Abstract
The bactericidal properties of chemically patterned lithium niobate substrates under a super-bandgap UV light source is established. UV irradiation of lithium niobate surfaces inoculated with bacteria leads to antimicrobial activity compared to a glass substrate under similar conditions, as determined by surface enhanced Raman spectroscopy and corroborated with a fluorescence-based live/dead assay. This finding may expand the possible biomedical applications of lithium niobate.
Keywords:
1. Introduction
Developing functional materials that inhibit microbial attachment and growth is of particular interest for human healthcare, public security, and food safety applications.[Citation1,Citation2] Several methods have been developed to create surfaces resistant to bacterial adhesion and colonization, e.g., by incorporating Ag.[Citation3] However, the use of Ag in the healthcare sector must be undertaken with caution due to the toxic effect of Ag.[Citation3] Semiconductor photocatalysis is also a promising antibacterial agent and has been used to degrade pollutants and eliminate microbes.[Citation3,Citation4] In particular, titanium dioxide (TiO2) nanomaterials are attractive for photocatalysis applications, where photogenerated charge accumulates on TiO2 surfaces upon irradiation with super-bandgap UV light leading to the formation of hydroxyl and hydroperoxide radicals (i.e., reactive oxygen species (ROS)). Elevated levels of ROS are a known bacteria disinfection pathway wherein the ROS disrupts bacterial cell function and damages the bacteria.[Citation4,Citation5] However, the drawback of TiO2-based photocatalytic nanomaterials is the relatively fast recombination time that necessitates continuous UV irradiation.[Citation1] Ferroelectric ceramics such as barium titanate and lead strontium zirconate titanate powders have also been used as photocatalytic antibacterial agents.[Citation6] The polarization-induced internal electric field of these ferroelectric materials facilitates the separation and migration of photo-induced electron-hole pairs.[Citation6] Ferroelectric lithium niobate (LN) powder has been reported to have high antimicrobial activity under cyclical thermal excitation that has been attributed to the pyroelectrocatalytic activity of the LN powder.[Citation7] Briefly, a change in temperature causes a change in polarization charge of LN particles, converting temperature changes into electrical energy that can drive catalytic reactions and the formation of ROS.[Citation7] In this context, LN, which has been demonstrated to be a suitable substrate for patterned photodeposition of Ag nanoparticles for Raman-based sensing and photocatalysis,[Citation8–10] provides a multi-functional platform through which to explore antimicrobial response.
In this work, we utilize periodically proton-exchanged LN (PPELN), on which both Ag and Au nanoparticles can be selectively deposited for the fabrication of metallic nanostructure arrays for Raman-based sensing of molecules[Citation9] with up to single-molecule sensitivity.[Citation11] We have further demonstrated PPELN as a cytocompatible[Citation12] substrate with tunable surface wettability,[Citation13] making it suitable for biosensing applications. Interestingly, the lifetime of photogenerated charge carriers in LN (hundreds of milliseconds to tens of seconds in LN[Citation14]) is longer than in TiO2 (pico- to microseconds in TiO2[Citation15]) resulting in longer lasting photocatalytic activity.[Citation10] Surface-enhanced Raman spectroscopy (SERS), known for its high sensitivity and rich intrinsic molecular resolution, which are important factors for bacteria detection,[Citation16–18] is implemented here to study the antimicrobial effect of PPELN. We demonstrate that PPELN with Ag and Au nanoparticles enables SERS detection of the presence of biofilms and allows live and dead bacteria to be distinguished. PPELN substrates with Ag nanoparticles are found to be highly antimicrobial. PPELN is further shown to have enhanced antimicrobial effects under UV irradiation. These findings may widen the use of PPELN in fields where bacterial detection or disinfection is required.
2. Materials and methods
2.1. PPELN
500 μm thick z-cut LN (CasTech Inc., China) was used to fabricate PPELN substrates on the –z surface via proton exchange in molten benzoic acid at 200 °C for 1440 minutes through photolithographically-defined reactive ion etching (RIE) openings (6.09 μm period; 50% duty cycle) in a titanium mask. After removing the mask by exposing the sample to hydrofluoric acid (48% solution in water) for 10 seconds, the surface comprised alternating stripes of LN and PE regions. The PE regions consisted of a central RIE region (i.e., proton exchange through the mask opening) ∼2.9 μm deep with lateral diffusion (LD) regions on either side (i.e., proton exchange under the mask).
2.2. Metal nanoparticle deposition
Ag nanoparticles and Au nanoparticles were photodeposited onto the PPELN surface. Prior to photodeposition, PPELN samples were dried with compressed nitrogen after being sonicated for 20 minutes each in acetone, isopropanol, deionized water, and ethanol. PPELN samples were placed on a glass slide, and 150 µl of 0.01 M AgNO3 solution (for Ag nanoparticles) or 0.01 M HAuCl4 (Sigma-Aldrich) (for Au nanoparticles) was pipetted onto the sample surface. The samples were illuminated with a 254 nm UV light source (11SC-2, Spectroline) located 2 cm above the surface for 10 minutes for the Ag nanoparticles and 20 minutes for the Au nanoparticles. The energy of this irradiation (4.88 eV) is greater than the bandgap of LN, ∼3.9 eV. After irradiation, the samples were blown dry with nitrogen after being immersed in deionized water for 1 minute. PPELN samples were reused following the cleaning steps described above, with an additional step of gently rubbing the sample with lens paper soaked in isopropanol in-between the acetone and isopropanol sonication steps and verifying by atomic force microscope (AFM) imaging that the cleaning steps resulted in surfaces free of nanoparticles.
For Au nanoparticle deposition on a glass slide (glass-Au): 150 µl of 15 nm diameter gold nanoparticle colloidal solution (777137, Sigma-Aldrich) was deposited on a 2 cm × 1 cm glass substrate and dried before use. Prior to Au nanoparticle deposition, the glass slide was sonicated for 20 minutes each in acetone, isopropanol, deionized water, and ethanol before being dried with compressed nitrogen.
All PPELN and glass slide samples with nanoparticles were immersed in ethanol for 3 minutes to disinfect them before exposure to bacteria and then washed three times in maximum recovery diluent (MRD) (OxoidCM0733) to remove the residual ethanol. This disinfection step and subsequent bacteria experiments were performed inside a UV-sterilized hood.
2.3. Atomic force microscopy
Amplitude modulation AFM (MFP-3D, Asylum Research) was used to image the surface topography and measure the surface roughness of each sample before and after photodeposition. AFM was employed here with cantilevers having a typical resonant frequency in air of ∼ 330 kHz and a spring constant of ∼ 42 N/m (PPP-NCH, Nanosensors). Roughness was determined as the mean and standard deviation of three 20 µm × 20 µm images.
2.4. Bacteria cultures and preparation
E. coli (ATCC25922) colonies were cultivated at 150 rpm in lysogeny broth (LB) medium (100 mL in 250 mL flasks) and then incubated for 24 hours at 37 °C. Bacteria were harvested in the stationary phase after cultivation for 24 hours, collected by centrifugation (3,000 rpm, 4 °C, 10 minutes) and washed three times in MRD to remove the residual LB medium. Bacteria were resuspended in MRD to a concentration equivalent to 108 colony-forming units per mL (108 cfu/mL). The suspension was then used for adhesion and biofilm cultivation.
2.5. Adhesion and biofilm cultivation
200 μL of the prepared suspension was added on the surface of PPELN, PPELN-Ag, and PPELN-Au and cultivated at room temperature for 1 hour to allow bacteria to adhere. For biofilm formation, substrates were subsequently immersed in LB medium and incubated without shaking at room temperature for 24 hours. The total incubation time was therefore 25 hours. The surfaces were gently washed three times with MRD after incubating to remove suspended bacteria and residual LB medium.
2.6. Staining bacteria in suspension
The live/dead viability kit (BacLight L7007, ThermoFisher) was used to examine the distribution of dead and live bacteria and observe biofilm formation. The kit employs two nucleic acid stains – a green fluorescent SYTO 9 stain and a red fluorescent propidium iodide (PI) stain – that differ in their ability to penetrate bacteria. SYTO 9 penetrates both live and dead bacteria whereas PI penetrates only bacteria with damaged membranes, thereby reducing SYTO 9 fluorescence when both dyes are present.[Citation19] Therefore, live bacteria with intact membranes exhibit a green fluorescence, while dead bacteria with damaged membranes display a red fluorescence. We prepared the stain solution by thoroughly mixing the entire 6 μL solution with 1 mL of filter-sterilized distilled water in a 16 mm × 125 mm glass culture tube. To distinguish between live and dead bacteria, antimicrobial test experiments were conducted by first pipetting 100 μL of each of the suspension mixtures into separate wells of a 12-well flat-bottom microplate containing PPELN substrates. After incubating for 24 hours at room temperature, 100 μL of the stain solution was pipetted using a new pipette tip for each well. The microplate was kept at room temperature in the dark for 15 minutes before capturing fluorescence images.
2.7. Fluorescence microscopy
Fluorescence images were captured using a Nikon Eclipse E400 optical microscope equipped with a CooLED PE300 white LED fluorescence source. Dual emission filters were used for simultaneous viewing of SYTO 9 and PI stains.
2.8. Raman spectroscopy
A 632.8 nm He–Ne excitation laser combined with a spectrograph (IsoPlane 630, Princeton Instruments), a back-illuminated electron multiplying charge-coupled device camera (ProEM-HS:512BX3, Princeton Instruments), and an optical microscope (IX71, Olympus) with a 100 × 0.8 NA objective were employed to record SERS spectra. The laser power at the sample was 0.5 mW. All Raman spectra were collected under the same conditions. The average spectrum of 10 measurements is displayed. Intensities at specific wavenumbers are reported as the mean and standard deviation from 3 spectra. Raman was collected starting 10 minutes after pipetting 200 μL of the 108 cfu/mL suspension.
2.9. Irradiation of E. coli bacteria on substrates
E. coli was deposited on PPELN-Au and glass-Au before irradiating the surface. The 254 nm UV lamp (11SC-2, Spectroline) was placed 2 cm from the sample and positioned to irradiate the same location from where Raman would be measured. The energy of this irradiation (4.88 eV) is greater than the bandgap of LN, ∼3.9 eV. The SERS spectra of PPLEN-Au and glass-Au surfaces were first measured 5 minutes after adding 200 μL of the 108 cfu/mL E. coli bacteria suspension on these two surfaces. This first Raman acquisition is considered as 0 minutes of irradiation. PPELN-Au and glass-Au were then irradiated with 254 nm UV light for 2 minutes, after which the lamp was turned off and the second Raman acquisitions were recorded. The UV light was subsequently turned on again to irradiate the surface for an additional 2 minutes, followed by a Raman measurement acquired while the UV lamp was turned off. This Raman spectrum is considered as 4 minutes of in-situ irradiation. The on/off sequence was continued for 18 minutes of total irradiation time.
2.10. Contact angle measurements
A contact angle measuring system (DSA10, Krüss, Germany) was employed here to measure the contact angles of sessile 2 μL deionized water droplets on PPELN surfaces. Contact angle measurements are reported as the mean and standard deviation of 3 measurements.
3. Results and discussion
3.1. AFM of substrates
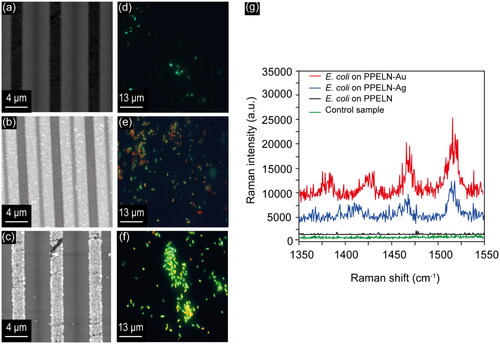
The AFM surface topography images for PPELN samples before and after Ag and Au photodeposition are shown in . Ag nanoparticle deposition occurred in the LD region where the electrons accumulated, as reported before,[Citation9] leading to an increase in surface roughness from 0.6 ± 0.1 to 1.1 ± 0.2 nm after Ag nanoparticle deposition. Au nanoparticle deposition occurred in the RIE region, as reported before,[Citation20] leading to an increase in surface roughness after Au nanoparticle deposition from 0.6 ± 0.1 to 19 ± 3 nm.
Figure 1. AFM images of PPELN (a) before deposition (PPELN), (b) after Ag deposition (PPELN-Ag), and (c) after Au deposition (PPELN-Au). Ag nanoparticle deposition occurred in the LD regions whereas Au nanoparticle deposition occurred in the RIE regions (i.e., the surface area covered by Ag is larger than that covered by Au). Fluorescence images of E. coli on (d) PPELN, (e) PPELN-Ag, and (f) PPELN-Au. (g) Raman spectra from E. coli on PPELN-Au (red), PPELN-Ag (blue), and PPELN (black), and also from PPELN with media and MRD in the absence of E. coli (Control sample; green).
3.2. Fluorescence from E. coli on substrates
To assess the E. coli viability, a live/dead assay was used. Images were acquired following an incubation time of 24 hours and a staining time of 1 hour. Fluorescence images of E. coli on PPELN, PPELN-Ag, and PPELN-Au are shown in , respectively. The percentage of live bacteria (green) was ∼10% on PPELN without nanoparticles, ∼30% on PPELN-Ag and ∼80% on PPELN-Au. These findings may be due to the difference in the surface roughness and chemistry between PPELN-Au, PPELN-Ag, and PPELN, which affects wettability.[Citation13] The contact angles were measured to be 84.2 ± 2.4° for PPELN-Ag, 59.9 ± 1.5° for PPELN-Au, and 58.3 ± 2.3° for PPELN, findings consistent with reports that E. coli bacteria attach preferentially to surfaces that are less hydrophillic.[Citation21]
3.3. Raman from E. coli on PPELN substrates
Raman spectra of E. coli on PPELN before and after deposition are shown in . A control sample has been prepared from Ag nanoparticles on PPELN immersed in LB media without using bacteria, which was then washed three times in MRD. The SERS spectrum of E. coli on PPELN-Au (, red) shows an enhanced signal compared to PPELN-Ag (, blue). However, almost no Raman peaks can be detected from bacteria on PPELN (, black) and the control sample (, green). The increased SERS signal on PPELN-Au compared to PPELN-Ag is accompanied by a noticeable decrease in the number of viable bacteria on PPELN-Ag.
The SERS signal of E. coli on PPELN-Ag and PPELN-Au showed bands associated with lipids, proteins, and DNA/RNA in bacterial cells.[Citation22] The peak at 1548 cm−1 is associated tryptophan N–H, C–H bending, and amide II.[Citation23,Citation24] Additionally, the peak at 1522 cm−1 is associated with cytosine DNA/RNA, the peak at 1455 cm−1 is associated with lipids (CH2 bending), and the peak at 1402 cm−1 is associated with COO2 symmetric stretching.[Citation23,Citation24] Finally, the peak at 1365 cm−1 is associated with COO and C–H bending of a protein.[Citation24,Citation25]
3.4. Raman-based discrimination between live and dead bacteria
The influence of PPELN-Ag and PPELN-Au on bacterial SERS spectral variance with time was investigated to distinguish between live and dead bacteria. To compare the effect of PPELN-Ag and PPELN-Au on E. coli bacteria, a plot of SERS signal at different times after adding E. coli is shown in . Background-subtracted intensity for the 1455.5 cm−1 lipid CH2 bending peak from E. coli on PPELN-Ag (black) and for 1453.5 cm−1 lipid CH2 bending peak from E. coli on PPELN-Au (red) is plotted in as a function of time. It is clear from the graph that the SERS signal of E. coli on PPELN-Ag dropped significantly (in the wavelength range between 1400–1600 cm−1) after 50 minutes. The reduced SERS signal of bacteria has been attributed to alterations to the bacterial cell wall and the loss of membrane potential during bacteria death.[Citation22] This suggests that the E. coli bacteria have been killed[Citation22] during this 50-minute period due to the effects of PPELN-Ag and laser irradiation. The SERS signal from E. coli on PPELN-Au dropped slightly during these 50 minutes, but the intensity was still higher than the SERS intensity from E. coli on PPELN-Ag after 10 minutes. This corroborates the observation that PPELN-Au has a less toxic effect on bacteria than PPELN-Ag. However, it has been previously reported that SERS spectra of bacteria on Au nanoparticles were stable. Only small spectral changes were observed during 4 hours, which might be due to the small changes in the physiological state of bacteria during these 4 hours.[Citation26] Here, a slightly faster spectral change of bacteria on PPELN-Au during 1 hour was observed, which might be due to the role of PPELN or the laser irradiation during Raman measurements. To study the effect of laser exposure on E. coli on PPELN-Au, a plot of SERS signal of E. coli on glass-Au as a function of irradiation time is shown in . It is clear from the graph that the SERS signal of E. coli on glass-Au dropped more slowly than the SERS signal of E. coli on PPELN-Au. Thus, PPELN has a more significant effect on dropping the SERS signal of E. coli on PPELN-Au than the laser, as will be discussed later in this paper.
Figure 2. (a) Background-subtracted intensity for the 1455.5 cm−1 peak from E. coli on PPELN-Ag (black) and for the 1453.5 cm−1 peaks from E. coli on PPELN-Au (red) and glass-Au (green) as a function of time. The 0-minute SERS spectra were collected 10 minutes after adding bacteria to the PPELN substrates; spectra were subsequently recorded from the same point every 10 minutes for 1 hour. Fluorescence images of E. coli on PPELN-Ag at (b) 20 minutes, (c) 40 minutes, and (d) 60 minutes.
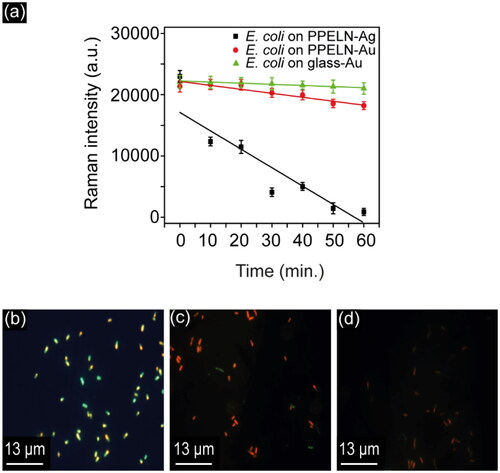
The live/dead bacterial viability kit was used to study the antimicrobial effect of PPELN-Ag on E. coli within 1 hour. As shown in , the green fluorescence in viable bacteria at 20 minutes ()) turned to the red color of dead bacteria within 40 minutes (), which shows the antimicrobial effect of PPELN-Ag on E. coli. The smaller size of the dead bacteria when exposed to PPELN-Ag, highlights the morphological changes ().[Citation27] At later times of exposure (60 minutes), the dead bacteria disappeared ()), due to the start of the cell lysis process.[Citation27]
The mechanism of Ag nanoparticle toxicity in environmental and biological media is affected by dissolved oxygen, pH, and other organic matter in the media. Exposure to light such as laser light can also alter the toxicity of Ag nanoparticles, presumably by light-induced transformation of Ag nanoparticles.[Citation28] One of the main changes that Ag nanoparticles undergo in environmental and biological media is surface oxidation and the release of Ag+ ions. Furthermore, Ag atoms (Ag0) on the surface of Ag nanoparticles can be oxidized in the presence of oxygen molecules and interact with other redox-active compounds to release Ag+. Also, the oxidation of Ag and the release of Ag+ ions can occur in environmental media, biological media, as well as inside the bacteria. Thus, Ag nanoparticles, whether as individual particles or as agglomerates/aggregates, can also be viewed as a source of Ag+ ions through a slow-release process.[Citation28] Furthermore, it has been found that when E. coli were present, the concentration of Ag+ ions decreased to a significantly lower value than that without E. coli as Ag+ ions bind to bacteria.[Citation27] This binding could induce inactivation of bacterial enzymes. Spectral changes of bacteria with increasing incubation time in PPELN-Ag nanoparticles are attributed to the toxicity of the released Ag+ ions.[Citation26]
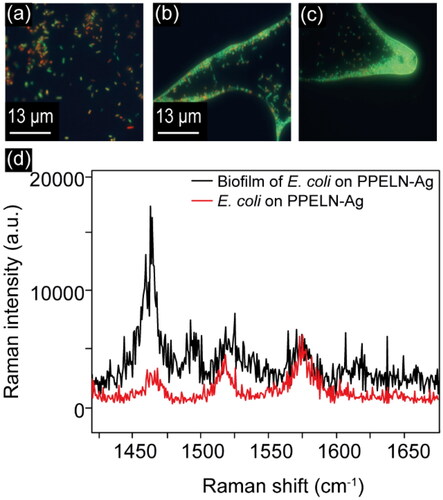
3.5. SERS for biofilm detection on PPELN-Ag
SERS spectra of Ag nanoparticles deposited on PPELN were used to evaluate the chemical components in the matrix of biofilm and distinguish them from the initially attached bacteria to highlight the ability of PPELN to identify biofilm matrix components. Here, E. coli bacteria have been incubated for a total of 25 hours on PPELN-Ag. Fluorescence images before ()) and after 25 hours of incubation on PPELN-Ag () show the formation of a biofilm in which the bacteria predominantly have intact bacterial cell walls. SERS results ()) show that the peak intensities of lipids at 1460 cm−1 in the biofilm matrix increased significantly compared to the same peaks 10 minutes after E. coli were added to PPELN-Ag. It was found previously that E. coli bacteria expressed high amounts of lipid in the biofilm matrix.[Citation29] Protein enrichment was found after incubation for 25 hours with peaks of amide I and amide II, at 1619 and 1571 cm−1 becoming more pronounced, in agreement with a previous study.[Citation29] This enhancement is due to the growth of a hydrogel-like biofilm matrix by bacteria, which contains extracellular polymeric substances.[Citation30] Furthermore, the SERS spectrum of the biofilm shows a significant peak at ∼ 1480 cm−1, which is missing from the spectrum of the initially attached bacteria. This peak is associated with C–H bonds in proteins,[Citation31] and might be caused by an increased concentration of proteins in extracellular polymeric substances, which consist of polysaccharides, proteins, nucleic acids, lipids, as well as humic-like substances.[Citation30] This detection of the variation of the chemical composition of E. coli on PPELN during biofilm formation might widen the use of PPELN in different areas such as in clinics, marine and food industries.[Citation29,Citation32]
3.6. The antibacterial activities of PPELN
To investigate the antibacterial properties of PPELN, SERS and fluorescence studies for E. coli on PPELN-Au and glass-Au were carried out while irradiating these surfaces with UV light. Due to the fast antimicrobial effect of PPELN-Ag on E. coli under irradiation of UV light, PPELN-Au was used here to monitor E. coli death. Plotting the SERS intensity against UV irradiation time ()) shows a decrease of E. coli SERS signal on PPELN-Au after 2 minutes irradiation and disappearance of this signal after 4 minutes irradiation. In contrast, the SERS signal on glass-Au disappeared after 14 minutes of irradiation.
Figure 4. (a) Background-subtracted intensity for 1520 cm−1 from E. coli on PPELN-Au (black square) and from E. coli on glass-Au (red circle) as a function of irradiation time. Fluorescence images of E. coli on PPELN-Au at (b) 0 minutes, (c) 2 minutes, and (d) at 4 minutes. Fluorescence images of E. coli on glass-Au at 0 minute, (f) 4 minutes, and (g) at 14 minutes.
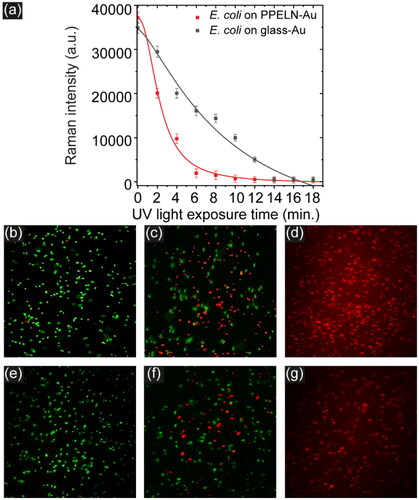
The classical use of UV light for sterilization is to cause inactivation of microorganisms by forming pyrimidine dimers in RNA and DNA, which can interfere with transcription and replication.[Citation33] This work shows that killing bacteria by UV light on PPELN-Au is faster than on glass-Au, due to the antibacterial role of PPELN. This effect originates from the energetic charge carriers (electron-hole pairs) that form on the PPELN surface upon illuminating the surface with a UV light source of energy higher than the LN bandgap, which damage the bacteria.[Citation1] Fluorescence images () confirm the effect of UV light to kill bacteria on PPELN within 4 minutes of irradiation. It is clear from the images that there are significant reductions in live bacterial populations after 2 minutes irradiation with UV light. The percentage of live bacteria decreased from 97.1% to 30.8% after 2 minutes irradiation ()) and all bacteria died after 4 minutes of irradiation ()). In contrast, the fluorescence images ()) for E. coli on glass-Au show no significant reductions in microbial populations after 4 minutes of irradiation when compared to the E. coli on PPELN-Au.
Regarding the possible mechanism of E. coli disinfection on a ferroelectric surface, besides the disruption or damage of various functions or structures by ROS, it has been reported that the electrostatic field can perforate the bacterial cell wall, causing death, when pyroelectric materials and bacteria are in intimate contact.[Citation6] In this work, we observe that the disinfection properties can be enhanced through the photo-generation of charge carriers. Further work might establish the relative roles of photogenerated charge and pyroelectric effects on the disinfection mechanism of LN surfaces.
4. Summary
PPELN-based templates are demonstrated as effective SERS substrates for bacterial detection, enabling label-free detection and distinction between live and dead bacteria and the presence of biofilms. PPELN substrates with Au nanoparticles lead to a reduction in SERS intensity compared to glass with Au nanoparticles, whereas PPELN with Ag nanoparticles completely disinfects the surface in around one hour. PPELN surfaces with photodeposited metal nanoparticles irradiated by super-bandgap UV light are shown to be highly antimicrobial. Disinfection under irradiation occurs three times faster on PPELN with Au nanoparticles than on the glass/Au nanoparticle control. Impressively, disinfection by PPELN with Ag nanoparticles under UV irradiation happened faster than we could observe and merits further investigation. Future efforts should elucidate the mechanism of disinfection in order to exploit the antimicrobial properties of lithium niobate crystals, thin films, and powders.
Additional information
Funding
References
- Prakash, J.; Sun, S.; Swart, H. C.; Gupta, R. K. Noble metals-TiO2 nanocomposites: from fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl. Mater. Today. 2018, 11, 82–135. DOI: 10.1016/j.apmt.2018.02.002.
- Monteiro, D. R.; Gorup, L. F.; Takamiya, A. S.; Ruvollo-Filho, A. C.; de Camargo, E. R.; Barbosa, D. B. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents. 2009, 34, 103–110. DOI: 10.1016/j.ijantimicag.2009.01.017.
- Stobie, N.; Duffy, B.; McCormack, D. E.; Colreavy, J.; Hidalgo, M.; McHale, P.; Hinder, S. J. Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol-gel coating. Biomaterials 2008, 29, 963–969. DOI: 10.1016/j.biomaterials.2007.10.057.
- Yu, J. C.; Ho, W.; Lin, J.; Yip, H.; Wong, P. K. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. DOI: 10.1021/es0259483.
- Van Acker, H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017, 25, 456–466. DOI: 10.1016/j.tim.2016.12.008.
- Kushwaha, H. S.; Halder, A.; Jain, D.; Vaish, R. Visible light-induced photocatalytic and antibacterial activity of Li-doped Bi0.5Na0.45K0.5TiO3-BaTiO3 ferroelectric ceramics. J. Elec. Mater. 2015, 44, 4334–4342. DOI: 10.1007/s11664-015-4007-y.
- Gutmann, E.; Benke, A.; Gerth, K.; Böttcher, H.; Mehner, E.; Klein, C.; Krause-Buchholz, U.; Bergmann, U.; Pompe, W.; Meyer, D. C.; et al. Pyroelectrocatalytic disinfection using the pyroelectric effect of nano- and microcrystalline LiNbO3 and LiTaO3 particles. J. Phys. Chem. C 2012, 116, 5383–5393. DOI: 10.1021/jp210686m.
- Damm, S.; Carville, N. C.; Rodriguez, B. J.; Manzo, M.; Gallo, K.; Rice, J. H. Plasmon enhanced Raman from Ag nanopatterns made using periodically poled lithium niobate and periodically proton exchanged template methods. J. Phys. Chem. C 2012, 116, 26543–26550. DOI: 10.1021/jp310248w.
- Carville, N. C.; Manzo, M.; Damm, S.; Castiella, M.; Collins, L.; Denning, D.; Weber, S. A. L.; Gallo, K.; Rice, J. H.; Rodriguez, B. J.; et al. Photoreduction of SERS-active metallic nanostructures on chemically patterned ferroelectric crystals. ACS Nano. 2012, 6, 7373–7380. DOI: 10.1021/nn3025145.
- Al-Shammari, R. M.; Baghban, M. A.; Al-Attar, N.; Gowen, A.; Gallo, K.; Rice, J. H.; Rodriguez, B. J. Photoinduced enhanced Raman from lithium niobate on insulator template. ACS Appl. Mater. Interfaces. 2018, 10, 30871–30878. DOI: 10.1021/acsami.8b10076.
- Al-Attar, N.; Al-Shammari, R. M.; Manzo, M.; et al. Wide-Field Surface-Enhanced Raman Scattering from Ferroelectrically Defined Au Nanoparticle Microarrays for Optical Sensing. 2018:AF2M.5.
- Carville, N. C.; Collins, L.; Manzo, M.; Gallo, K.; Lukasz, B. I.; McKayed, K. K.; Simpson, J. C.; Rodriguez, B. J. Biocompatibility of ferroelectric lithium niobate and the influence of polarization charge on osteoblast proliferation and function. J. Biomed. Mater. Res. A 2015, 103, 2540–2548. DOI: 10.1002/jbm.a.35390.
- Al-Shammari, R. M.; Manzo, M.; Gallo, K.; Rice, J. H.; Rodriguez, B. J. Tunable wettability of ferroelectric lithium niobate surfaces: the role of engineered microstructure and tailored metallic nanostructures. J. Phys. Chem. C 2017, 121, 6643–6649. DOI: 10.1021/acs.jpcc.6b12336.
- Volk, T.; Wohlecke, M. Lithium niobate: defects, photorefraction and ferroelectric switching. Lithium Niobate: Defects, Photorefraction and Ferroelectric Switching. Springer Series in Materials Science. 2008, 115, 1–247. https://doi.org/10.1007/978-3-540-70766-0
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron-hole recombination time at TiO2 single-crystal surfaces: influence of surface band bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957. DOI: 10.1021/jz500770c.
- Mosier-Boss, P. A. Review on SERS of Bacteria. Biosensors (Basel) 2017, 7, 51. DOI: 10.3390/bios7040051.
- Miccio, L.; Marchesano, V.; Mugnano, M.; Grilli, S.; Ferraro, P. Light induced DEP for immobilizing and orienting Escherichia coli bacteria. Opt. Lasers Eng. 2016, 76, 34–39. DOI: 10.1016/j.optlaseng.2015.03.025.
- Alattar, N.; Daud, H.; Al-Majmaie, R.; Zeulla, D.; Al-Rubeai, M.; Rice, J. H. Surface-enhanced Raman scattering for rapid hematopoietic stem cell differentiation analysis. Appl. Opt. 2018, 57, E184–E189. DOI: 10.1364/AO.57.00E184.
- Gatti, M.; Bernini, V.; Lazzi, C.; Neviani, E. Fluorescence microscopy for studying the viability of micro-organisms in natural whey starters. Lett. Appl. Microbiol. 2006, 42, 338–343. DOI: 10.1111/j.1472-765X.2006.01859.x.
- Carville, N. C.; Neumayer, S. M.; Manzo, M.; Gallo, K.; Rodriguez, B. J. Biocompatible gold nanoparticle arrays photodeposited on periodically proton exchanged lithium niobate. ACS Biomater. Sci. Eng. 2016, 2, 1351–1356. DOI: 10.1021/acsbiomaterials.6b00264.
- Tuson, H. H.; Weibel, D. B. Bacteria-surface interactions. Soft Matter. 2013, 9, 4368–4380. DOI: 10.1039/C3SM27705D.
- Zhou, H.; Yang, D.; Ivleva, N. P.; Mircescu, N. E.; Schubert, S.; Niessner, R.; Wieser, A.; Haisch, C. Label-free in situ discrimination of live and dead bacteria by surface-enhanced Raman scattering. Anal. Chem. 2015, 87, 6553–6561.
- Mushtaq, A.; Nawaz, H.; Majeed, M. I.; et al. Surface-enhanced raman spectroscopy (SERS) for monitoring colistin-resistant and susceptible E. coli strains. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy 2022, 278, 121315. DOI: 10.1016/j.saa.2022.121315
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. DOI: 10.1016/j.bios.2017.02.032.
- Walter, A.; März, A.; Schumacher, W.; Rösch, P.; Popp, J. Towards a fast, high specific and reliable discrimination of bacteria on strain level by means of SERS in a microfluidic device. Lab Chip. 2011, 11, 1013–1021. DOI: 10.1039/c0lc00536c.
- Cui, L.; Chen, S. D.; Zhang, K. S. Effect of toxicity of Ag nanoparticles on SERS spectral variance of bacteria. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 137, 1061–1066. DOI: 10.1016/j.saa.2014.08.155.
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-phase bactericidal mechanism of silver nanoparticles against Burkholderia pseudomallei. PLoS One. 2016, 11, e0168098. DOI: 10.1371/journal.pone.0168098.
- McShan, D.; Ray, P. C.; Yu, H. T. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. DOI: 10.1016/j.jfda.2014.01.010.
- Keleştemur, S.; Avci, E.; Çulha, M. Raman and surface-enhanced raman scattering for biofilm characterization. Chemosensors 2018, 6, 5. DOI: 10.3390/chemosensors6010005.
- Chao, Y. Q.; Zhang, T. Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: from initial attachment to mature biofilm. Anal. Bioanal. Chem. 2012, 404, 1465–1475. DOI: 10.1007/s00216-012-6225-y.
- Goeller, L. J.; Riley, M. R. Discrimination of bacteria and bacteriophages by Raman spectroscopy and surface-enhanced Raman spectroscopy. Appl. Spectrosc. 2007, 61, 679–685. DOI: 10.1366/000370207781393217.
- Efeoglu, E.; Culha, M. In situ-monitoring of biofilm formation by using surface-enhanced Raman scattering. Appl. Spectrosc. 2013, 67, 498–505. DOI: 10.1366/12-06896.
- Pereira, R. V.; Bicalho, M. L.; Machado, V. S.; Lima, S.; Teixeira, A. G.; Warnick, L. D.; Bicalho, R. C. Evaluation of the effects of ultraviolet light on bacterial contaminants inoculated into whole milk and colostrum, and on colostrum immunoglobulin G. J. Dairy Sci. 2014, 97, 2866–2875. DOI: 10.3168/jds.2013-7601.