs contain six predicted membrane-spanning domains and two Ca2+-binding EF-hand motifs in an N-terminal cytoplasmic region, which is absent in the homologue of mammalian NOX1-4/gp91phox.Citation47–49 RBOHs are activated upon Ca2+ binding the EF-hand and phosphorylation in the N-terminal region of RBOH.Citation42,Citation50,Citation51 In Arabidopsis, root hairs are initiated correctly but incapable of elongation in rbohc (root hair defective 2, rhd2) mutants grown on medium buffered to pH 5.0.28,Citation34 In maize, RHT5 (ROOTHAIRLESS 5), encoding a monocot-specific NADPH oxidase, is responsible for establishing the high levels of ROS in the tips of growing root hairs, which is required for cell elongation.Citation52 Consistent with these findings, the elongation of wild-type hairs of Arabidopsis was inhibited by diphenyleneiodonium chloride (DPI), an inhibitor of NADPH oxidases.Citation40 In addition, OH• partially rescued the growth of rbohc mutants, but this growth lacked spatial control.Citation53 Interestingly, supercentipedel1 (SCN1), a RhoGTPase GDP dissociation inhibitor (RhoGDI), can spatially restrict ROS to a single point on the trichoblast.Citation39 Therefore, in scn1 mutants, multiple tip-growing axes were formed from single bulges,Citation39,Citation54 and ROS were produced at ectopic foci in root hair cells.Citation39 Trans-Golgi network-localized YPT-INTERACTING PROTEIN 4a and YPT-INTERACTING PROTEIN 4b (YIP4a/b) can induce activation and plasma membrane accumulation of ROP GTPases during root hair initiation.Citation22 RHD2 accumulated at a single location in the wild type whereas it appeared at several distinct loci in the scn1 mutant.Citation51 These results suggested that SCN1 RhoGDI controls the spatial accumulation of RHD2 to regulate the ROS distribution in root hairs.Citation39,Citation51 In addition, SCN1 RhoGDI is likely to act by regulating ROP2 GTPase that is located to the hair tip, and regulates actin microfilament dynamics in root hair cells.Citation21,Citation39,Citation55 Further study indicates that such microfilaments are required for the localization of RHD2 to the growing tip.Citation51 These findings reveal that normal, long, polarized root hair growth requires not only ROS production but also correct ROS localization.
In addition to NADPH oxidase, class III PRXs can be secreted into the apoplastic space and modulate ROS levels in apoplasts to mediate root hair growth.Citation35 Class III PRXs are involved in the consumption or release of H2O2 and the generation of other ROS. In the hydroxylic cycle, OH• is generated from H2O2 and oxygen by Class III PRXs. In peroxidative and hydroxylic cycles, the apoplast H2O2 concentration is regulated by Class III PRXs.Citation35,Citation56 In the oxidative cycle, together with NADPH oxidase/RBOH proteins, PRXs contribute to O2•− production by oxidizing 1O2.Citation57 In this regard, some PRXs appear to promote the polymerization of cell wall components, whereas others likely cause cell wall loosening via polysaccharide cleavage to promote polarized growth.Citation37,Citation58,Citation59 Mutations in two apoplastic class III peroxidases, PRX44 and PRX57, led to root hair cell wall rupture because the walls of root hair cells of the mutants were thin and mechanically weakened.Citation60 Moreover, PRX62 and PRX69 stimulate Arabidopsis root hair elongation at low temperatures, likely through modulating ROS homeostasis and cell wall extensin-insolubilization.Citation6 PRX01, PRX44, and PRX73 function in ROS homeostasis and control Tyr-crosslinking of cell wall extensins during polar expansion of root hair cells.Citation7,Citation61 RSL4 directly binds to the promoter regions of RBOHC and PRX1, 44, 73, and 69 to increase their expression, triggering apoplast oxidation and polarized root hair elongation.Citation6,Citation7
3. Regulation of root hair elongation by Ca2+
During root hair elongation Ca2+ is distributed primarily at the tip apex (about 1 μM) and reduced to 0.1–0.2 μM at the base of the root hair.Citation46,Citation62,Citation63 When root hair elongation ceases, the Ca2+ gradient is changed.Citation1,Citation46 An increased cytoplasmic Ca2+ concentration provokes polar secretion, rearrangement of the actin cytoskeleton, movement of organelles, and enzyme activity,Citation64 facilitating the elongation of tip-growing cells in roots.Citation65,Citation66 In addition to the Ca2+ concentration gradient, Ca2+ oscillations play a key role in transmitting signaling events for tip growth.Citation46,Citation66,Citation67 Early studies primarily focused on the importance of the Ca2+ gradient and oscillations in root hair growth. Recently, Ca2+ channels contributing to the regulation of Ca2+ signatures to control polarized tip growth of root hairs have been identified.Citation9 The CNGC14 nonselective cation channel mediates Ca2+ influx, which is required for maintaining the correct Ca2+ signature for the integrity of root hairs.Citation68 Cyclic nucleotide-gated channel 14 (cngc14) mutants exhibited very short and some branched root hairs, with altered amplitude and frequency of Ca2+ oscillations compared to the wild type.Citation68 Moreover, CNGC14 physically interacts with calmodulin 7 (CaM7) to suppress CNGC14 activity, potentially affecting Ca2+ oscillations during polarized growth of root hair.Citation69 Also, CNGC5, CNGC6, and CNGC9 maintain tip-focused Ca2+ oscillations, which are required for root hair growth and polarity. CNGC6, CNGC9, and CNGC14 triple mutants showed root hair burst after transition to the rapid growth phase.Citation70 Similarly, triple cngc5/6/9 mutants exhibit markedly attenuated cytosolic Ca2+ oscillations in shorter and branched root hairs.Citation71
An increase in Ca2+ concentration could impact the pH at the cell surface and ROS generation.Citation72 In root hairs, apoplastic ROS, apoplastic and cytoplasmic pH, and cytoplasmic Ca2+ oscillations occurred with a frequency of two to four peaks per minute and lag behind the growth oscillations by 8.0, 7.0, and 5.3 s, respectively.Citation34,Citation73 RBOH proteins are activated by Ca2+ via direct binding of Ca2+ to the EF-hand motifs of RBOH proteins and Ca2+-induced phosphorylation of RBOH by kinases such as Ca2+-dependent protein kinase 5 (CPK5) and calcineurin B-interacting protein kinase (CIPK26).Citation10,Citation74,Citation75 ROS and Ca2+ form a positive feedback loop in root hair elongation.Citation34,Citation51,Citation76 ROS produced by RBOHC oxidase activate hyperpolarization-activated Ca2+ channels (HACCs) and stimulate Ca2+ influx into the cytoplasm.Citation40,Citation77 In turn, a high level of Ca2+ triggers RBOHC oxidase activity by binding to EF-hands and promoting the phosphorylation of the residues S318/322, maintaining active growth.Citation51 Recent studies showed that calcineurin B-like protein (CBL1)-CBL-interacting protein kinase (CIPK26) complex activates RBOHC by phosphorylation, triggering ROS generation.Citation10
Surface pH affects cell wall viscosity by mediating the regulation of expansins, non-hydrolytic cell wall-loosening proteins, and certain cell wall-modifying enzymes.Citation78–81 Low pH promotes not only root hair initiation but also elongation. At root hair initiation sites, the pH in the apoplastic space is reduced from 5.0 to 4.562. Low pH induces rapid extension of plant cell walls.Citation82,Citation83 Membrane H+-ATPases (AHAs) directly regulate apoplastic pH, and AHA2 is highly expressed in growing root hairs.Citation84,Citation85 FER interacts with RALF1 peptide to inhibit the activity of AHA2 possibly via Ser899 phosphorylation, thereby alkalizing the apoplast and suppressing cell expansion.Citation86,Citation87 Ca2+ modulates extracellular pH via H+-ATPases, which alter cell wall structure during growth.Citation88 However, the root hair elongation stopped in rbohc mutants when exposed to pH 5.0, whereas rbohc mutants restored root hair elongation under pH 6.034. This indicates that extracellular pH and ROS function in a coordinated and complementary manner to regulate the expansion of the growing root hair.Citation34 However, little is known about the interaction of ROS and ions in modulating plant root hair development and growth.
4. Conclusions
Polarized root hair growth is tightly regulated by internal and external signals. ROS and Ca2+ are essential for the regulation of root hair elongation. Gradients and oscillations of apoplastic ROS, cytoplasmic Ca2+, and H+ are tightly linked and modulate cell wall dynamics during polar growth of root hairs. A better understanding of the mechanism underlying root hair expansion by ROS and Ca2+ has been achieved. However, it is unclear how ROS, Ca2+, and H+ regulate each other. Visualization of ROS and H+ dynamics in plant tissues and cells is challenging. Despite the identification of genes responsible for Ca2+ transport, further research is clearly needed to identify the Ca2+ transporters or channels that regulate final root hair size and to clarify how the channels are regulated by ROS. Information on the mechanism underlying root hair growth will enable the breeding of crops that can thrive under nutrient-deficient conditions and thereby increase yields.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grierson C, Nielsen E, Ketelaarc T, Schiefelbein J. Root hairs. Arabidopsis Book/American Soc Plant Bio. 2014;12:e0172. doi:10.1199/tab.0172.
- Cormack RGH. Investigations on the development of root hairs. New Phytol. 1935;34(1):30–6. doi:10.1111/j.1469-8137.1935.tb06826.x.
- Bünning E. Über die Differenzierungsvorgänge in der Cruciferenwurzel. Planta. 1951;39(2):126–153. doi:10.1007/BF01910114.
- Dolan L, Duckett CM, Grierson CS, Linstead PJ, Roberts K, Dean C, Poethig S, Roberts K. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development. 1994;120(9):2465–2474. doi:10.1101/gad.8.18.2241.
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166(2):740–754. doi:10.1006/dbio.1994.1352.
- Pacheco JM, Ranocha P, Kasulin L, Fusari CM, Servi L, Aptekmann AA, Gabarain VB, Peralta JM, Borassi C, Marzol E, et al. Apoplastic class III peroxidases PRX62 and PRX69 promote Arabidopsis root hair growth at low temperature. Nat Commun. 2022;13(1):1–14. doi:10.1038/s41467-022-28833-4.
- Mangano S, Denita-Juarez SP, Choi H-S, Estevez JM, Hwang Y, Ranocha P, Velasquez SM, Borassi C, Barberini ML, Aptekmann AA. Molecular link between auxin and ROS-mediated polar growth. Proc Natl Acad Sci USA. 2017;114(20):5289–5294. doi:10.1073/pnas.1701536114.
- Mangano S, Juárez SPD, Estevez JM. ROS regulation of polar growth in plant cells. Plant Physiol. 2016;171(3):1593–1605. doi:10.1104/pp.16.00191.
- Tian W, Wang C, Gao Q, Li L, Luan S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants. 2020;6(7):750–759. doi:10.1038/s41477-020-0667-6.
- Zhang X, Köster P, Schlücking K, Balcerowicz D, Hashimoto K, Kuchitsu K, Vissenberg K, Kudla J. CBL1-CIPK26-mediated phosphorylation enhances activity of the NADPH oxidase RBOHC, but is dispensable for root hair growth. FEBS Lett. 2018;592(15):2582–2593. doi:10.1002/1873-3468.13187.
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119(1):71–84. doi:10.1242/dev.119.1.71.
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks DM, Shimura Y, Okada K. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development. 2002;129(23):5409–5419. doi:10.1242/dev.00111.
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog. CPC Sci. 1997;277(5329):1113–1116. doi:10.1126/science.277.5329.1113.
- Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 1994;106(4):1335–1346. doi:10.1104/pp.106.4.1335.
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122(4):1253–1260. doi:10.1242/dev.1224.1253.
- Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, Ruberti I, Morelli G. The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J. 1996;10(3):393–402. doi:10.1046/j.1365-313X.1996.10030393.x.
- Lin Q, Ohashi Y, Kato M, Tsuge T, Gu H, Qu L-J, Aoyama T. GLABRA2 directly suppresses basic helix-loop-helix transcription factor genes with diverse functions in root hair development. Plant Cell. 2015;27(10):2894–2906. doi:10.1105/tpc.15.00607.
- Menand B, Yi K, Jouannic S, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L. An ancient mechanism controls the development of cells with a rooting function in land plants. Science. 2007;316(5803):1477–1480. doi:10.1126/science.1142618.
- Yi K, Menand B, Bell E, Dolan L. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet. 2010;42(3):264–267. doi:10.1038/ng.529.
- Molendijk AJ, Bischoff F, Rajendrakumar CSV, Friml J, Braun M, Gilroy S, Palme K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20(11):2779–2788. doi:10.1093/emboj/20.11.2779.
- Jones MA, Shen -J-J, Fu Y, Li H, Yang Z, Grierson CS. The Arabidopsis ROP2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14(4):763–776. doi:10.1105/tpc.010359.
- Gendre D, Baral A, Dang X, Esnay N, Boutté Y, Stanislas T, Vain T, Claverol S, Gustavsson A, Lin D, et al. Rho-of-plant activated root hair formation requires Arabidopsis YIP4a/b gene function. Development. 2019;146(5):dev168559. doi:10.1242/dev.168559.
- Li C, Yeh F-L, Cheung AY, Duan Q, Kita D, Liu M-C, Maman J, Luu EJ, Wu BW, Gates L. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife. 2015;4:e06587. doi:10.7554/eLife.06587.
- Liao H, Tang R, Zhang X, Luan S, Yu F. FERONIA receptor kinase at the crossroads of hormone signaling and stress responses. Plant Cell Physiol. 2017;58(7):1143–1150. https://doi.org/10.1093/pcp/pcx048.
- Franck CM, Westermann J, Boisson-Dernier A. Plant malectin-like receptor kinases: from cell wall integrity to immunity and beyond. Annu Rev Plant Biol. 2018;69:301–328. doi:10.1146/annurev-arplant-042817-040557.
- Li C, Wu H-M, Cheung AY. FERONIA and her pals: functions and mechanisms. Plant Physiol. 2016;171(4):2379–2392. doi:10.1104/pp.16.00667.
- Zhu S, Martínez Pacheco J, Estevez JM, Yu F. Autocrine regulation of root hair size by the RALF-FERONIA-RSL4 signaling pathway. New Phytol. 2020;227(1):45–49. doi:10.1111/nph.16497.
- Zhu S, Estévez JM, Liao H, Zhu Y, Yang T, Li C, Wang Y, Li L, Liu XM, Pacheco JM, et al. The RALF1–FERONIA complex phosphorylates eIF4E1 to promote protein synthesis and polar root hair growth. Mol Plant. 2020;13(5):698–716. doi:10.1016/j.molp.2019.12.014.
- Akkerman M, Franssen‐verheijen M, Immerzeel P, Hollander L, Schel J, Emons AMC. Texture of cellulose microfibrils of root hair cell walls of Arabidopsis thaliana. Medicago Truncatula Vicia Sativa J Microsc. 2012;247(1):60–67. doi:10.1111/j.1365-2818.2012.03611.x.
- Velasquez SM, Ricardi MM, Dorosz JG, Fernandez PV, Nadra AD, Pol-Fachin L, Egelund J, Gille S, Harholt J, Clancia M, et al. O-glycosylated cell wall proteins are essential in root hair growth. Science. 2011;332(6036):1401–1403. doi:10.1126/science.1206657.
- Nielsen E. Plant cell wall biogenesis during tip growth in root hair cells. : Springer, Berlin; 2008. doi:10.1007/978-3-540-79405-9_11.
- Hocq L, Pelloux J, Lefebvre V. Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci. 2017;22(1):20–29. doi:10.1016/j.tplants.2016.10.009.
- Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–19. doi:10.1016/j.tplants.2016.08.002.
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104(52):20996–21001. doi:10.1073/pnas.0708586104.
- Passardi F, Penel C, Dunand C. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 2004;9(11):534–540. doi:10.1016/j.tplants.2004.09.002.
- Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 2007;174(2):332–341. doi:10.1111/j.1469-8137.2007.01995.x.
- Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J. 1998;332(2):507–515. doi:10.1042/bj3320507.
- Schopfer P. Hydroxyl radical‐induced cell‐wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 2001;28(6):679–688. doi:10.1046/j.1365-313x.2001.01187.x.
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438(7010):1013–1106. doi:10.1038/nature04198.
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422(6930):442–446. doi:10.1038/nature01485.
- Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. J Exp Bot. 2009;60(2):391–408. doi:10.1093/jxb/ern318.
- Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta. 2012;1823(2):398–405. doi:10.1016/j.bbamcr.2011.09.011.
- Marino D, Dunand C, Puppo A, Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012;17(1):9–15. doi:10.1016/j.tplants.2011.10.001.
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol. 2011;14(6):691–699. doi:10.1016/j.pbi.2011.07.014.
- Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2(3):235–243. doi:10.1105/tpc.2.3.235.
- Wymer CL, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997;12(2):427–439. doi:10.1046/j.1365-313X.1997.12020427.x.
- Oda T, Hashimoto H, Kuwabara N, Akashi S, Hayashi K, Kojima C, Wong HL, Kawasaki T, Shimamoto K, and Sato M, et al. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J Biol Chem. 2010;285(2):1435–1445. doi:10.1074/jbc.M109.058909.
- Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8(4):397–403. doi:10.1016/j.pbi.2005.05.014.
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 1998;14(3):365–370. doi:10.1046/j.1365-313X.1998.00136.x.
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283(14):8885–8892. doi:10.1074/jbc.M708106200.
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319(5867):1241–1244. doi:10.1126/science.1152505.
- Nestler J, Liu S, Wen T-J, Paschold A, Marcon C, Tang HM, Li D, Li L, Meeley RB, and Sakai H, et al. Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J. 2014;79(5):729–740. doi:10.1111/tpj.12578.
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot. 2006;57(8):1829–1834. doi:10.1093/jxb/erj201.
- Parker JS, Cavell AC, Dolan L, Roberts K, Grierson CS. Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell. 2000;12(10):1961–1974. doi:10.1105/tpc.12.10.1961.
- Xu J, Scheres B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell. 2005;17(2):525–536. doi:10.1105/tpc.104.028449.
- Francoz E, Ranocha P, Nguyen-Kim H, Jamet E, Burlat V, Dunand C. Roles of cell wall peroxidases in plant development. Phytochemistry. 2015;112:15–21. doi:10.1016/j.phytochem.2014.07.020.
- Plieth C, Vollbehr S. Calcium promotes activity and confers heat stability on plant peroxidases. Plant Signal Behav. 2012;7(6):650–660. doi:10.4161/psb.20065.
- Passardi F, Tognolli M, De Meyer M, Penel C, Dunand C. Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta. 2006;223(5):965–974. doi:10.1007/s00425-005-0153-4.
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta. 2002;214(6):821–828. doi:10.1007/s00425-001-0699-8.
- Kwon T, Sparks JA, Nakashima J, Allen SN, Tang Y, Blancaflor EB. Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am J Bot. 2015;102(1):21–35. doi:10.3732/ajb.1400458.
- Marzol E, Borassi C, Carignani Sardoy M, Ranocha P, Aptekmann AA, Bringas M, Pennington J, Paez-Valencia J, Peralta JM, Fleming M, et al. Class III peroxidases PRX01, PRX44, and PRX73 control root hair growth in Arabidopsis thaliana. Int J Mol Sci. 2022;23(10):5375. doi:10.3390/ijms23105375.
- Bibikova TN, Zhigilei A, and Gilroy S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997;203(4):495–505. doi:10.1007/s004250050219.
- Ketelaar T. The actin cytoskeleton in root hairs: all is fine at the tip. Curr Opin Plant Biol. 2013;16(6):749–756. doi:10.1016/j.pbi.2013.10.003.
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi:10.1105/tpc.002899.
- Carroll AD, Moyen C, Van Kesteren P, Tooke F, Battey NH, Brownlee C. Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell. 1998;10(8):1267–1276. doi:10.1105/tpc.10.8.1267.
- Cramer GR, Jones RL. Osmotic stress and abscisic acid reduce cytosolic calcium activities in roots of Arabidopsis thaliana. Plant Cell Environ. 1996;19(11):1291–1298. doi:10.1111/j.1365-3040.1996.tb00007.
- Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi:10.1016/j.cell.2007.11.028.
- Zhang S, Pan Y, Tian W, Dong M, Zhu H, Luan S, Li L. Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Mol Plant. 2017;10(7):1004–1006. doi:10.1016/j.molp.2017.02.007.
- Zeb Q, Wang X, Hou C, Zhang X, Dong M, Zhang S, Zhang Q, Ren ZJ, Tian W, Zhu HF, et al. The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J Integr Plant Biol. 2020;62(7):887–896. doi:10.1111/jipb.12890.
- Brost C, Studtrucker T, Reimann R, Denninger P, Czekalla J, Krebs M, Fabry B, Schumacher K, Grossmann G, Dietrich P. Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J. 2019;99(5):910–923. doi:10.1111/tpj.14371.
- Tan Y-Q, Yang Y, Zhang A, Fei C-F, L-L G, Sun S-J, Xu W, Wang LL, Liu HT, Wang YF. Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels. Plant Commun. 2020;1(1):100001. doi:10.1016/j.xplc.2019.100001.
- Maciver SK, Hussey PJ. The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 2002;3(5):reviews3007. doi:10.1186/gb-2002-3-5-reviews3007.
- Monshausen GB, Messerli MA, Gilroy S. Imaging of the yellow cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147(4):1690–1698. doi:10.1104/pp.108.123638.
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mitter R. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014;19(10):623–630. doi:10.1016/j.tplants.2014.06.013.
- Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J. The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant. 2013;6(2):559–569. doi:10.1093/mp/sst009.
- Jones MA, Raymond MJ, Yang Z, Smirnoff N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot. 2007;58(6):1261–1270. doi:10.1093/jxb/erl279.
- Miedema H, Demidchik V, Véry -A-A, Bothwell JHF, Brownlee C, Davies JM. Two voltage-dependent calcium channels co-exist in the apical plasma membrane of Arabidopsis thaliana root hairs. New Phytol. 2008;179(2):378–385. doi:10.1111/j.1469-8137.2008.02465.x.
- Cosgrove DJ. Characterization of long-term extension of isolated cell walls from growing cucumber hypocotyls. Planta. 1989;177(1):121–130. doi:10.1007/BF00392162.
- McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4(11):1425–1433. doi:10.1105/tpc.4.11.1425.
- Rayle DL, Cleland R. Enhancement of wall loosening and elongation by acid solutions. Plant Physiol. 1970;46(2):250–253. doi:10.1104/pp.46.2.250.
- Eklöf JM, Brumer H. The XTH gene family: an update on enzyme structure, function, and phylogeny in xyloglucan remodeling. Plant Physiol. 2010;153(2):456–466. doi:10.1104/pp.110.156844.
- Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99(4):1271–1274. doi:10.1104/pp.99.4.1271.
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407(6802):321–326. doi:10.1038/35030000.
- Altartouri B, Geitmann A. Understanding plant cell morphogenesis requires real-time monitoring of cell wall polymers. Curr Opin Plant Biol. 2014 11;2015(23):76–82. doi:10.1016/j.pbi.
- Hoffmann RD, Olsen LI, Ezike CV, Pedersen JT, Manstretta R, López-Marqués RL, and Palmgren M. Roles of plasma membrane proton ATPases AHA2 and AHA7 in normal growth of roots and root hairs in Arabidopsis thaliana. Physiol Plant. 2019; 166(3): 848–861. doi:10.1111/ppl.12842
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343(6169):408–411. doi:10.1126/science.1244454.
- Mangano S, Martínez Pacheco J, Marino-Buslje C, Estevez JM. How does pH fit in with oscillating polar growth? Trends Plant Sci. 2018;23(6):479–489. doi:10.1016/j.tplants.2018.02.008.
- Feijó JA. The pollen tube oscillator: towards a molecular mechanism of tip growth? Fertilization in higher plants. : Springer, Berlin; 1999. 317–336. doi:10.1007/978-3-642-59969-9_22.
