ABSTRACT
In nature, co-evolution shaped balanced entities of host plants and their associated microorganism. Plants maintain this balance by detecting their associated microorganism and coordinating responses to them. Quorum sensing (QS) is a widespread bacterial cell-to-cell communication mechanism to modulate the collective behavior of bacteria. As a well-characterized QS signal, N-acyl homoserine lactones (AHL) also influence plant fitness. Plants need to coordinate their responses to diverse AHL molecules since they might host bacteria producing various AHL. This opinion paper discusses plants response to a mixture of multiple AHL molecules. The function of various phytohormones and WRKY transcription factors seems to be characteristic for plants’ response to multiple AHL. Additionally, the perspectives and possible approaches to facilitate further research and the application of AHL-producing bacteria are discussed.
N-acyl homoserine lactones-mediated interactions between plants and associated microorganisms
The host plants and their associated microorganisms together shape balanced holobiont.Citation1 Beneficial microorganisms help maintain the balance of the holobiont through complex interactions. Additionally, the plant immune system contributes to the stability of the holobiont by monitoring microorganism-associated signals, thereby modulating the reaction to associated microorganisms. The coordinated response to those various signals ultimately drives plant performance.
Quorum sensing (QS) signals may function as mediators in interactions between plants and bacteria.Citation2 N-acyl homoserine lactones (AHLs) are well-characterized QS molecules that influence plant fitness as evidenced by the application of AHL-producing bacteria or AHL molecules.Citation3,Citation4 In nature, plants might host various AHL-producing bacteria. Moreover, enhanced resistance was the predominant response when plants encountered a mixture of AHL molecules (thereafter termed AHL mix).Citation4,Citation5 Plants are able to acquire a conditioned state of increased defense capacity against invading pathogens and pests upon prior inoculation with specific stimuli. This phenomenon is known as induced resistance (IR).Citation6 AHL may also evoke IR (AHL-IR), a phenomenon known as AHL priming. Since the latter term was used in previous studies,Citation2,Citation7–10 we proposed to use the term AHL priming, for consistency issues. We assume that a better understanding of AHL priming will support further application of beneficial AHL-producing bacteria.
Jasmonate metabolism participates in priming with AHL mix
Benefits conferred by different AHL molecules depend on diverse phytohormones () such as abscisic acid, jasmonic acid (JA), salicylic acid (SA), and auxin.Citation3,Citation5,Citation11,Citation12 For instance, SA-signaling is required for enhanced resistance to Pseudomonas syringae pv. tomato (Pst) in N-3-oxo-octanoyl homoserine lactone (oxo-C8-HSL)-primed Arabidopsis thaliana (Arabidopsis). Whereas JA and auxin-signaling are required for enhanced resistance to Pectobacterium carotovorum ssp. carotovorum (Pcc) in oxo-C8-HSL-primed Chinese cabbage and Arabidopsis.Citation11,Citation12 Although AHL priming, induced by single AHL molecules, depends on different phytohormone signaling pathways, a novel mechanism was proposed for the response of host plants to AHL mix. Measurement of phytohormones and related molecules, performed when plants were treated with four single AHL molecules or a mixture of those AHL molecules,Citation7 revealed the distinct characteristics between single AHL molecule priming and priming with AHL mix priming.
Figure 1. Phytohormone signaling differs between single molecule AHL priming and AHL mix priming. Induced resistance (IR) in primed plants conferred by N-acyl homoserine lactones (AHL) depends on various phytohormones. In single AHL-primed plants, accumulated jasmonates (JA), salicylic acid (SA), auxin, and OPDA were observed when primed plants encounter secondary stressors (A). Whereas, the impact of a mixture of four AHL molecules (AHL mix) on plants appeared to depend on a novel mechanism. Unlike the single AHL-primed plants, AHL mix primed-plants inhibited the catabolism of JA (B).
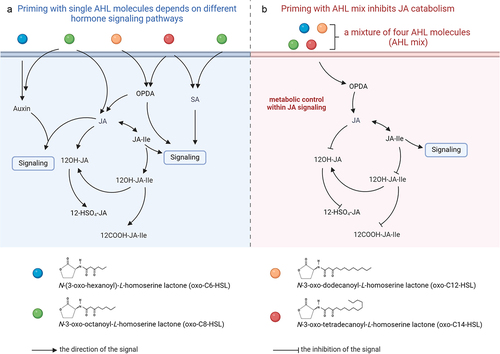
It is important to note that the degradation of jasmonates decreased in plants primed by AHL mix. For instance, the content of the jasmonate-catabolic derivative dicarboxy-JA-Ile (COOH-JA-Ile) decreased in plants primed by AHL mix, when those plants were challenged with flg22. This phenomenon was not observed in plants treated with any of the four single AHL molecules, individually.Citation5 Whereas the content of jasmonic acid and its precursor cis-OPDA were higher in the oxo-C8-HSL, N-3-oxo-dodecanoyl-L-homoserine lactone (oxo-C12-HSL), or N-3-oxo-tetradecanoyl-L-homoserine lactone (oxo-C14-HSL) primed plants before and after exposure to secondary stressors.Citation3,Citation5,Citation12 Genetic analyses further revealed impaired AHL mix priming in mutants deficient in jasmonate homeostasis. Induced resistance against Pst was lost in the lox2 mutant, Lipoxygenase 2 (LOX2) performs the oxidation of unsaturated fatty acids to the JA precursor (oxylipins), as well as in the jar1 mutant, Jasmonoyl-Isoleucine Synthetase (JAR1) conjugates JA to isoleucine. Additionally, jasmonate metabolism-related genes such as Cytochrome P450 94B1 (CYP94B1), Sulfotransferase 2A (ST2A), Jasmonate-Induced Oxygenase 2, 3 and 4 (JAO2, JAO3, and JAO4) were downregulated when AHL mix primed-plants encountered flg22.Citation5 Hence, we postulate that JA-signaling, especially its metabolic control, participates in AHL mix priming, rather than the accumulation of the active jasmonoyl-isoleucine (JA-Ile) ().
The plasticity of the jasmonate metabolic pathway might offer an explanation of complex functions of phytohormones in AHL priming. Long-chain AHL molecules such as oxo-C12-HSL and oxo-C14-HSL triggered plant immune resistance via cis-OPDA and SA,Citation3 whereas short-chain AHL molecules such as N-(3-oxo-hexanoyl)-L-homoserine lactone (oxo-C6-HSL) and oxo-C8-HSL induced the accumulation of jasmonates and auxins.Citation12 The crosstalk among multiple phytohormones needs to be considered when the host plant encounters four different AHL molecules including oxo-C6-HSL, oxo-C8-HSL, oxo-C12-HSL, and oxo-C14-HSL, at the same time. Our previous study revealed two situations: I) Before challenge, the content of SA, JA, and other phytohormone-related molecules is lower or not changed in AHL mix primed-Arabidopsis. II) Post challenge, the content of JA catabolites significantly decreased in AHL mix primed-Arabidopsis.Citation5 The fact that accumulation of JA and SA was missed in AHL mix-primed plants might minimize their antagonistic effects.
However, how does the flux of jasmonate metabolism contribute to the immune response in the AHL mix primed-plants? Generally, the accumulation of JA and SA was observed in single AHL molecule priming.Citation3,Citation11,Citation12 Notably, the content of JA catabolites affected the defense output. For instance, the deficiency of JA oxidation in the jao2 mutant, compromised in Jasmonic Acid Oxidase 2, led to a constitutive enhanced resistance to biotic stressors.Citation13 However, an elevated level of the active JA-Ile was not observed in naive jao2 mutant. Similarly, the content of JA catabolites only decreased upon flg22 challenge in AHL mix primed plants.Citation5 Thus, we proposed that similar molecular mechanism may persist in AHL mix priming. AHL mix primed plants could modulate their immune response via the control of jasmonate metabolism rather than via simply the accumulation of phytohormones.
WRKY transcription factors regulate defense response to AHL mix
WRKY transcription factors (TFs) are known to be parts of signaling cascades, involved in various responses including AHL priming. The expression of WRKY22 and WRKY29 was differently regulated in single AHL molecule- or AHL mix- primed Arabidopsis upon flg22 challenge.Citation3,Citation4 Similarly, expression of WRKY18, WRKY22, WRKY29, WRKY40, WRKY48, and WRKY70 was quickly regulated in response to AHL mix.Citation5,Citation14 We proposed thus, that the function of WRKY is highly correlated with AHL priming.
How do the AHL mix-primed plants trigger their defense response when they encounter various stressors? The chromatin modification in the promoter regions of defense-related genes could be a possible explanation for the fast activation and subsequent stronger defense response in AHL-primed plants (). During the flg22-triggered transcriptome reprogramming, multiple WRKY TFs such as WRKY18, WRKY33, and WRKY40 preferentially bind to the cis-regulatory elements (termed enhancers).Citation15 The binding makes it possible for WRKY and enhancers to modulate the expression of defense-related genes together. We further proposed that chromatin modification, such as the binding of enhancers and WRKY TFs, might be correlated to AHL priming. WRKY TFs such as WRKY7 and WRKY11 interact with OBERON histone-binding proteins, forming complexes modulating long-term stress response. These protein complexes bind to the promoter region of stress-defense-related genes.Citation16 The loss of functions of OBERON protein or WRKY11 led to increased expression of stress-defense-related genes such as WRKY18, WRKY48, and WRKY53.Citation16–18 Since those WRKY TFs are regulated upon AHL priming, they might be, probably with other associated regulatory elements, part of the complex interaction network in AHL-primed plants ().
Figure 2. Altered availability of transcription factors affects the expression of defense-related genes. During plant response to various AHL molecules, chromatin remodeling and histone modification might be influenced by AHL molecules directly or by AHL-related factors including proteins, calcium ions, lipids, and others. Open chromatin facilitates gene expression, whereas the availability and interactive regulation of transcription factors (TFs) may limit the expression of the defense-related gene in AHL-primed plants (A). Pathogens or microorganism-associated molecular patterns could induce the gene expression of WRKY TFs. More abundant TFs would shift the balance of the gene regulation network (B). The increased TF availability would thus strongly promote the expression of defense-related genes in AHL-primed plants.
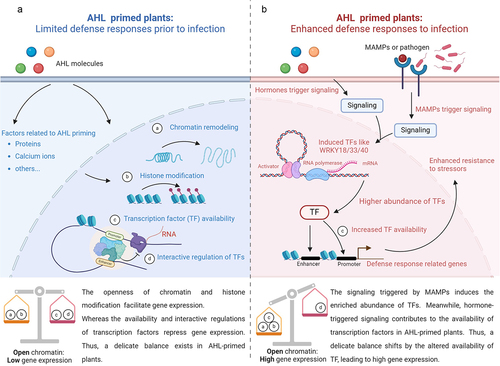
Similar indications were discovered in plants primed by other stimuli such as the salicylic acid analog acibenzolar S-methyl (BTH) or β-aminobutyric acid (BABA).Citation6,Citation19 The number of histone modifications increased on promoters of defense-related WRKY6, WRKY29, and WRKY53 in BTH-primed plants. Although the gene expression was not active, the memory stored in the form of histone modification conferred faster and stronger gene responses when BTH-primed plants were exposed to subsequent stressors (e.g. flg22). The epigenetic regulatory systems and WRKY TFs were involved in plant response to various stressors. For instance, the binding of WRKY33 to its target YODA DOWNSTREAM (YDD) gene promoters promoted the epigenetic modification of YDD genes. Upon a fungal infection, the function of WRKY33 and epigenetic modification mutually potentiated the expression of YDD in Arabidopsis.Citation20 Thus, we supposed that similar patterns may exist in AHL-primed plants. The chromatin modification might help to enhance the expression of defense-related WRKY genes in AHL-primed plants exposed to a secondary challenge. The elevated level of WRKY may disturb the original balance in primed plants, eventually leading to increased expression of defense-related genes ().
Other potential signals involved in AHL priming
Calcium ions (Ca2+) and G-protein related proteins seemed to be involved in AHL priming. Expression of two candidate G-protein Coupled Receptors (GPCRs) genes, Cand2 and Cand7, was elevated in Arabidopsis 24 h after exposition to oxo-C6-HSL or oxo-C8-HSL.Citation21 Another two GPCRs, GCR1 and GPA1, and the acidic Ca2+-binding protein calmodulin (CaM) were reported to be involved in oxo-C6-HSL- or oxo-C8-HSL-induced root elongation in Arabidopsis.Citation22 AHL molecules such as C4-HSL or C10-HSL could induce a transient increased concentration of the free cytosolic Ca2+. In addition, the activation of plasma membrane Ca2+ channels might be involved in AHL priming.Citation23,Citation24 Furthermore, the effects of C10-HSL on increased reactive oxygen species (ROS) and nitric oxide (NO) production could be significantly alleviated by inhibiting the activity of Ca2+ channels.Citation23 Overall, these studies indicated that the initial response to AHL partially depends on the regulation of Ca2+-signaling.
Conclusions and future perspective
AHL priming is an important component in plant-bacteria interactions. This opinion discussed the master role of WRKY TFs in plant response to various AHL molecules (). The chromatin modifications may determine the openness of gene promoter regions and the accessibility of transcription factors, which would further influence the regulation of expression of defense-related genes. The function of WRKY TFs might be an essential factor in AHL priming. However, the complex interaction among WRKY TFs requires further investigations. To address the functions of WRKY TFs in AHL priming, epigenetic studies on chromatin modifications would be a valuable addition.
Notably, the possible application of AHL-producing bacteria and AHL priming has drawn attention in sustainable agriculture.Citation25 For instance, the AHL-producing bacterium Ensifer meliloti revealed good ability in promoting plant growth and enhancing plant resistance against various pathogens.Citation26–29 Additionally, the application of AHL-producing bacteria may improve the quality of crop production. The bioactive secondary metabolites such as flavonoids in plants have wide potential applications including anti-cancer, anti-inflammatory, and antibacterial.Citation30 The expression of flavonoids biosynthesis genes was modulated in barley when plants were inoculated with the AHL-producing bacterium Acidovorax radicis N35.Citation31 In addition, iron homeostasis-related genes were regulated in barley when plants were inoculated with different AHL-producing bacteria such as E. meliloti and Pantoea sp.Citation5 However, some issues would require further clarification before the application of AHL-producing bacteria in sustainable agriculture. For instance, the AHL priming phenomenon varied in different cultivars and species.Citation25,Citation27 Nonetheless, we proposed that the application of AHL-producing bacteria could broaden the means of biocontrol and biofertilizer agents in sustainable agriculture.
Authors’ contributions
YD and AS conceived the idea. YD and MH analyzed and organized the references. YD wrote the manuscript. MH and AS contributed to writing, reviewing, and editing the manuscript. All authors agreed with the publication of this manuscript.
Acknowledgments
Figures were created with BioRender.com, license numbers DP26UEQHBY and OB26UEQHFP.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mesny F, Hacquard S, Thomma BP. Co-evolution within the plant holobiont drives host performance. EMBO Rep. 2023;24(9):e57455. doi:10.15252/embr.202357455.
- Schikora A, Schenk ST, Hartmann A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol Biol. 2016;90(6):605–5. doi:10.1007/s11103-016-0457-8.
- Schenk ST, Hernandez-Reyes C, Samans B, Stein E, Neumann C, Schikora M, Reichelt M, Mithofer A, Becker A, Kogel KH. et al. N-Acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell. 2014;26(6):2708–2723. doi:10.1105/tpc.114.126763.
- Shrestha A, Grimm M, Ojiro I, Krumwiede J, Schikora A. Impact of quorum sensing molecules on plant growth and immune system. Front Microbiol. 2020;11:1545. doi:10.3389/fmicb.2020.01545.
- Duan Y, Han M, Grimm M, Ponath J, Reichelt M, Mithofer A, Schikora A. Combination of bacterial N-acyl homoserine lactones primes Arabidopsis defenses via jasmonate metabolism. Plant Physiol. 2023;191(3):2027–2044. doi:10.1093/plphys/kiad017.
- Cooper A, Ton J, Kanyuka K, Hammond-Kosack K. Immune priming in plants: from the onset to transgenerational maintenance. Essays Biochem. 2022;66(5):635–646. doi:10.1042/EBC20210082.
- Duan Y, Han M, Grimm M, Schierstaedt J, Imani J, Cardinale M, Le Jean M, Nesme J, Sorensen SJ, Schikora A. Hordeum vulgare differentiates its response to beneficial bacteria. BMC Plant Biol. 2023;23(1):460. doi:10.1186/s12870-023-04484-5.
- Hartmann A, Schikora A. Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. J Chem Ecol. 2012;38(6):704–713. doi:10.1007/s10886-012-0141-7.
- Schikora A, Schenk ST, Stein E, Molitor A, Zuccaro A, Kogel KH. N-acyl-homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011;157(3):1407–1418. doi:10.1104/pp.111.180604.
- Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmid M, Van Breusegem F, Eberl L. et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant, Cell & Environ. 2006;29(5):909–918. doi:10.1111/j.1365-3040.2005.01471.x.
- Liu F, Zhao Q, Jia Z, Song C, Huang Y, Ma H, Song S. N-3-oxo-octanoyl-homoserine lactone-mediated priming of resistance to Pseudomonas syringae requires the salicylic acid signaling pathway in Arabidopsis thaliana. BMC Plant Biol. 2020;20(1):38. doi:10.1186/s12870-019-2228-6.
- Liu F, Zhao Q, Jia Z, Zhang S, Wang J, Song S, Jia Y. N-3-Oxo-octanoyl homoserine lactone primes plant resistance against necrotrophic pathogen pectobacterium carotovorum by coordinating jasmonic acid and auxin-signaling pathways. Front Plant Sci. 2022;13:886268. doi:10.3389/fpls.2022.886268.
- Caarls L, Elberse J, Awwanah M, Ludwig NR, de Vries M, Zeilmaker T, Van Wees SCM, Schuurink RC, Van den Ackerveken G. Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc Natl Acad Sci USA. 2017;114(24):6388–6393. doi:10.1073/pnas.1701101114.
- Duan Y, Han M, Grimm M, Schikora A. Network analysis uncovers the master role of WRKY transcription factors in Arabidopsis thaliana response to N-acyl homoserine lactones. CABI Agric Biosci. 2024;5(1):6. doi:10.1186/s43170-023-00206-x.
- Zhang Y, Tang M, Huang M, Xie J, Cheng J, Fu Y, Jiang D, Yu X, Li B. Dynamic enhancer transcription associates with reprogramming of immune genes during pattern triggered immunity in Arabidopsis. BMC Biol. 2022;20(1):165. doi:10.1186/s12915-022-01362-8.
- Du P, Wang Q, Yuan DY, Chen SS, Su YN, Li L, Chen S, He XJ. WRKY transcription factors and OBERON histone-binding proteins form complexes to balance plant growth and stress tolerance. Embo J. 2023;42(19):e113639. doi:10.15252/embj.2023113639.
- Birkenbihl RP, Kracher B, Roccaro M, Somssich IE. Induced genome-wide binding of three Arabidopsis WRKY transcription factors during early MAMP-Triggered immunity. Plant Cell. 2017;29(1):20–38. doi:10.1105/tpc.16.00681.
- Birkenbihl RP, Kracher B, Ross A, Kramer K, Finkemeier I, Somssich IE. Principles and characteristics of the Arabidopsis WRKY regulatory network during early MAMP-triggered immunity. Plant Journal. 2018;96(3):487–502. doi:10.1111/tpj.14043.
- Jaskiewicz M, Conrath U, Peterhansel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12(1):50–55. doi:10.1038/embor.2010.186.
- Cai H, Huang Y, Chen F, Liu L, Chai M, Zhang M, Yan M, Aslam M, He Q, Qin Y. ERECTA signaling regulates plant immune responses via chromatin-mediated promotion of WRKY33 binding to target genes. New Phytol. 2021;230(2):737–756. doi:10.1111/nph.17200.
- Jin GP, Liu F, Ma H, Hao SY, Zhao Q, Bian ZR, Jia ZH, Song SS. Two G-protein-coupled-receptor candidates, Cand2 and Cand7, are involved in Arabidopsis root growth mediated by the bacterial quorum-sensing signals N-acyl-homoserine lactones. Biochem Bioph Res Co. 2012;417(3):991–995. doi:10.1016/j.bbrc.2011.12.066.
- Liu F, Bian Z, Jia Z, Zhao Q, Song S. The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-Acyl-homoserine lactones, the bacterial quorum-sensing signals. MPMI. 2012;25(5):677–683. doi:10.1094/MPMI-10-11-0274.
- Cao XY, Zhao Q, Sun YN, Yu MX, Liu F, Zhang Z, Jia ZH, Song SS. Cellular messengers involved in the inhibition of the Arabidopsis primary root growth by bacterial quorum-sensing signal N-decanoyl-L-homoserine lactone. BMC Plant Biol. 2022;22(1):488. doi:10.1186/s12870-022-03865-6.
- Song S, Jia Z, Xu J, Zhang Z, Bian Z. N-butyryl-homoserine lactone, a bacterial quorum-sensing signaling molecule, induces intracellular calcium elevation in Arabidopsis root cells. Biochem Biophys Res Commun. 2011;414(2):355–360. doi:10.1016/j.bbrc.2011.09.076.
- Shrestha A, Schikora A. AHL-priming for enhanced resistance as a tool in sustainable agriculture. FEMS Microbiol Ecol. 2020;96(12). doi:10.1093/femsec/fiaa226.
- Hernandez-Reyes C, Schenk ST, Neumann C, Kogel KH, Schikora A. N-acyl-homoserine lactones-producing bacteria protect plants against plant and human pathogens. Microb Biotechnol. 2014;7(6):580–588. doi:10.1111/1751-7915.12177.
- Shrestha A, Elhady A, Adss S, Wehner G, Böttcher C, Heuer H, Ordon F, Schikora A. Genetic differences in Barley Govern the responsiveness to N-Acyl homoserine lactone. Phytobiomes J. 2019;3(3):191–202. doi:10.1094/PBIOMES-03-19-0015-R.
- Veliz-Vallejos DF, van Noorden GE, Yuan MQ, Mathesius U. A Sinorhizobium meliloti-specific N-acyl homoserine lactone quorum-sensing signal increases nodule numbers in Medicago truncatula independent of autoregulation. Front Plant Sci. 2014;5:551. doi:10.3389/fpls.2014.00551.
- Zarkani AA, Stein E, Rohrich CR, Schikora M, Evguenieva-Hackenberg E, Degenkolb T, Vilcinskas A, Klug G, Kogel KH, Schikora A. Homoserine lactones influence the reaction of plants to rhizobia. Int J Mol Sci. 2013;14(8):17122–17146. doi:10.3390/ijms140817122.
- Tariq H, Asif S, Andleeb A, Hano C, Abbasi BH. Flavonoid production: current trends in plant metabolic engineering and de novo microbial production. Metabolites. 2023;13(1):124. doi:10.3390/metabo13010124.
- Han S, Li D, Trost E, Mayer KF, Vlot AC, Heller W, Schmid M, Hartmann A, Rothballer M. Systemic responses of Barley to the 3-hydroxy-decanoyl-homoserine lactone producing plant beneficial endophyte acidovorax radicis N35. Front Plant Sci. 2016;7:1868. doi:10.3389/fpls.2016.01868.
