Abstract
Accurate and reliable measurements of surface tension at the interface of immiscible phases are crucial to understanding various physico-chemical reactions taking place between those. Based on the pendant drop method, an optical (graphical)-numerical procedure was developed to determine surface tension and its dependency on the surrounding temperature. For modeling and experimental verification, chemically inert and thermally stable perfluorocarbon (PFC) oil and water was used. Starting with geometrical force balance, governing equations were derived to provide non-dimensional parameters which were later used to extract values for surface tension. Comparative study verified the accuracy and reliability of the proposed method.
NOMENCLATURE
| β | = |
dimensionless parameter expressed by ρg b 2 /σ where b is length scale |
| g | = |
gravitational acceleration, 9.8 m2/sec |
| ΔP | = |
differential pressure, N/m2 |
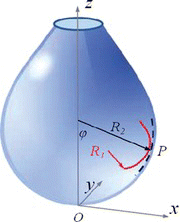
| R 1, R 2 | = |
principal radii of curvature |
| ρ | = |
density, kg/m3 |
| σ | = |
surface tension, N/m |
| ϕ | = |
angle that R 1 makes with z axis |
| E w | = |
work of adhesion between two phases |
| Ψ | = |
correction factor |
1. INTRODUCTION
For most fluids, surface (interfacial) tension decreases with the increase of temperature. Surface tension gradients created as a result of thermal gradients give rise to fluid motion, which is generally known as thermocapillary flow. Motion of bubbles and droplets (dispersed phase) in other fluids (continuous phase) due to gradients of temperature is an example of such flows (Young et al. Citation1959; Bratukhin Citation1975; Balasubramaniam et al. Citation1996; Xie et al. Citation2005). It has been shown that droplets of water resting at the air-liquid interface of a shallow layer of an immiscible liquid can be set in motion in the direction of increasing or decreasing temperature based on the configuration of droplets at the interface (Greco and Grigoriev Citation2009; Yakhshi-Tafti et al. Citation2010). Vela et al. (Citation2009) used infra red laser-induced thermocapillary-driven flow to manipulate objects on a liquid platform, where the the local surface/interfacial tension varies due to the radiative heating. Knowledge of the interfacial tension at the air-liquid and liquid-liquid interfaces, and especially the temperature dependency of tension values are essential in the study of thermocapillary phenomena. A comprehensive theory capable of predicting surface tension and its dependency on temperature does not exist and semi empirical methods and equations are used (Eotvos and van der Waals-Guggenheim equations) (Tropea et al. Citation2007).
In this study we measured the temperature-dependency of surface (interfacial) tension of a perfluorocarbon (PFC) liquid (FC-43 – Perfluorotributylamine) in air and in water. Due to chemical inertness and thermal stability, PFC liquids have broad applications ranging from electronic cooling to biomedical applications, and recently have been used as working fluids in multiphase droplet-based microfluidic devices (Selimovic et al. Citation2009). In multiphasic applications, it is mostly likely that PFCs would have interfaces with water and/or air; therefore, the method and data provided in this article is of benefit for practical applications and modeling purposes. For example, droplet based microfluidic platforms are used in the study of artificial cells because small scale droplets act as self-contained carriers of cells and are convenient for controlling and manipulating quantitative chemical experiments (Takinoue and Takeuchi Citation2011). Droplet-based micro systems have been used for generating discrete reactors ranging from nano- to femtoliters, synthesizing particles, and encapsulating biological entities for biomedicine and biotechnology applications (Shia-Yen et al. Citation2008). The performance of these systems is strongly dependent on the surface/interfacial tension of the fluids involved. Considering widespread interest in the aforementioned applications, the availability of a simple but accurate measurement technique for surface/interfacial tension based on commonly accessible experimental tools is in great demand. In most multiphase (liquid/liquid) configurations, there is an aqueous phase (dispersed) and a non-aqueous phase (carrier liquid), which is to be chemically inert, electrically insulating and thermally stable. PFCs are well-suited for this purpose. Based on the pendant drop method, an optical (graphical)-numerical procedure is described for measuring the interfacial tension of PFC-air and PFC-water as a function of temperature.
Variations of the pendant drop method have been reported and used for the measurement of surface/interfacial tension (Lopez de Ramos et al. Citation1993; Schreyeck and Marie Citation1999). The method has been proven to be versatile and with enough accuracy for measurements even for extremely low surface-tension liquids (∼0.06 mN/m). Based on this concept, a simple, yet accurate procedure is described in this work for measuring temperature dependent surface (interfacial) tension of a fluorocarbon oil.
2. THEORETICAL MODEL
2.1. Pendant Drop Method
More than 40 methods have been documented for measuring surface (interface) tension at the liquid-fluid and solid-fluid boundaries (Rusanov and Prokhorov Citation1996). Among the various techniques, the drop (bubble) profile analysis tensiometry has been most frequently used in customized measurement setups. While the general aspects of the pendant drop shape method are well-understood and straightforward, the collections of hardware and software involved are usually proprietary (Woodward 1984; Rotenberg et al. Citation1983; Cheng et al. Citation1990) or require expertise in programming and image analysis. A laboratory technique that can be easily deployed using commonly available image processing/mathematics software, hardware and laboratory equipment, is provided here.
A pendant drop is formed in air or in another immiscible liquid. The profile of the droplet is captured by imaging the droplet and using backlighting. Based on the Young-Laplace equations, the shape of a pendant droplet can be found theoretically. By comparing the actual droplet curvature and the theoretically-predicted shape, the appropriate value for surface (interface) tension can be determined.
2.2. Governing Equations
Bashforth and Adams first developed the drop-shape method by numerically solving the theoretical equations describing the shape of a static droplet in a gravitation field (Bashforth and Adams Citation1883). Consider R 1 and R 2 to be the principal radii of curvature at point P on the surface, R 1 corresponds to the curvature in a drop cross section that includes the z axis and R 2 is measured in a plane perpendicular to the former (x-z) (Figure ). The equations that describe the interface curve (drop shape) are derived in the following manner.
2.3. Parameterization
Using and grouping physical properties and constants in the gravitational parameter,
the dimensionless differential equation is found as follows:
From the definition of sinϕ it can be seen that since the differential curve length is written as: ds = R
1
dϕ; similarly
.
Considering equation 3, and the auxiliary equations for the coordinates, the set of first order differential equations that are solved simultaneously to give the drop shape is as follows:
3. EXPERIMENTAL RESULTS
3.1. Test Setup
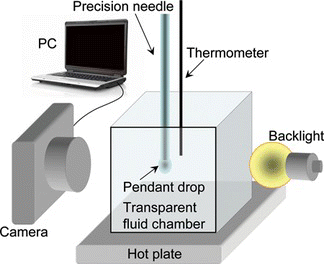
The experimental setup shown in (Figure ) is used to take images of pendant drops. A pendant drop is formed at the tip of a precision needle in the fluid medium of interest (air or water) in a transparent chamber with temperature control. The profile of the droplet is captured by imaging the droplet using backlighting. The droplet is imaged using a digital camera (ProSilica GE680, Allied Vision Technologies GmbH, Germany) with a normal magnifying lens (Navitar Macro 7000 Video Lens, Navitar, Inc., USA). The 10X Macro Zoom lens allows a close-up image capture without extension tubes or close-up lenses and the up-to-10X magnification variable zooming capability allows small objects to be expanded for close-up observation. Backlighting using a diffuse light source makes the detection of the curvature of the drop convenient since both the drop liquid (FC43, n = 1.291) and the surrounding fluid (water, n = 1.333) are transparent.
Figure 2 Experimental setup: Images of pendant drops formed in a fluidic chamber are transferred to a computer for processing (color figure available online).
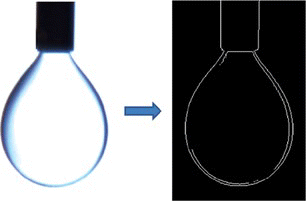
Imaging the pendant drop of FC43 formed in air or water using a diffuse light source for backlighting results in the profile image of a droplet with a sharp edge (Figure left). This could be achieved using available edge detection routines (for example, MATLAB's image processing toolbox, Photoshop, etc.). The image processing procedure consists of converting the indexed “RGB” image to “GrayScale,” enhancing the contrast and applying an edge detection routine. The resulting data from edge detection is a binary file which is stored in a matrix containing 0 and 1 values, representing values of the edge pixels (black) and transparent pixels (white), respectively (Figure right). The x and z coordinates of the edge curve correspond to the row (z) and column (x) values of the edge pixels, which are found and stored in vectors by scanning the matrix for 1 (white edge pixels). The outer edge is compared to the theoretical curve in order to back calculate the surface/interfacial tension. Five droplet images per a given condition were taken on an average for calculation.
Figure 3 Left) Actual image of a pendant drop; Right) Binary image resulting from edge detection (color figure available online).
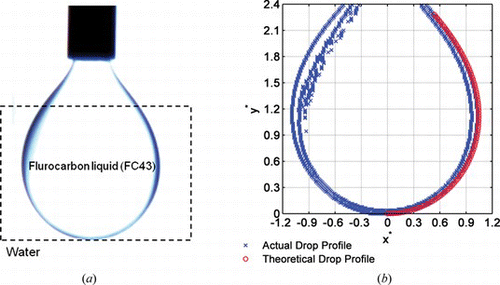
Based on the Young-Laplace equations developed in the previous section, the shape of a pendant droplet can be predicted theoretically. By comparing the actual droplet curvature and the theoretically-predicted shape, the appropriate value for surface (interface) tension can be determined. A computer code reads the image files and detects the boundaries; the drop is then rescaled for plotting. A comprehensive shape comparing procedure known as “axisymmetric drop shape analysis-profile (ADSA-P) has been developed by Neumann and coworkers (Rotenberg et al. Citation1983; Cheng et al. Citation1990), where the calculated theoretical (Laplacian) curve is compared to the actual drop profile until the specified overall is achieved. The surface tension is obtained from the gravitational parameter that results in the closest overlap between the theoretical curve and the actual drop profile (Figure ).
Figure 4 Left) Actual image of a pendant oil drop immersed in water (transparent); Right) By choosing an appropriate value of interfacial tension, s, the theoretical and actual drop profile overlap. The interfacial tension calculated for oil in water drops was found to be 52 dyne/cm (color figure available online).
3.2. Sensitivity, Accuracy and Reproducibility
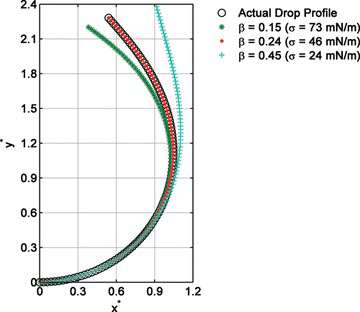
The measured surface (interface) tension is highly sensitive to the gravitational parameter β used in the graphical fitting (overlapping) procedure . For example, the measured interfacial tension for FC43 drops formed in water can vary 2 mN/m as a result of 0.01 increment in the corresponding gravitational fitting parameter. Figure shows the sensitivity of the graphical method to the gravitational parameter.
Figure 5 Effect of the gravitational parameter on the theoretical curve calculated for a pendant drop of FC-43 in water (T = 55°C) (color figure available online).
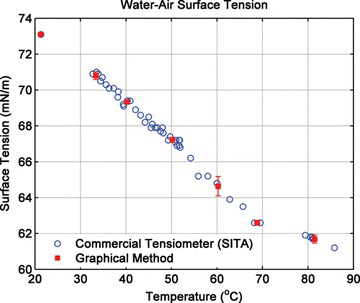
Consistency, accuracy and reproducibility of results based on this method was verified by comparing the measurements with data for water available in references, as well as measurements made by a commercially-available tensiometer (SITA bubble pressure tensiometer, SITA Messtechnik GmbH, Germany) as shown in Figure . The tensiometer can measure surface tension over a range of 10–100 mN/m and temperature range of 0–100°C, with a resolution of 0.1 mN/m. The tensiometer operates based on the following mechanism: air is pumped through a capillary into the liquid, forming bubbles that grow in size until they burst and break away from the capillary. During this process the pressure inside decreases as the bubble radius increases. The surface tension is calculated by correlating the maximum pressure to the smallest radius of curvature (radius of the capillary) using the Young-Laplace equation.
Figure 6 Surface tension of water as a function of temperature measured by commercially available tensiometer (SITA bubble pressure tensiometer) and the in-house graphical method (dσ/dT = −0.203 mN/m/°C) (color figure available online).
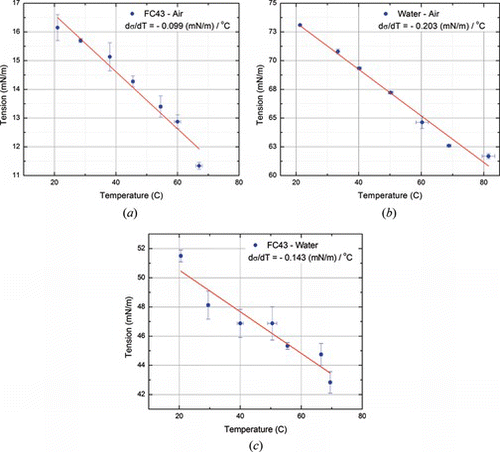
In the graphical method used here, the unknown interfacial tension between the oil (FC-43) and water at room temperature was extracted in multiple runs at identical conditions which yielded the result with less than 5% in repeatability error. It should be noted that the performance of the developed system matches with that of commercially available products, while being far less expensive and having the capability of providing the linear rate at which surface (interfacial) tension decreases with increasing temperature. In experiments, the temperature was increased in increments from room temperature up to 85°C in the imaging chamber. Images were taken when steady temperatures were reached. Figure shows the measurements obtained for temperature dependent surface tension (liquid-air) and interfacial tension (liquid-water) for FC43 perfluorocarbon liquid. For liquid-liquid pairs, e.g., FC43 droplets in water, at higher temperatures, convective currents inside the fluid chamber caused refractive index changes resulting in blurred edges. At such high temperatures, the graphical method starts losing accuracy.
Figure 7 Variation of surface tension with temperature of fluid-medium pairs (FC43-air, water-air, FC43-water) (color figure available online).
Girifalco and Good (Citation1957) provided an equation that related the free energy of cohesion of separate dense phases to the free energy of adhesion of each of the phases; i.e., the relationship between the interfacial tension among to two phases (σ AB ) to the surface tension of each phase (σ A , σ B ).
4. CONCLUSION
A graphical-numerical procedure based on the pendant drop method was developed for making temperature dependent measurement of surface and interfacial tension of fluids. Measurements using this method were compared to those made by the commercially available tensiometer. Results are accurate and reproducible with 5% error. Specifically, surface tension (FC43-Air) and interfacial tension (FC43-Water) was measured as a function of temperature. As expected, tension values decreased linearly with increasing temperature. The linear slopes for FC43-Air and FC-43-Water interfacial tension were found to be −0.099 and −0.143 mN/m/°C, respectively. As droplet-based microfluidic transport platforms are currently being studied and developed more widely, the information and methods described in this article are of practical importance for the design and modeling of such systems.
uopt_a_633206_sup_22284199.zip
Download Zip (209.7 KB)ACKNOWLEDGEMENTS
This work was supported by the National Science Foundation (ECCS1102280), USA.
REFERENCES
- Balasubramaniam , R. , C. E. Lacy , G. Woniak , and R. S. Subramanian . 1996 . Thermocapillary migration of bubbles and drops at moderate values of the Marangoni number in reduced gravity . Physics of Fluids 8 ( 4 ): 872 – 880 .
- Bashforth , F. and J. C. Adams . 1883 . An attempt to test the theories of capillary action: By comparing the theoretical and measured forms of drops of fluid . Cambridge : University Press .
- Bratukhin , Y. K. 1975 . Thermocapillary drift of a droplet of viscous liquid . Fluid Dynamics 10 ( 5 ): 833 – 837 .
- Cheng , P. , D. Li , L. Boruvka , Y. Rotenberg , and A. W. Neumann . 1990 . Automation of axisymmetric drop shape analysis for measurements of interfacial tensions and contact angles . Colloids and Surfaces 43 ( 2 ): 151 – 167 .
- Girifalco , L. A. and R. J. Good . 1957. A theory for the estimation of surface and interfacial energies, I: Derivation and application to interfacial tension. The Journal of Physical Chemistry 61 (7): 904–909.
- Greco , E. F. and R. O. Grigoriev . 2009 . Thermocapillary migration of interfacial droplets . Physics of Fluids 21 ( 4 ): 1 – 19 .
- Lopez de Ramos , A. , R. A. Redner , and R. L. Cerro . 1993 . Surface tension from pendant drop curvature . Langmuir 9 ( 12 ): 3691 – 3694 .
- Owens , D. K. and R. C. Wendt . 1969 . Estimation of the surface free energy of polymers . Journal of Applied Polymer Science 13 ( 8 ): 1741 – 1747 .
- Rotenberg , Y. , L. Boruvka , and A. W. Neumann . 1983 . Determination of surface tension and contact angle from the shapes of axisymmetric fluid interfaces . Journal of Colloid and Interface Science 93 ( 1 ): 169 – 183 .
- Rusanov , A. I. and V. A. Prokhorov . 1996 . Interfacial tensiometry . Amsterdam : Elsevier .
- Schreyeck , G. and P. Marie . 1999 . Kinetics of the adsorption of a PDMS-g-PEO copolymer at the PDMS/PEO interface . Langmuir 15 ( 23 ): 8212 – 8219 .
- Selimovic , S. , Y. Jia , and S. Fraden . 2009 . Measuring the nucleation rate of Lysozyme using microfluidics . Crystal Growth & Design 9 ( 4 ): 1806 – 1810 .
- Shia-Yen , T. , R. Lin , H. Lung-Hsin , and A. P. Lee . 2008 . Droplet microfluidics . Lab on a Chip 8 ( 2 ): 198 – 220 .
- Takinoue , M. M. and S. Takeuchi . 2011 . Droplet microfluidics for the study of artificial cells . Analytical and Bioanalytical Chemistry 400 ( 6 ): 1705 – 1716 .
- Tropea , C. , A. L. Yarin , and J. F. Foss . 2007 . Handbook of experimental fluid mechanics . Berlin : Springer-Verlag .
- Vela , E. , M. Hafez , and S. Regnier . 2009 . Laser-Induced thermocapillary convection for mesoscale manipulation . International Journal of Optomechatronics 3 ( 4 ): 289 – 302 .
- Woodward , R. P. Surface tension measurements using the drop shape method . Accessed October 10, 2010. http://www.firsttenangstroms.com/pdfdocs/STPaper.pdf .
- Xie , J.-C. , H. Lin , P. Zhang , F. Liu , and W.-R. Hu . 2005 . Experimental investigation on thermocapillary drop migration at large Marangoni number in reduced gravity . Journal of Colloid and Interface Science 285 ( 2 ): 737 – 743 .
- Yakhshi-Tafti , E. , H. J. Cho , and R. Kumar . 2010 . Droplet actuation on a liquid layer due to thermocapillary motion: Shape effect . Applied Physics Letters 96 ( 26 ): 264101.1 – 3 .
- Young , N. O. , J. S. Goldstein , and M. J. Block . 1959 . The motion of bubbles in a vertical temperature gradient . Journal of Fluid Mechanics Digital Archive 6 ( 3 ): 350 – 356 .
SUPPLEMENTARY MATERIAL
“TensionCalculator.xmcd” is a MathCad spreadsheet including the procedure for solving the theoretical drop curve equations. For a given pair of fluids, the surface (interfacial tension) is found from the gravitation parameter that results in the best overlap between the actual drop profile and the theoretical curve. “BinaryImage.dat” is a sample input file generated from applying edge detection to drop images.