ABSTRACT
Arabidopsis DYNAMIN-RELATED PROTEIN1A (AtDRP1A) and AtDRP2B are large GTPases that function together in endocytosis for effective cytokinesis, cell enlargement and development. A recent study shows that these DRPs contribute to ligand-induced endocytosis of the immune receptor FLAGELLIN SENSING2 (AtFLS2) to modulate flg22-immune signaling, and they are required for immunity against Pseudomonas syringae pv. tomato bacteria. Here, we demonstrate that atdrp1a and atdrp2b single mutants showed increased susceptibility to Botrytis cinerea indicating that AtDRP1A and AtDRP2B are necessary for effective resistance against this necrotrophic fungus. Thus, we expanded our limited understanding of clathrin endocytic accessory proteins in immunity against plant pathogens.
KEYWORDS:
Main text
Endocytosis is the process by which eukaryotic cells take up extracellular components and plasma membrane (PM)-localized proteins to regulate cell surface-derived responses. During ligand-induced endocytosis, receptors are removed from the PM in response to specific ligands to desensitize cells to the stimulus and attenuate signaling.Citation1–3 Plant studies have focused on clathrin-mediated endocytosis (CME), during which evolutionarily conserved and plant-specific clathrin core, adaptor and accessory proteins are recruited to the PM in a coordinated spatiotemporal manner.Citation1,Citation3
In eukaryotes, dynamins and DYNAMIN-RELATED PROTEINs (DRPs) are high-molecular weight GTPases functioning in the mechanochemical fission of organelles or membranes. Arabidopsis thaliana (Arabidopsis) DRPs are divided into six subfamilies with DRP1 and DRP2 family members being required for CME. Based on their domain structure, the plant-specific AtDRP1 family contains five members (DRP1A-1E) whereas the evolutionary conserved bone fide dynamin family consists of the two AtDRP2 members (AtDRP2A-2B).Citation4,Citation5 AtDRP1A and AtDRP2B are the best studied DRPs contributing to constitutive CME of PM proteins and bulk PM for effective cytokinesis, cell enlargement and developmental processes.Citation3–5 Consistent with their colocalization and biochemical interaction,Citation5 we recently uncovered synergistic genetic interactions between AtDRP1A and AtDRP2B in plant growth and development as atdrp1a atdrp2b double mutants exhibit severely stunted roots and cotyledons, defective cell shape, cytokinesis and seedling lethality.Citation6 In the absence of any stimulus, these double mutants also hyperaccumulate the Arabidopsis immune receptor FLAGELLIN SENSING2 (AtFLS2) in the PM indicating combinatorial roles for AtDRP1A and AtDRP2B in governing PM abundance of AtFLS2.Citation6
Using single loss-of-function atdrp mutants, we have also shown that a) like AtDRP2B,Citation7 AtDRP1A is required for effective defense signaling in response to flg22, a Pseudomonas flagellin peptide that is perceived by AtFLS2 and induces host defense signaling; and b) AtDRP1A plays a more prominent role than AtDRP2B in flg22-induced endocytosis of FLS2.Citation6 Consistent with ligand-induced endocytosis of FLS2 attenuating flg22-signaling,Citation8 the delay in flg22-induced removal of FLS2 from the PM correlates with increased early flg22-signaling in atdrp1a and atdrp2b single mutants.Citation6,Citation7 AtDRP1A and AtDRP2B also contribute to effective immunity against bacterial pathogens because atdrp1a and atdrp2b single mutants display increased susceptibility to flagellated Pseudomonas syringae pv. tomato (Pto) DC3000 bacterial strains.Citation6,Citation7 The roles of DRP1A and DRP2B orthologues in defense signaling and host immunity extend to other pathogens; however, these DRPs have distinct contributions depending on the type of stimuli or pathogens.Citation9–12 So far, however, it remains unknown whether AtDRP1A and/or AtDRP2B contribute to immunity against fungal infection.
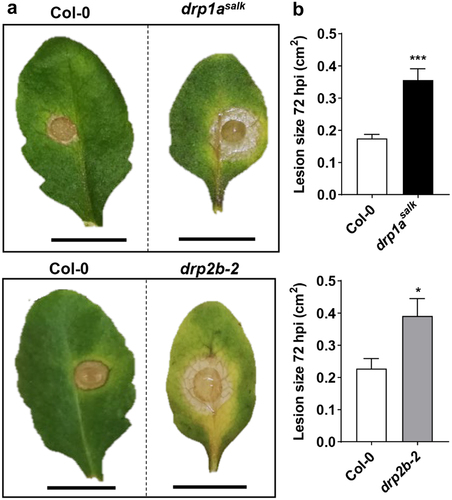
To test this, we infected atdrp1a and atdrp2b single mutants with Botrytis cinerea, a highly destructive necrotrophic fungal pathogen with a broad host range including agricultural crops.Citation13 We focused on altered susceptibility against this fungus in the single mutant plants because of severe seedling stunting and lethality of drp1a drp2b double mutants.Citation6 Arabidopsis Col-0 (wild-type), atdrp1asalk (SALK_069077)Citation6,Citation14 and atdrp2b-2 (SALK_134887)Citation7,Citation15 seeds were sown onto a 1:1 mixture of peat (Jiffy Products, Norway) and vermiculite. After stratification in the dark at 4°C for 3 days, plants were grown under 16 h light/8 h dark at 22°C and cool white fluorescent light of 80–100 µmol m−2 s−1. Botrytis cinerea pepper isolate was sub-cultured on apricot halves in the dark at 25°C two weeks prior to use of the spores. To determine the susceptibility of Col-0, atdrp1asalk and atdrp2b-2 plants to B. cinerea, a maximum of two detached leaves from individual four-week-old plants were inoculated with 10 μL of half-strength grape juice containing 5 × 104 spores mL−1 as previously described.Citation16 Special care was taken to select leaves at a similar developmental stage and age, in that the youngest fully expanded leaves from each genotype were chosen for the fungal infection assays. Half-strength grape juice served as the mock infection control. Lesions were photographed 72 h post-inoculation (hpi) (). The size of each lesion was determined using IMAGEJ (http://rsbweb.nih.gov/ij/). Lesion areas were transformed by square rooting to meet assumptions of normality for parametric tests prior to statistical analysis using Student’s t-test (). As shown in , B. cinerea-induced lesions were significantly larger for atdrp1asalkand atdrp2b-2 single mutant leaves compared to Col-0 indicating that atdrp1asalk and atdrp2b-2 were more susceptible to this necrotrophic fungus. We conclude that both AtDRP1A and AtDRP2B contribute positively to effective resistance against B. cinerea. Prior to our study, the only other Arabidopsis DRP1/2 member implicated in plant immunity against fungal infection is AtDRP1E, originally identified as enhanced disease resistance3 (edr3).Citation17 In contrast to loss-of-function mutations in atdrp1a and atdrp2b resulting in increased susceptibility (this study), edr3 mutant plants display increased resistance to Erysiphe cichoracearum and B. cinerea,Citation17 likely due to a potential gain-of-function point mutation in the GTPase domain of AtDRP1E because atdrp1e null mutant plants do not show altered resistance to these fungal pathogens.Citation17
Figure 1. Single atdrp1a and atdrp2b mutants are more susceptible to the necrotrophic fungus Botrytis cinerea. Detached leaves from four-week-old Col-0 (wildtype), atdrp1asalk and atdrp2b-2 single mutant plants were inoculated with B. cinerea spores for 72 h. (a) Representative leaves with lesions at 72 h post-infection (hpi). Stippled line indicates images taken from same experiment. Scale bar, 1 cm. (b) Lesion size (cm2) was measured at 72 hpi (n ≥ 15/ genotype), and values presented are means plus standard error of the mean (SEM). Using Student’s t-test, statistically significant differences were identified between Col-0 and atdrp1asalk (P= .0001) or drp2b-2 (P = .0134). All experiments were performed five times with similar results.
In conclusion, we have expanded our limited understanding of the CME accessory proteins AtDRP1A and AtDRP2B in plant immunity, providing evidence that AtDRP1A and AtDRP2B contributed to effective resistance against the necrotrophic fungus B. cinerea. The increased susceptibility in atdrp1a and atdrp2b single mutants may be caused by incorrect cell surface accumulation of cellular components that play critical roles during early stages of fungal infection. In future studies, it will be interesting to determine whether these Arabidopsis DRPs regulate the abundance of PM proteins involved in fungal perception and/or signaling.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Reynolds GD, Wang C, Pan J, Bednarek SY. Inroads into internalization: five years of endocytic exploration. Plant Physiol. 2018;176(1):208. doi:10.1104/pp.17.01117.
- Claus LAN, Savatin DV, Russinova E. The crossroads of receptor-mediated signaling and endocytosis in plants. J Integr Plant Biol. 2018;60(9):827–3. doi:10.1111/jipb.12672.
- Ekanayake G, LaMontagne ED, Heese A. Never walk alone: Clathrin-Coated Vesicle (CCV) components in plant immunity. Ann Rev Phytopath. 2019;57(1):387–409. doi:10.1146/annurev-phyto-080417-045841.
- Bednarek SY, Backues SK. Plant dynamin-related protein families DRP1 and DRP2 in plant development. Biochem Soc Trans. 2010;38(3):797–806. doi:10.1042/BST0380797.
- Fujimoto M, Tsutsumi N. Dynamin-related proteins in plant post-Golgi traffic. Front Plant Sci. 2014;5:408. doi:10.3389/fpls.2014.00408.
- Ekanayake G, Smith JM, Jones KB, Stiers HM, Robinson SJ, LaMontagne ED, Kostos PH, Cornish PV, Bednarek SY, Heese A. Dynamin-related protein DRP1A functions with DRP2B in plant growth, flg22-immune responses, and endocytosis. Plant Physiol. 2021;185(4):1986–2002. doi:10.1093/plphys/kiab024.
- Smith JM, Leslie ME, Robinson SJ, Korasick DA, Zhang T, Backues SK, Cornish PV, Koo AJ, Bednarek SY, Heese A. Loss of arabidopsis thaliana dynamin-related protein 2B reveals separation of innate immune signaling pathways. PLoS Pathog. 2014;10(12):e1004578. doi:10.1371/journal.ppat.1004578.
- Smith JM, Salamango DJ, Leslie ME, Collins CA, Heese A. Sensitivity to Flg22 is modulated by ligand-induced degradation and de novo synthesis of the endogenous flagellin-receptor FLAGELLIN-SENSING2. Plant Physiol. 2014;164(1):440–454. doi:10.1104/pp.113.229179.
- Chaparro-Garcia A, Schwizer S, Sklenar J, Yoshida K, Petre B, Bos JI, Schornack S, Jones AM, Bozkurt TO, Kamoun S. Phytophthora infestans RXLR-WY effector AVR3a associates with dynamin-related protein 2 required for endocytosis of the plant pattern recognition receptor FLS2. PLoS One. 2015;10(9):e0137071. doi:10.1371/journal.pone.0137071.
- Wu G, Cui X, Chen H, Renaud JB, Yu K, Chen X, Wang A, Simon AE. Dynamin-like proteins of endocytosis in plants are coopted by potyviruses to enhance virus infection. J Virol. 2018;92(23):e01320–18. doi:10.1128/jvi.01320-18.
- Pizarro L, Leibman-Markus M, Schuster S, Bar M, Avni A. Tomato dynamin related protein 2A associates with LeEIX2 and enhances PRR mediated defense by modulating receptor trafficking. Front Plant Sci. 2019;10:936. doi:10.3389/fpls.2019.00936.
- Leibman-Markus M, Schuster S, Vasquez-Soto B, Bar M, Avni A, Pizarro L. Dynamin-related proteins enhance tomato immunity by mediating pattern recognition receptor trafficking. Membranes. 2022;12(8):760. doi:10.3390/membranes12080760.
- Cheung N, Tian L, Liu X, Xin L. The destructive fungal pathogen botrytis cinerea-insights from genes studied with mutant analysis. Pathogens. 2020;9(11):923. doi:10.3390/pathogens9110923.
- Boutté Y, Frescatada-Rosa M, Men S, Chow C-M, Ebine K, Gustavsson A, Johansson L, Ueda T, Moore I, Jürgens G, et al. Endocytosis restricts arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 2010;29(3):546–558. doi:10.1038/emboj.2009.363.
- Backues SK, Korasick DA, Heese A, Bednarek SY. The arabidopsis dynamin-related protein2 family is essential for gametophyte development. Plant Cell. 2010;22(10):3218–3231. doi:10.1105/tpc.110.077727.
- Ingle RA, Roden LC. Circadian regulation of plant immunity to pathogens. Methods Mol Biol. 2014;1158:273–283. doi:10.1007/978-1-4939-0700-7_18.
- Tang D, Ade J, Frye CA, Innes RW. A mutation in the GTP hydrolysis site of Arabidopsis dynamin related protein 1E confers enhanced cell death in response to powdery mildew infection. Plant J. 2006;47(1):75–84. doi:10.1111/j.1365-313X.2006.02769.x.
