ABSTRACT
Rapeseed (Brassica napus L.) is an important oilseed crop widely cultivated worldwide, and drought is the main environmental factor limiting its yield enhancement and the expansion of planted areas. SIMILAR TO RCD ONE (SRO) is a plant-specific small gene family that plays a crucial role in plant growth, development, and responses to abiotic stresses such as drought. However, the functional role of SROs in rapeseed remains poorly understood. In this study, 19 BnaSROs were identified from the rapeseed genome, with 9, 10, 10, 18, and 20 members identified from the genomes of Brassica rapa, Brassica nigra, Brassica oleracea, Brassica juncea, and Brassica carinata, respectively. We then analyzed their sequence characteristics, phylogenetic relationships, gene structures, and conserved domains, and explored the collinearity relationships of the SRO members in Brassica napus and Brassica juncea. Next, we focused on the analysis of tissue expression and stress-responsive expression patterns of rapeseed SRO members and examined their expression profiles under ABA, MeJA and water-deficit drought treatments using qPCR. Transcriptome data analysis and qPCR detection indicated that BnaSROs exhibit multiple stress-responsive expression patterns. BnaSRO1 and BnaSRO11, which are likely to function through interactions with NAC transcription factors, were screened as major drought-regulated members. Our results provide a solid foundation for functional analysis of the role of the SRO gene family in abiotic stress responses, especially drought stress responses, in rapeseed.
Introduction
Rapeseed (Brassica napus L.) is an important oilseed crop that is grown worldwide for its edible oil and protein-rich meal.Citation1 As the main source of edible vegetable oil in China, rapeseed is grown mainly in the Yangtze River valley.Citation2 However, due to the uneven distribution of rainfall in the region, less rainfall in the fall and winter, seasonal droughts are severe, resulting in rapeseed seedlings often suffering from drought stress, which in turn affects their later growth and development, leading to lower yields.Citation3 Drought stress has become a major factor limiting the yield improvement of rapeseed and the expansion of its cultivation area.Citation3,Citation4 Therefore, it is particularly important to identify drought response genes and analyze their molecular mechanisms to cultivate drought-resistant rapeseed cultivars.
The SIMILAR TO RCD ONE (SRO) gene family is a group of plant-specific genes that have been shown to play important roles in plant responses to various environmental stresses, including drought stress.Citation5–7 Arabidopsis RADICAL-INDUCED CELL DEATH1 (RCD1) was the first SRO member to be identified.Citation8 This family was subsequently identified based on the conserved domains of Poly ADP-ribose polymerase (PARP, PF00644) and RCD-SRO-TAF4 (RST, PF12174) at the C-terminus. In addition, some SRO proteins also had a WWE domain (PF02825) at the N-terminus.Citation5 Among these three structural domains, PARP is mainly responsible for various biological functions, while RST and WWE are mainly involved in protein interactions.Citation8,Citation9 In Arabidopsis, there are six members (AtRCD1 and AtSRO1-5) of this family.Citation9 Among them, AtRCD1 and AtSRO1 share the same structural domains and exhibit partially redundant functions.Citation8,Citation10 Both are involved in the oxidative stress response in plants and respond to a variety of stress-related hormones.Citation8 Double mutations strongly affect the growth and development of plants.Citation10,Citation11 Functional mutations in AtRCD1 increase plant sensitivity to salt and osmotic stress.Citation10 In addition, AtRCD1 elevates oxidative stress and drought tolerance in plants by interacting with SOS1 and DREB2A, respectively, in Arabidopsis.Citation12,Citation13
As an increasing number of plant genomes have been sequenced, the SRO gene family has been consecutively identified in different plants, such as rice,Citation6 maize,Citation14 wheat,Citation15 tomato,Citation16 cotton,Citation17 potato,Citation18 sesame,Citation19 banana,Citation20 tea plantCitation21 and poplar,Citation22 and the understanding of their functions is expanding. In rice, the five SRO genes can respond to a multitude of abiotic stresses,Citation23 in which OsSRO1c is directly regulated by the transcription factor gene SNAC1 to enhance rice drought and oxidative stress tolerance.Citation6 In wheat, 30 SRO members showed different response patterns to biological and abiotic stresses,Citation15 and the TaSRO1 gene was able to enhance seedling growth and abiotic stress resistance by regulating redox homeostasis and maintaining genomic integrity.Citation24 In maize, all six SRO1 genes are inducible by a variety of abiotic stressors,Citation14 and overexpression of ZmSRO1e in maize and Arabidopsis increases the sensitivity of transgenic lines to abiotic stress. Additionally, ZmSRO1e responds to abiotic stress by interfering with the formation of the MBW complex and inhibiting anthocyanin synthesis.Citation25 In banana, the MaSRO4 gene regulates downstream signaling pathways by interacting with MaNAC6 and MaMYB4 in a positive response to biotic and abiotic stressors.Citation20 However, currently, only one SRO gene has been reported in rapeseed, and its study is limited to sequence analysis.Citation26 The number of members of the SRO gene family in rapeseed and their response to adverse stressors, especially drought stress, are unknown.
In this study, we first performed genome-wide identification of the SRO gene family in rapeseed and five other genotypes of Brassica crops (Brassica rapa, Brassica nigra, Brassica oleracea, Brassica juncea, and Brassica carinata). We then analyzed their sequence characteristics, phylogenetic relationships, gene structures, and conserved domains, and explored the collinearity relationships of the SRO members in Brassica napus and Brassica juncea. Next, we focused on the analysis of tissue expression and stress-responsive expression patterns of rapeseed SRO members and examined their expression profiles under ABA, MeJA and water-deficit drought treatments using qPCR. Finally, we screened two potential drought-responsive members and constructed their initial transcriptional regulatory networks. Our results lay the foundation for analyzing the function and stress tolerance regulation of SROs and provide theoretical references for the mining of drought-tolerance genes and drought-tolerance breeding in rapeseed.
Materials and methods
Identification of SRO genes in six Brassica species
The genome sequences of Brassica rapa ssp.pekinensis (Chiifu.v4), Brassica nigra (Ni100 LR v2.0), Brassica oleracea var.italica (HDEM.v0) and Brassica napus ssp.oleifera (ZS11.v0) were downloaded from the BnIR databaseCitation27 (https://yanglab.hzau.edu.cn/BnIR). The genome sequences of Brassica juncea (Braju_AU213_V1.0) and Brassica carinata (zd-1) were downloaded from the BRAD databaseCitation28 (http://www.brassicadb.cn/#/) and BIO2DB database (http://brassicadb.bio2db.com/),Citation29 respectively. The amino acid sequences of the SRO genes of Arabidopsis thaliana (AtRCD1, AtSRO1–5) were acquired from the TAIR database (https://www.arabidopsis.org/) and used as queries for BLASTp searches against the local annotated protein database of the above six Brassica genomes with an E-value cutoff <1e−5. The candidate protein sequences were first filtered out if they were less than 100 amino acids (AAs) in length and then further identified using Pfam (https://www.ebi.ac.uk/Tools/pfa/pfamscan/), CDD (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) and SMART (https://smart.embl.de/). The members containing at least one PARP domain (PF00644) or RST domain (PF12174) were reserved for subsequent analysis.
The GFF annotation files of the identified SRO genes were used to visualize their genomic distribution by TBtools software.Citation30 Then, they were renamed separately according to their distribution order on different genomic chromosomes. The isoelectric points (pIs), molecular weights (MWs) and grand average hydropathicity (GRAVY) values of the SRO proteins were predicted using an online tool on the ExPASy server (https://web.expasy.org/protparam/). The subcellular localization of each protein was predicted using WoLF PSORT (https://wolfpsort.hgc.jp/).Citation31
Phylogenetic, gene structure, and conserved domain analyses
The complete amino acid sequences of the different SRO genes were used for phylogenetic analysis. Sequence alignment was first performed using ClustalX software, and then phylogenetic tree construction based on the neighbor-joining (NJ) method with 1000 bootstrap replicates was carried out using MEGA6 software.Citation32 The phylogenetic tree was visualized using the iTOL online tool (https://itol.embl.de/).Citation33 The exon-intron structures of each SRO gene were analyzed and visualized based on their GFF annotation files using the TBtools software. The conserved motifs of each SRO protein were predicted using the Multiple Em for Motif Elicitation (MEME) online tool (http://meme-suite.org/tools/meme)Citation34 based on the default parameters, and the maximum motif number was set to 15. The conserved motifs were subsequently visualized with TBtools software.
Genome collinearity analysis and Ka and Ks calculations
Genome-wide collinearity analyses of Brassica napus (Bna), Brassica juncea (Bju), and Bna and Bju were performed using the “One Step MCScanX” plugin of TBtools software and visualized by the “Advanced Circos” or “Dual Systeny Plot for MCScanX” plugins. The nonsynonymous substitution rate (Ka) and synonymous substitution rate (Ks) were estimated for SRO gene pairs collinear between Bna and Bju using TBtools software. Ka/Ks < 1 indicates purifying selection or negative selection, and Ka/Ks > 1 indicates positive selection.Citation35
Cis-acting element prediction and expression pattern analysis
The 2 kb upstream sequence of the start codon of each gene was considered the promoter region, and the presumed cis-regulatory elements of each promoter were analyzed using the PlantCARE online tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).Citation36 Data on the expression of BnaSROs in different tissues of rapeseed and in rapeseed leaves and roots in response to salt, drought, freezing, cold, heat, osmotic stress, and ABA and JA treatments were downloaded from the BnIR database. The visualized heatmaps were drawn using TBtools software.
Plant materials and treatments
In this study, the ‘Zhongshuang 11’ rapeseed cultivar was used as materials. Seedlings were planted in nutrient soil in the greenhouse of the Oil Crops Research Institute of Guizhou Academy of Agricultural Sciences under 16 h of light (22°C) and 8 h of darkness (20°C). The seedlings were treated when they had four complete leaves. For the ABA and MeJA treatments, final concentrations of 100 μM ABA and 50 μM MeJA, respectively, were prepared and dissolved in sterile water containing 0.05% anhydrous ethanol and 0.01% Tween 20. A uniform spray was applied to the front and back leaves of the seedlings, and samples were taken at 0, 0.5, 1, 2, 4, and 8 h after spraying. For the drought treatment, 2 d after the watering of seedlings stopped under normal cultivation management was used as the starting point (0 d) for the drought treatment. Samples were taken at 0, 1, 3, 5, 7, and 10 days after drought treatment. In all the treatments, the top two young leaves of the seedlings were sampled, and the samples were snap-frozen in liquid nitrogen and stored in a −80 refrigerator for later use. Three biological replicates were used for each treatment.
RNA extraction, reverse transcription and quantitative real-time PCR (qPCR) analysis
For RNA extraction, leaves were first ground to a powder under liquid nitrogen, and then total RNA was extracted using a TaKaRa MiniBEST Plant RNA Extraction Kit (TaKaRa, Japan) according to the manufacturer’s instructions. RNA quality and concentration were detected using 1.5% agarose gel electrophoresis and a nucleic acid concentration meter (ND-1000 spectrophotometer, Thermo Fisher, USA), followed by reverse transcription using the EasyScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) Kit (TIANGEN, China). The TB Green Premix Ex Taq™ II (TaKaRa, Japan) was used for qPCR. The 10 µL reaction system contained 5 µL of TB Green Premix Ex Taq, 0.3 µL of forward and reverse primers, 1 µL of template, and 3.4 µL of RNA-free sterile water. The qPCR was performed using a Bio-Rad CFX96 instrument (Bio-Rad, USA) as previously described.Citation37 The BnaActin gene was used as an internal control. The analysis of each sample was repeated three times, and the relative expression levels of each gene were calculated using the 2−ΔΔCT method.Citation38 The primers used for qPCR analysis are listed in Table S1.
Results
Identification of the SRO genes in rapeseed and five other Brassica species
In this study, a total of 19 SRO genes were identified from the ‘Zhongshuang 11’ rapeseed genome (AACC, BnaSRO1-BnaSRO19). In addition, 9, 10, 10, 18, and 20 SRO genes were also identified from Brassica rapa (AA, BraSRO1-BraSRO9), Brassica nigra (BB, BniSRO1-BniSRO10), Brassica oleracea (CC, BolSRO1-BolSRO10), Brassica juncea (AABB, BjuSRO1-BjuSRO18), and Brassica carinata (BBCC, BcaSRO1-BcaSRO20), respectively. The genes showed a variable distribution across the genomes of these six Brassica crops (Table S2). Further analysis of these SROs for protein physicochemical properties revealed that their amino acid numbers ranged from 291(BcaSRO14 and BcaSRO15) to 584 (BolSRO7), their molecular weights ranged from 31.94 kDa (BcaSRO15) to 64.39 kDa (BolSRO7), their isoelectric points ranged from 5.12 (BnaSRO19) to 9.33 (BniSRO6), and their GRAVY values ranged from −0.22 to −0.5. Subcellular localization prediction analyses showed that most of these proteins may be localized in the nucleus, while the rest may be localized in the cytoplasm, chloroplasts, or peroxisomes.
Phylogenetic analysis of SROs in angiosperms
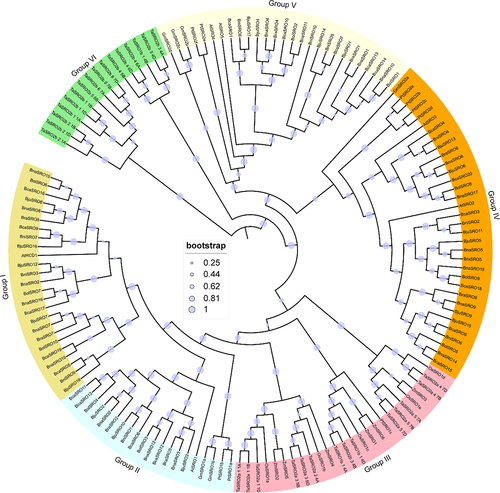
To investigate the evolutionary relationships of the SROs of these six Brassica crops, a phylogenetic tree was constructed with them using SROs from Arabidopsis thaliana (At), rice (Oryza sativa, Os), maize (Zea mays, Zm), soybean (Glycine max, Gm), wheat (Triticum aestivum, Ta), and Populus trichocarpa (Pt). As shown in , these SROs were categorized into six subgroups (Group I-Group VI). The SROs of Brassica crops were distributed in Groups I, II, IV, and V, which correspond to the AtRCD1, AtSRO1, AtSRO2 and AtSRO3, and AtSRO4 and AtSRO5 members of Arabidopsis, respectively, and members of these subgroups were all derived from dicots. All the members of Group VI were from wheat, and all the members of Group III except PtSRO1c were monocotyledons.
Gene structure and conserved motif analysis of SROs in Brassica
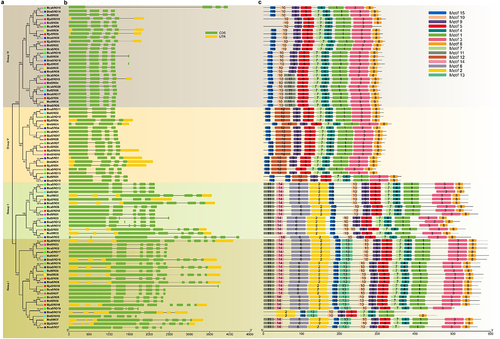
The phylogenetic tree reconstruction of only the 86 SROs from these six Brassica species also supported their classification into four subgroups (). Members within the same clade exhibited similar intron and exon structures (). Among them, members of Group IV possess 3–4 introns, members of Group V have 2–3 introns, members of Group II contain no less than 4 introns except BraSRO2, BjuSRO2 and BnaSRO2, and members of Group I possess 4–5 introns. In addition, except for BnaSRO3 in Group II, the length of the first exon of all members of Groups I and II was longer than that of Groups IV and V. Based on the amino acid sequences of these 86 SROs, a total of 15 conserved structural domains were identified (). The composition and distribution of the conserved structural domains also showed a high degree of similarity within subgroups. Among these 15 conserved motifs, only Motif 1 and Motif 5 were present in all the members, Motif 12 was present only in the members of Group V, Motif 13 was present only in the members of Group I, and Motif 2, Motif 6 and Motif 14 were found only in the members of Groups I and II.
Collinearity analysis of SROs in Brassica napus and Brassica juncea
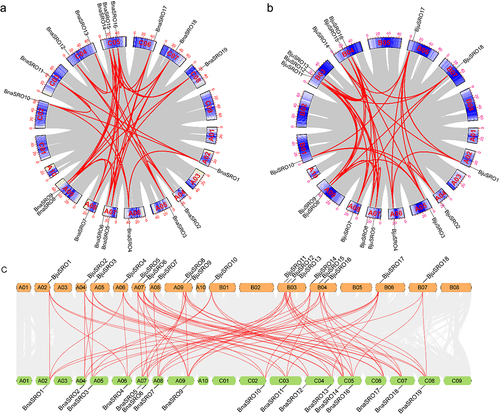
To further characterize the evolution of SROs in rapeseed, collinearity analyses of SRO genes in Brassica napus and Brassica juncea were performed. As shown in , a total of 37 pairs of segmentally duplicated genes were found in the Brassica napus genome (), and 37 collinear gene pairs were also identified in the Brassica juncea genome (). A total of 72 syntenic gene pairs were identified between the Brassica napus and Brassica juncea genomes (). A large number of multigene colineages existed both within the genome and between these two genomes. For example, BnaSRO1, BnaSRO6, and BnaSRO7 have 3, 4, and 4 collinearity genes, respectively, in the Brassica napus genome. The genes BjuSRO2, BjuSRO5, and BjuSRO7 had 4, 5, and 4 collinearity genes, respectively, in the Brassica juncea genome. BnaSRO1 is simultaneously collinear with the BjuSRO1, BjuSRO4, BjuSRO14, and BjuSRO17 genes. However, the BnaSRO8 gene has no collinear genes in the Brassica juncea genome. We further analyzed the Ka/Ks values of the 72 collinear gene pairs between the Brassica napus and Brassica juncea genomes, and all of the Ka/Ks ratios were less than 1, indicating that they all underwent purifying selection during evolution (Table S4).
Figure 3. Collinearity of the SRO gene pairs. (a) Collinearity analysis of the SRO gene family in Brassica napus. (b) Collinearity analysis of the SRO gene family in Brassica juncea. (c) collinearity analysis of SRO genes between Brassica napus and Brassica juncea. The identified SRO gene pairs are connected by red lines.
Promoter analysis of BnaSROs
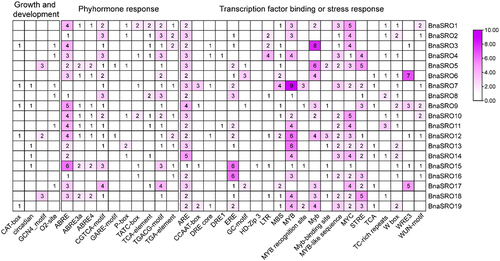
To explore the potential functions of rapeseed SRO genes, the cis-acting elements in their promoter regions were predicted and analyzed. As shown in , these elements can be classified into three categories, namely, growth and development-related, hormone-responsive, and transcription factor binding or stress-responsive. For these hormone response-related elements, ABA (ABRE) and MeJA response-related elements (TGACG-motif and CGTCA-motif) were the most abundant and most widely distributed among the 19 SRO members in rapeseed, suggesting that the function of BnaSROs may be regulated by ABA and MeJA. Transcription factor binding or stress-responsive elements showed diverse distribution characteristics among different members of the rapeseed SRO family. Among them, three classes of transcription factors, MYB, MYC, and WRKY, had the greatest number of binding elements associated with them, indicating that the same SRO gene may be regulated by multiple transcription factors at the same time. In addition, low temperature-responsive element (LTR), drought-responsive element (MBS), defense and stress-responsive element (TC rich repeats), and wound-responsive element (WRE3 and WUN-motif) also exhibited variable distributions among the different members, which suggests diverse stress responses in the rapeseed SRO family along with functional differences among these members.
Figure 4. Statistics on the number of cis-acting elements in the promoter region of the BnaSRO genes. The elements were categorized into three groups based on their functions: plant growth and development, phytohormone response, and transcription factor binding or stress response. Heatmaps were drawn based on the number of different elements using TBtools software.
Expression pattern analysis of BnaSROs based on transcriptome data
To further investigate their functional diversity, we analyzed the expression patterns of individual members of the rapeseed SRO family in different tissues of rapeseed and under various adverse conditions and hormonal treatments using transcriptome data from public databases (). Expression patterns based on 91 different tissues or developmental periods showed the diversity of tissue expression of these 19 members, with different members exhibiting tissue-specific expression patterns (). Among these genes, BnaSRO7, BnaSRO8, BnaSRO13, and BnaSRO15 presented relatively high expression levels in leaf tissues, and BnaSRO5, BnaSRO9, BnaSRO14, and BnaSRO18 were highly expressed in early developing seeds. Moreover, BnaSRO4 was specifically expressed in filaments, petals and sepals; BnaSRO6 and BnaSRO17 were exclusively expressed in seed_14DAF and seed_16DAF; BnaSRO3 and BnaSRO12 were mainly expressed in seed_62DAF and seed_64DAF; and BnaSRO19 was predominantly expressed in siliques in the late flowering period.
Figure 5. The expression patterns of BnaSROs in rapeseed based on transcriptome data. (a) The expression patterns of BnaSROs in different tissues or developmental periods of rapeseed. (b) The expression patterns of BnaSROs under salt, drought, freezing, cold, heat, and osmotic stresses, as well as under ABA and JA treatments, in rapeseed leaves and roots (c).
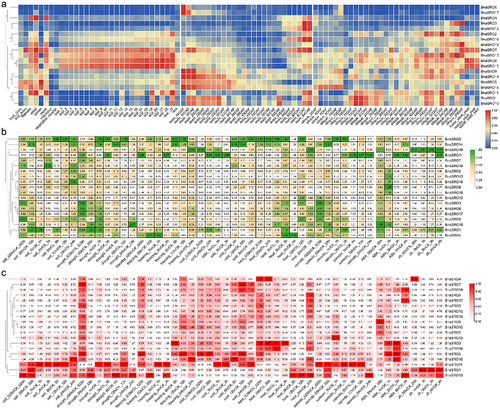
For stress and hormone responses, we analyzed the expression patterns of these 19 members under salt, drought, freezing, cold, heat, and osmotic stresses, as well as under ABA and JA treatments in rapeseed leaves () and roots (), respectively. They also showed a variety of expression patterns in response to different stresses, and the degree of response in leaf tissue and root tissue was not consistent. In terms of expression in leaf tissues (), BnaSRO11 showed the most dramatic response to osmotic stress, and its expression level was up-regulated more than 40-fold compared with that of the control at 6 h after treatment and remained up-regulated nearly 10-fold at 24 h after treatment. In addition, its expression was also substantially induced by freezing and cold treatments as well as ABA and JA treatments. The expression pattern of BnaSRO10 was basically the same as that of BnaSRO11, but to a lesser degree. The expression of BnaSRO9 and BnaSRO14 was induced by almost all of the above treatments, and both were induced to the greatest extent at 12 h after the drought and cold treatments. Both BnaSRO10 and BnaSRO1 were significantly induced in response to the JA treatments with a consistent trend. In terms of expression in root tissues (), BnaSRO1, BnaSRO10 and BnaSRO11 were significantly induced by salt, drought, freezing, heat, osmotic and JA treatments, especially drought treatment. In addition, in both leaf and root tissues, BnaSRO9 and BnaSRO14 as well as BnaSRO5 and BnaSRO18, showed similar expression patterns in response to stress, suggesting redundancy in their functions.
Expression profiles of BnaSROs under ABA, MeJA and drought treatments
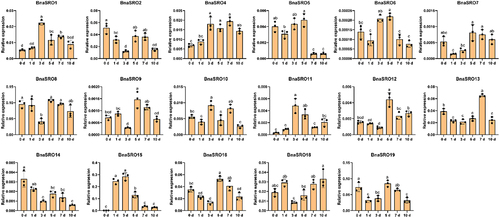
To further clarify the potential biological functions of rapeseed SRO family members, we analyzed their expression under ABA, MeJA and drought treatments using qPCR. As shown in , since the expression levels of BnaSRO3 and BnaSRO17 were too low to be accurately detected by qPCR, we analyzed the expression characteristics of the remaining 17 members under ABA () and MeJA () treatments. With the exception of BnaSRO6, BnaSRO7, BnaSRO9, BnaSRO12 and BnaSRO16, the expression of the remaining 12 members increased at 0.5 h after ABA treatment. However, all members were down-regulated to varying degrees afterward, except for BnaSRO4, BnaSRO5, BnaSRO10 and BnaSRO11, which recovered to the pre-treatment level, the expression levels of the remaining members were lower than the pre-treatment level. In the MeJA treatment group, all members exhibited distinct expression characteristics compared with those in the ABA treatment group. Among them, except for BnaSRO5, the expression of the other members increased to different degrees after treatment, but the time of induction was inconsistent. BnaSRO1, BnaSRO10 and BnaSRO11 were up-regulated to significant levels at 0.5 and 1 h after treatment, while BnaSRO7, BnaSRO8, BnaSRO9, BnaSRO13 and BnaSRO14 reached significant levels at 4 and 8 h after treatment. Under drought stress, as shown in , more than half of the members were induced by drought stress. Among these genes, BnaSRO1, BnaSRO10, BnaSRO11 and BnaSRO15 were induced to the greatest extent at 3 d after treatment, BnaSRO6, BnaSRO9, BnaSRO12 and BnaSRO16 peaked at 5 d after treatment, and BnaSRO4 and BnaSRO13 were induced to the greatest degree at 7 d after treatment. Notably, BnaSRO1, BnaSRO4 and BnaSRO11 were still induced 10 d after drought, suggesting that they have specific functions in the drought stress response in rapeseed.
Figure 6. The relative expression levels of the BnaSROs in rapeseed leaves at different time points after exogenous ABA (a) and MeJA (b) treatments. The expression levels were calculated based on the 2−ΔCT method relative to the internal reference gene. The bars represent the mean ± SD (n = 3). The different letters indicate significant differences at p < 0.05 according to Duncan’s test.
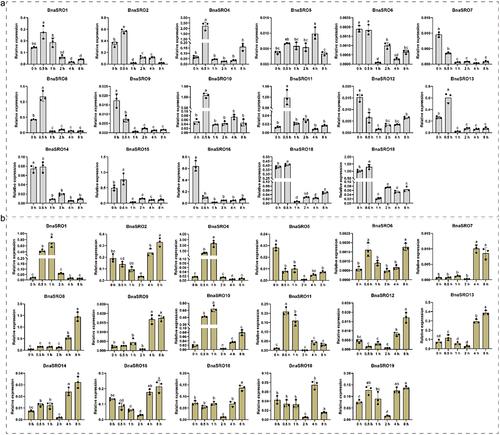
Figure 7. The relative expression levels of BnaSROs in rapeseed leaves after a different number of days of water deficit drought treatment. The expression levels were calculated based on the 2−ΔCT method relative to the internal reference gene. The bars represent the mean ± SD (n = 3). The different letters indicate significant differences at p < 0.05 according to Duncan’s test.
Co-expression network construction of BnaSRO1 and BnaSRO11
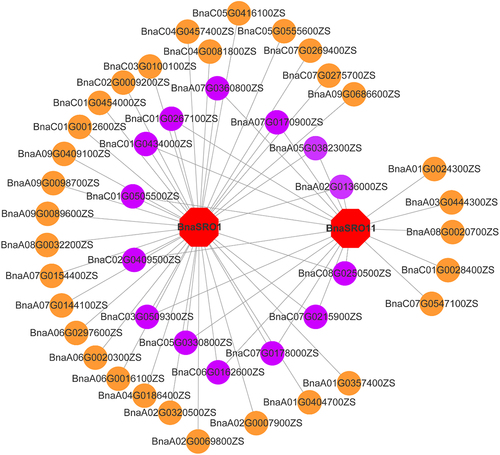
Synthesizing the transcriptome data and qPCR results, we initially screened BnaSRO1 and BnaSRO11 as functionally important members of the rapeseed SRO family for subsequent functional studies. We constructed transcription factor (TF) regulatory networks of these two members based on public transcriptome data. As shown in , a total of 45 TFs were identified, 14 of which may regulate both BnaSRO1 and BnaSRO11. The functional annotation of these TFs showed that most were NAC family members (Table S5), implying that the functions of BnaSRO1 and BnaSRO11 are likely regulated by NAC transcription factors.
Figure 8. Transcriptional regulatory network of the BnaSRO1 and BnaSRO11 genes. The transcription factor genes are indicated by circles, and those in purple indicate transcription factors that have regulatory relationships with both BnaSRO1 and BnaSRO11. The homologous gene information for these transcription factor genes in Arabidopsis is listed in Table S5.
Discussion
Plants have developed complex mechanisms to cope with and adapt to a variety of environmental stressors during long-term evolution, and plant-specific genes play an indispensable role in this process.Citation39,Citation40 SROs, as plant-specific genes, have been consistently identified in various plants, indicating their the conserved distribution in plants. They are receiving sustained attention for their special roles in plant responses to abiotic and oxidative stressors.Citation5,Citation16,Citation22 However, studies on their molecular functions and regulatory mechanisms are currently limited to partial members of model plants such as Arabidopsis,Citation8,Citation12 rice,Citation6,Citation23 and maize.Citation25 Nothing is known about their function in rapeseed. In this study, we systematically identified SRO family members in six Brassica crops, including rapeseed, and analyzed their sequence characteristics, physicochemical properties, structural features, and evolutionary relationships. Unlike the common diploid crops, which have 5–6 members of the SRO family,Citation6,Citation14,Citation16 the tetraploid Brassica napus, Brassica juncea, and Brassica carinata have 19, 18, and 20 members, respectively, while Brassica rapa, Brassica nigra, and Brassica oleracea, which are also diploids, have 9, 10, and 10 members, respectively. This finding predicts the expansion of SRO family members in Brassica crops. This phenomenon was also detected in diploid tea plants, which possess 9 members.Citation21 Given that Brassica napus and Brassica juncea are co-cultivated in southwestern China, we focused on comparing the collinearity characteristics of SRO genes in these two crops. A large number of segmental duplication gene pairs were identified both within and between these two genomes, and multiple duplicated genes also existed for the same gene. This explains the expansion of SRO family members in Brassica. Interestingly, BnaSRO8 is the only member that does not have a collinear gene in Brassica juncea, suggesting that it is unique to Brassica napus. Phylogenetic analyses with different plant SROs revealed that the 86 SRO members of the abovementioned six Brassica species could be categorized into four subgroups, and members within the same subgroup presented similar gene structures and conserved motif compositions. This phenomenon has also been found in other plant SROs studies as well as in other gene family identifications,Citation18,Citation19,Citation41,Citation42 further demonstrating their close evolutionary relationship. In addition, our evolutionary tree results showed that, with the exception of individual members, the SROs of monocotyledonous and dicotyledonous plants were in separate subgroups. It has been suggested that the genome evolution of SROs accompanied the divergence of monocotyledons and dicotyledons.Citation15
According to the results of the cis-acting elements analysis of the promoters of BnaSROs, each member has a large number of stress-responsive and hormone-responsive related elements, indicating that, similar to SROs in other plants, BnaSROs could be involved in the regulation of the stress response in rapeseed. Among these hormone response elements, the ABA and MeJA response elements were the most abundant. The phytohormone ABA plays a crucial role in drought responses,Citation43 and the MdRCD1 gene in apple plants positively regulates drought tolerance through the ABA signaling pathway.Citation44 This indicates that members of the BnaSRO family regulate the response to drought stress in rapeseed. In addition, it has been demonstrated that SROs are also regulated by TFs during the drought stress response in plants,Citation9,Citation23 and we also identified a large number of TF binding elements on the promoters of BnaSROs, suggesting that this regulatory role is conserved among plants. Meanwhile, that the composition of the cis-acting elements in the promoter regions of the different BnaSRO family members varied, indicating the diversity of their functions. This functional diversity was further validated by transcription patterns. Based on public transcriptome data, we found that BnaSROs responded to a variety of stresses, including salt, drought, freezing, cold, heat, and osmotic stressors, to varying degrees. Under the same conditions, the response in the root tissue was greater than that in the leaves. This suggests that, similar to SROs in other plants,Citation19,Citation20 BnaSROs may also act as key functional genes in the abiotic stress response pathway, and our results provide additional evidence for the multiple stress response functions of SROs in plants. In addition, we further examined the expression characteristics of BnaSROs under ABA, MeJA and water deficit drought stress conditions using qPCR. However, although different members presented diverse response expression patterns, none of them showed regular changes. This phenomenon has been found in studies of other plant SROs, such as those of rice,Citation6 maize,Citation14 poplar,Citation22 tea plant,Citation21 potatoCitation18 and tomato,Citation16 and has also been presented in the analysis of other gene families. This might be related to the different regulatory mechanisms they were subjected to during the different treatment periods. This reveals the complexity of SROs expression patterns while also mirroring the complexity of plant responses to stressors.
Although there were no members who were regularly expressed throughout the treatments, we screened for members who responded significantly to the different treatments. In response to ABA treatment, BnaSRO1, BnaSRO2, BnaSRO4, BnaSRO8, BnaSRO10, BnaSRO11, BnaSRO13, BnaSRO15, BnaSRO18, and BnaSRO19 were significantly up-regulated at 0.5 h post-treatment, while BnaSRO7, BnaSRO9, BnaSRO12, and BnaSRO16 were significantly down-regulated. In tomato, SolySRO3, SolySRO4, and SolySRO6 were significantly up-regulated after ABA treatment, whereas SolySRO1 and SolySRO2 were significantly down-regulated.Citation16 In potato, rice and poplar, the expression of all members of the SRO family was induced upon ABA treatment.Citation6,Citation18,Citation22 In the MeJA treatment group, BnaSRO1, BnaSRO4, BnaSRO6, BnaSRO10, BnaSRO11, and BnaSRO19 were significantly up-regulated in the early treatment group, whereas BnaSRO2, BnaSRO7, BnaSRO8, BnaSRO9, BnaSRO12, BnaSRO13, BnaSRO14, BnaSRO15, and BnaSRO16 were up-regulated in the later treatment group. In tomato, the SolySRO1 gene was significantly up-regulated starting 6 h after MeSA treatment and consistently continued for 24 h, while SolySRO3 and SolySRO4 were significantly up-regulated only at 12 h16. Although MeJA also plays a key role in the plant drought stress response,Citation45 plant responses to ABA and MeJA treatments were inconsistent or even opposite. This may be related to the inconsistency in the signaling pathways through which they regulate the plant drought stress response. For drought treatment, most studies have used PEG treatment to simulate drought.Citation16,Citation18,Citation21,Citation22 In this study, we used the direct drought form of the water deficit treatment to simulate real-world conditions. We found that the expression of BnaSRO1, BnaSRO4, BnaSRO10, BnaSRO11, and BnaSRO15 peaked 3 d after drought treatment; that of BnaSRO6, BnaSRO9, BnaSRO12, and BnaSRO16 peaked 5 d after drought treatment; and that of BnaSRO13 peaked 7 d after drought treatment. Combining the results of the different treatments, we concluded that BnaSRO1, BnaSRO4, BnaSRO10, BnaSRO11, and BnaSRO15 deserve in-depth functional studies as important members of the drought response in rapeseed. In this study, based on the results of transcriptome expression patterns and qPCR analysis, we screened BnaSRO1 and BnaSRO11 as potential major drought-responsive members of the BnaSRO family. Both of these genes may be target genes of many transcription factor genes, especially NAC transcription factors. The NACs regulation of SROs has been identified and partially validated in previous studies. For example, in poplar, a recent study identified a number of NAC-SRO chains, such as ANAC002-PtSRO2f, ANAC017-PtSRO2e and ANAC087-PtSRO2e, through co-expression network construction, predicting that these SROs may be induced to respond to drought stress after interacting with NAC family proteins.Citation22 In sesame, SiSROs may also be involved in abiotic stress response through interactions with NAC016 and NAC017.Citation19 In rice, OsSRO1c is directly regulated by SNAC1 to enhance drought and oxidative stress tolerance.Citation6 In banana, MaSRO4 directly interacts with MaNAC6 through the PARP domain to regulate downstream signaling pathways.Citation20 Notably, we found that BnaSRO1, BnaSRO11, PtSRO2e and PtSRO2f were all in the same subgroup, further suggesting that BnaSRO1 and BnaSRO11 may be target genes of NACs. This deserves a subsequent investigated in depth. Moreover, subcellular localization predictions showed that BnaSRO1 and BnaSRO11 were localized in the nucleus, suggesting that they may also regulate other drought-responsive genes.
Conclusions
In summary, in this study, we systematically characterized SRO genes in six Brassica crops, including Brassica napus, Brassica rapa, Brassica nigra, Brassica oleracea, Brassica juncea and Brassica carinata, and identified 19, 9, 10, 10, 18, and 20 members, respectively. Based on phylogenetic analysis, these 86 members were divided into 4 subgroups, corresponding to AtRCD1, AtSRO1, AtSRO2 and AtSRO3, and AtSRO4 and AtSRO5 of Arabidopsis, respectively. The number of SRO family members underwent significant expansion in these six Brassica species, with segmental duplication being the main mode of expansion. With the exception of BnaSRO8, the remaining 18 members had collinear genes in the Brassica juncea genome. The promoter regions of BnaSROs contain a large number of ABA- and MeJA-responsive elements, as well as transcription factor-binding and stress-responsive elements. Transcriptome data analysis and qPCR detection indicated that BnaSROs have multiple stress-responsive expression patterns. BnaSRO1 and BnaSRO11 were screened as two potential major drought-responsive members. Both of them may be target genes of NACs involved in drought regulation. Our study laid a foundation for the functional exploration of SRO genes in rapeseed and provided new genetic resources for the biological breeding of drought-tolerant rapeseed.
Author contributions
H.J: Conceptualization, Methodology, Investigation, Visualization, Writing-original draft preparation. Y.L.Z and J.L: Methodology, Investigation, Validation. R.Z.T, F.L, R.T and Y.Y.Z: Formal analysis, Resources, Validation. C.Z: Supervision, Writing review & editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Supplementary_materials_R.docx
Download MS Word (514.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data relevant to this study are included in the article or uploaded as Supplementary Materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592324.2024.2379128
Additional information
Funding
References
- Hu J, Chen B, Zhao J, Zhang F, Xie T, Xu K, Gao G, Yan G, Li H, Li L. et al. Genomic selection and genetic architecture of agronomic traits during modern rapeseed breeding. Nat Genet. 2022;54(5):694–13. doi:10.1038/s41588-022-01055-6.
- Ning N, Mo J, Hu B, Li D, Lou H, Wang Y, Bai C, Kuai J, Wang B, Wang J. et al. Comparative study on the processing quality of winter rape in different ecological zones of the Yangtze River valley. Acta Agronomica Sin. 2023;49:3315–3327.
- Li Y, Wu D, Xu J, Chen Z, Xu X, Xu J, Tang Z, Zhang Y, Zhu L, Yan Z. et al. Identification of candidate genes associated with drought tolerance based on QTL and transcriptome sequencing in Brassica napus L. Acta Agronomica Sin. 2024;50:820–835.
- Li J, Duan Y, Sun N, Wang L, Feng S, Fang Y, Wang Y. The miR169n-NF-YA8 regulation module involved in drought resistance in Brassica napus L. Plant Sci. 2021;313:111062. doi:10.1016/j.plantsci.2021.111062.
- Jaspers P, Overmyer K, Wrzaczek M, Vainonen JP, Blomster T, Salojarvi J, Reddy RA, Kangasjarvi J. The RST and PARP-like domain containing SRO protein family: analysis of protein structure, function and conservation in land plants. BMC Genomics. 2010;11(1):170. doi:10.1186/1471-2164-11-170.
- You J, Zong W, Li X, Ning J, Hu H, Li X, Xiao J, Xiong L. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J Exp Bot. 2013;64(2):569–583. doi:10.1093/jxb/ers349.
- Yuan B, Chen M, Li S. Isolation and identification of Ipomoea cairica (L.) sweet gene IcSRO1 encoding a SIMILAR to RCD-ONE protein, which improves salt and drought tolerance in transgenic Arabidopsis. Int J Mol Sci. 2020;21(3):1017. doi:10.3390/ijms21031017.
- Ahlfors R, Lång S, Overmyer K, Jaspers P, Brosché M, Tauriainen A, Kollist H, Tuominen H, Belles-Boix E, Piippo M. et al. Arabidopsis RADICAL-INDUCED CELL DEATH1 belongs to the WWE protein–protein interaction domain protein family and modulates abscisic acid, ethylene, and methyl jasmonate responses. The Plant Cell. 2004;16(7):1925–1937. doi:10.1105/tpc.021832.
- Jaspers P, Blomster T, Brosche M, Salojarvi J, Ahlfors R, Vainonen JP, Reddy RA, Immink R, Angenent G, Turck F. et al. Unequally redundant RCD1 and SRO1 mediate stress and developmental responses and interact with transcription factors. Plant J. 2009;60(2):268–279. doi:10.1111/j.1365-313X.2009.03951.x.
- Teotia S, Lamb RS. The paralogous genes RADICAL-INDUCED CELL DEATH1 and SIMILAR to RCD ONE1 have partially redundant functions during Arabidopsis development. Plant Physiol. 2009;151(1):180–198. doi:10.1104/pp.109.142786.
- Teotia S, Lamb RS. RCD1 and SRO1 are necessary to maintain meristematic fate in Arabidopsis thaliana. J Exp Bot. 2011;62(3):1271–1284. doi:10.1093/jxb/erq363.
- Katiyar-Agarwal S, Zhu J, Kim K, Agarwal M, Fu X, Huang A, Zhu JK. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in arabidopsis. Proc Natl Acad Sci USA. 2006;103(49):18816–18821. doi:10.1073/pnas.0604711103.
- Vainonen JP, Jaspers P, Wrzaczek M, Lamminmaki A, Reddy RA, Vaahtera L, Brosche M, Kangasjarvi J. RCD1–DREB2A interaction in leaf senescence and stress responses in Arabidopsis thaliana. Biochem J. 2012;442(3):573–581. doi:10.1042/BJ20111739.
- Jiang H, Xiao Y, Zhu S. Genome-wide identification, systematic analysis and characterization of SRO family genes in maize (zea mays L.). Acta Physiol Plant. 2018;40(10):40. doi:10.1007/s11738-018-2738-0.
- Jiang W, Geng Y, Liu Y, Chen S, Cao S, Li W, Chen H, Ma D, Yin J. Genome-wide identification and characterization of SRO gene family in wheat: molecular evolution and expression profiles during different stresses. Plant Physiol Biochem. 2020;154:590–611. doi:10.1016/j.plaphy.2020.07.006.
- Li N, Xu R, Wang B, Wang J, Huang S, Yu Q, Gao J. Genome-wide identification and evolutionary analysis of the SRO gene family in tomato. Front Genet. 2021;12:12. doi:10.3389/fgene.2021.753638.
- Lyu Y, Yang W, Zhao L, Yao J, Chen W, Li Y, Zhang Y. Genome-wide identification and expression analysis of SRO genes family in Gossypium hirsutum L. Acta Agronomica Sin. 2017;43(10):1468–1479. doi:10.3724/SP.J.1006.2017.01468.
- Ma Y, Zhou X, Liu Z, Wu B. Comprehensive analysis of StSRO gene family and its expression in response to different abiotic stresses in potato. Int J Mol Sci. 2022;23(21):13518. doi:10.3390/ijms232113518.
- Liu A, Wei M, Zhou Y, Li D, Zhou R, Zhang Y, Zhang X, Wang L, You J. Comprehensive analysis of SRO gene family in sesamum indicum (L.) reveals its association with abiotic stress responses. Int J Mol Sci. 2021;22(23):13048. doi:10.3390/ijms222313048.
- Zhang L, Zhou D, Hu H, Li W, Hu Y, Xie J, Huang S, Wang W. Genome-wide characterization of a SRO gene family involved in response to biotic and abiotic stresses in banana (Musa spp.). BMC Plant Biol. 2019;19(1). doi:10.1186/s12870-019-1807-x.
- Guo Y, Wang P, Chen D, Zheng Y, Chen X, Ye N. Genome-wide identification and expression analysis of SRO gene family in camellia sinensis. J Tea Sci. 2019;39:392–402. doi:10.13305/j.cnki.jts.2019.04.004.
- Wang Y, Wang R, Yu Y, Gu Y, Wang S, Liao S, Xu X, Jiang T, Yao W. Genome-wide analysis of SIMILAR to RCD ONE (SRO) family revealed their roles in abiotic stress in poplar. Int J Mol Sci. 2023;24(4):4146. doi:10.3390/ijms24044146.
- You J, Zong W, Du H, Hu H, Xiong L. A special member of the rice SRO family, OsSRO1c, mediates responses to multiple abiotic stresses through interaction with various transcription factors. Plant Mol Biol. 2014;84(6):693–705. doi:10.1007/s11103-013-0163-8.
- Liu S, Liu S, Wang M, Wei T, Meng C, Wang M, Xia G. A wheat SIMILAR to RCD-ONE gene enhances seedling growth and abiotic stress resistance by modulating redox homeostasis and maintaining genomic integrity. The Plant Cell. 2014;26(1):164–180. doi:10.1105/tpc.113.118687.
- Qin L, Sun L, Wei L, Yuan J, Kong F, Zhang Y, Miao X, Xia G, Liu S. Maize SRO1e represses anthocyanin synthesis through regulating the MBW complex in response to abiotic stress. The Plant J. 2021;105(4):1010–1025. doi:10.1111/tpj.15083.
- Anjum S, Raza S, Azhar A, Qamarunnisa S. Bnsro1: a new homologue of Arabidopsis thaliana rcd1 from Brassica napus. Biologia (Bratisl). 2015;70(5):588–598. doi:10.1515/biolog-2015-0073.
- Yang Z, Wang S, Wei L, Huang Y, Liu D, Jia Y, Luo C, Lin Y, Liang C, Hu Y. et al. BnIR: a multi-omics database with various tools for Brassica napus research and breeding. Mol Plant. 2023;16(4):775–789. doi:10.1016/j.molp.2023.03.007.
- Chen H, Wang T, He X, Cai X, Lin R, Liang J, Wu J, King G, Wang X. BRAD V3.0: an upgraded Brassicaceae database. Nucleic Acids Res. 2022;50(D1):D1432–D1441. doi:10.1093/nar/gkab1057.
- Song X, Wei Y, Xiao D, Gong K, Sun P, Ren Y, Yuan J, Wu T, Yang Q, Li X. et al. Brassica carinata genome characterization clarifies U’s triangle model of evolution and polyploidy in Brassica. Plant Physiol. 2021;186(1):388–406. doi:10.1093/plphys/kiab048.
- Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y. et al. TBtools-II: a “one for all, all for one” bioinformatics platform for biological big-data mining. Mol Plant. 2023;16(11):1733–1742. doi:10.1016/j.molp.2023.09.010.
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(Web Server):W585–W587. doi:10.1093/nar/gkm259.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi:10.1093/molbev/mst197.
- Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–W296. doi:10.1093/nar/gkab301.
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server):W202–W208. doi:10.1093/nar/gkp335.
- Zhang Z, Li J, Zhao XQ, Wang J, Wong GK, Yu J. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics, Proteomics & Bioinf. 2006;4(4):259–263. doi:10.1016/S1672-0229(07)60007-2.
- Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi:10.1093/nar/30.1.325.
- Lei S, Chen L, Liang F, Zhang Y, Zhang C, Xiao H, Tang R, Yang B, Wang L, Jiang H. Identification of a major QTL and candidate genes analysis for branch angle in rapeseed (Brassica napus L.) using QTL-seq and RNA-seq. Front Plant Sci. 2024;15:15. doi:10.3389/fpls.2024.1340892.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262.
- Lahmy S, Guilleminot J, Cheng CM, Bechtold N, Albert S, Pelletier G, Delseny M, Devic M. DOMINO1, a member of a small plant-specific gene family, encodes a protein essential for nuclear and nucleolar functions. Plant J. 2004;39(6):809–820. doi:10.1111/j.1365-313X.2004.02166.x.
- Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167(2):313–324. doi:10.1016/j.cell.2016.08.029.
- Zuo X, Yang C, Yan Y, Huang G, Li R. Systematic analysis of the thioredoxin gene family in Citrus sinensis: identification, phylogenetic analysis, and gene expression patterns. Plant Signal Behav. 2023;18(1):2294426. doi:10.1080/15592324.2023.2294426.
- Zhang Z, Long Y, Yin X, Wang W, Li W, Jiang L, Chen X, Wang B, Ma J. Genome-wide identification and expression patterns of the laccase gene family in response to kiwifruit bacterial canker infection. BMC Plant Biol. 2023;23(1):591. doi:10.1186/s12870-023-04606-z.
- Kim J, Kidokoro S, Yamaguchi-Shinozaki K, Shinozaki K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024;195(1):170–189. doi:10.1093/plphys/kiae105.
- Li H, Li R, Qu F, Yao J, Hao Y, Wang X, You C. Identification of the SRO gene family in apples (Malus×domestica) with a functional characterization of MdRCD1. Tree Genet Genomes. 2017;13(5):94. doi:10.1007/s11295-017-1175-3.
- Yu X, Zhang W, Zhang Y, Zhang X, Lang D, Zhang X. The roles of methyl jasmonate to stress in plants. Funct Plant Biol. 2019;46(3):197–212. doi:10.1071/FP18106.