Abstract
These practical guidelines for the biological treatment of unipolar depressive disorders in primary care settings were developed by an international Task Force of the World Federation of Societies of Biological Psychiatry (WFSBP). They embody the results of a systematic review of all available clinical and scientific evidence pertaining to the treatment of unipolar depressive disorders and offer practical recommendations for general practitioners encountering patients with these conditions. The guidelines cover disease definition, classification, epidemiology and course of unipolar depressive disorders, and the principles of management in the acute, continuation and maintenance phase. They deal primarily with biological treatment (including antidepressants, other psychopharmacological and hormonal medications, electroconvulsive therapy, light therapy).
Contents
Abstract………………………………………………………………………….…....67
Executive summary of recommendations………………………………………………..68
General recommendations………………………………………………………68
Biological treatment recommendations…………………………………………..69
1 Unipolar depressive disorders………………………………………………………69
1.1 Introduction…………………………………………………………....69
1.2 Goal and target audience of WFSBP guidelines…………………….…..70
1.3 Methods of literature research and data extraction………………….…..70
1.4 Evidence-based classification of recommendations……………………...70
1.5 Epidemiology and course of major depressive disorder………………....71
1.6 Indications and goals of treatment for major depressive disorder………...73
2 Acute-phase treatment of major depressive disorder………………………….……..74
2.1 Antidepressants…………………………………………………….…..74
2.2 Herbal remedies…………………………………………………….….82
2.3 Electroconvulsive therapy………………………………………….…...82
2.4 Psychotherapy…………………………………………………….…...82
2.5 Light therapy……………………………………………………….…..83
2.6 Adjunctive therapy………………………………………………….….84
2.7 Other treatment options………………………………………………...85
3 Continuation-phase treatment of major depressive disorder…………………….……85
4 Maintenance-phase treatment of major depressive disorder……………………….…85
4.1 General treatment principles of maintenance treatment……………….….85
4.2 Pharmacotherapy of maintenance treatment………………………….….86
4.3 Duration and discontinuation of maintenance treatment…………………..89
4.4 Switching from unipolar depression to bipolar disorder……………….….90
4.5 Psychotherapy……………………………………………………….…90
5 Treatment of chronic depressive disorders……………………………………….…..90
5.1 Dysthymic disorder……………………………………………………..90
5.2 Pharmacotherapy of dysthymic disorder………………………………...91
6 Treatment in special circumstances…………………………………………….…….91
6.1 Comorbidity of depression with other psychiatric disorders……………...91
6.2 Treatment of depression in older adults………………………………….92
6.3 Treatment-resistant depression………………………………………….92
6.4 Treatment of depression in children and adolescents……………………..93
6.5 Treatment during pregnancy and breast-feeding………………………….93
Update of guideline……………………………………………………………………....94
References……………………………………………………………………………….94
Executive summary of recommendations
General recommendations
For patients who meet the diagnostic criteria for a depressive episode (ICD-10) or a major depressive disorder (DSM-IV), biological treatment (pharmacological and non-pharmacological approaches) should in general be considered. Before treatment is begun, a comprehensive treatment plan should be developed on the basis of the patient's history and experience with previous treatments, current clinical subtype, current findings, severity of illness and risk of suicide. Concurrent psychiatric and somatic disorders, non-psychiatric medications or psychosocial stress factors should be thoroughly considered, as they can contribute to a depressive syndrome or interfere with treatment. Family history for mood disorders should be assessed. Whichever biological treatment intervention is chosen, clinical and psychiatric management should be initiated and continued throughout the treatment. This includes determining treatment plan and setting, establishing and maintaining a therapeutic alliance, monitoring and reassessing psychiatric status including risk of suicide, reassessing the adequacy of the diagnosis, monitoring the patient's treatment response, side effects and general medical condition, and educating patients and families as to the importance of adhering to treatment. The ultimate goal of the acute treatment phase is remission. After a period of about 2–4 weeks of antidepressant treatment response should be evaluated and, if insufficient, optimization strategies should be implemented. At least 8–10 weeks may be required to define the full extent of symptom reduction which must be achieved before entering the continuation phase of treatment. The more severe the depression is, the greater are the potential benefits derived from adequate treatment. The goal of continuation treatment is to prevent a relapse, to eliminate any residual symptoms and to restore the patient's prior level of psychosocial and occupational functioning. Maintenance (prophylactic) treatment is aimed at preventing a new episode of depression and suicide, and is definitely indicated in situations in which the risk of recurrence is high, i.e. for patients who have had three or more episodes of major depression and in patients with a high prior frequency of recurrences (e.g., two episodes within 5 years). Maintenance treatment may last from 3 years to a lifetime. In general, the graver the prognosis, the longer the maintenance therapy should last. Successful treatment of depressed patients with antidepressants includes educating the patients and the families about available treatment options, time of first noticible response, early side effects and what to do about them, and the expected course of treatment.
Biological treatment recommendations
Antidepressants are the first-line treatments for a major depressive episode (moderate to severe depressive episode). Depending on individual characteristics and/or requests of the patient, antidepressant treatment might also be indicated in mild depressive episodes, otherwise psycho- and sociotherapeutic approaches alone may be sufficient.
Factors to take into account when choosing an antidepressant are: the patient's prior experience with medication (response, tolerability, adverse effects), concurrent medical conditions and use of nonpsychiatric drugs, a drug's short- and long-term side effects, the physician's own experience with the medication, the patient's history of adherence to medication, history of first-degree relatives responding to a medication, patient preferences, and the costs and availability of specific antidepressants.
No one class of antidepressants has proved to be more effective or have a more rapid onset than another, although some tricyclic antidepressants (TCAs) (amitriptyline and clomipramine), and venlafaxine are slightly more effective than SSRIs in severely depressed hospitalized patients. Antidepressants differ considerably in their side effects profile, potential for interacting with other drugs and in the danger they pose when taken in overdose. Second-generation (e.g., bupropion, maprotiline, mianserin, trazodone) and third-generation (e.g., SSRIs, SNRIs, mirtazapine and reboxetine) (“newer”) antidepressants are generally tolerated better than are the first-generation (“older”) TCAs, and patients are thus less likely to discontinue them.
In at least 30% of depressive episodes, patients will not respond sufficiently to an adequately performed first-line treatment with any chosen antidepressant. This situation warrants a careful review of the correctness of diagnosis and sufficiency of drug dosing and compliance. Following this, potential strategies are (1) switching to another antidepressant from a different pharmacological class, (2) switching to another antidepressant within the same pharmacological class, (3) combining two antidepressants from different classes, (4) augmenting the antidepressant with other agents (e.g., lithium, thyroid hormone, pindolol, estrogen, buspirone, atypical antipsychotics) to enhance antidepressant efficacy, and (5) combining the antidepressant with a psychotherapeutic intervention. Of these alternatives, augmentation with lithium is the foremost and best documented strategy.
Electroconvulsive therapy (ECT) should be considered as a first-line strategy only in special situations calling for rapid relief from depression (e.g., severe psychotic depression, severe depression with psychomotor retardation, “true” treatment-resistant depression, persistent refusal of food, severe suicidality) and for patients with previous positive response to ECT. These patients should be referred to a specialist.
The medication of choice for the maintenance treatment of major depressive disorder (MDD) is either the same dose of the same antidepressant with which remission was achieved in the acute and continuation phase or lithium. In the latter case, serum lithium levels (at 12 h after last lithium intake) of 0.5–0.8 mmol/l (mEq/l) are usually recommended and should be monitored regularly. None of the other mood stabilizers used for bipolar affective disorders (e.g., valproate [divalproex], lamotrigine or gabapentin) have been studied for maintenance treatment of MDD in randomized-controlled trials. Although the data from controlled studies is still limited, results verify the efficacy of a variety of antidepressants (TCAs, SSRIs, SNRIs and other “newer” antidepressants) for dysthymic disorder.
1 Unipolar depressive disorders
1.1 Introduction
Unipolar depressive disorders present depressive symptoms only, without any manic symptomatology or history thereof. This distinguishes them from bipolar affective disorders. Unipolar depressive disorders have been classified into three main diagnostic groups (ICD-10 diagnoses, World Health Organization Citation1992; corresponding DSM-IV diagnoses (American Psychiatric Association Citation1994a) are given in parentheses):
depressive episode or recurrent depressive disorder (DSM-IV: major depressive disorder (MDD) – single episode or recurrent);
dysthymia (DSM-IV: dysthymic disorder and other chronic depressive disorders (MDD in incomplete remission and chronic MDD); and
depressive episode, unspecified, brief recurrent depressions (DSM-IV “subthreshold depressions”).
1.2 Goal and target audience of WFSBP guidelines
These WFSBP guidelines provide an update of contemporary knowledge of unipolar depressive disorders and evidence-based recommendations for their treatment. They were developed by the authors and approved by the WFSBP Task Force on Unipolar Depressive Disorders consisting of 46 international researchers and clinicians. The recommendations presented in these guidelines are based on a systematic review of all available evidence pertaining to the treatment of unipolar depressive disorders and embody important clinical and scientific advancements. They also incorporate the opinions of scientifically respected experts and international representatives of the state-of-the-art treatment of these disorders. In the few questions for which a consensus could not be reached, the authors were mandated to make a final judgement.
The guidelines were originally published in 2002 in two parts (Bauer et al. Citation2002a,Citationb) for use by all physicians, particularly psychiatric specialists. The guidelines presented here have been comprehensively revised and are intended for use by general practitioners encountering patients with depressive conditions, and are thus restricted to the issues most important in the primary care context and to those treatments which are possible within the scope of a general practice. They can serve only as guidelines, because the final judgment regarding any particular treatment procedure must be made by the responsible treating physician in light of the clinical picture presented by the patient and the diagnostic and treatment options available.
These guidelines deal primarily with biological (somatic) treatment (e.g., antidepressants). Psychotherapeutic treatment interventions are covered only briefly. The guidelines do not address depressive disorders occurring in bipolar affective disorders (which are covered by separate WFSBP guidelines, Grunze et al. Citation2002, Citation2003, Citation2004). Since the availability of medications, treatments and diagnostic procedures varies considerably from country to country, several different treatment options are included in the guidelines.
1.3 Methods of literature search and data extraction
The data used for the development of these guidelines have been extracted from the following sources: Agency for Health Care Policy and Research (AHCPR) Depression Guidelines Panel (1993); AHCPR Evidence Report on Treatment of Depression: Newer Pharmacotherapies (1999); American Psychiatric Association (APA) Practice Guideline for the Treatment of Patients with Major Depressive Disorder, Revision (2000); British Association for Psychopharmacology Revised Guidelines for Treating Depressive Disorders (Anderson et al. Citation2000); CitationCanadian Psychiatric Association and the Canadian Network for Mood and Anxiety Treatments, CANMAT, Clinical Guidelines for the Treatment of Depressive Disorders (2000); Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder (Lam and Levitt Citation1999); CitationDeutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde, DGPPN, Praxisleitlinien in Psychiatrie und Psychotherapie, Affektive Erkrankungen (2000); The Cochrane Library; World Federation of Societies of Biological Psychiatry WFSBP Guidelines for biological treatment of unipolar depressive disorders (2002); meta-analyses on the efficacy of antidepressant medications identified by a search in the MEDLINE database (up to 1 February 2005); major pertinent review articles identified by a search in the MEDLINE database and textbooks, and individual clinical experience of the authors and members of the WFSBP Task Force on Unipolar Depressive Disorders. All original data quoted were taken from research articles published in peer-reviewed journals in English before 1 February 2005. Important evidence published after this deadline is added and labeled by footnotes.
1.4 Evidence-based classification of recommendations
Each treatment recommendation was evaluated for the strength of evidence for its efficacy, safety and feasibility.Footnote1 Given the disparities in medication costs across the world, daily treatment costs were not taken into consideration. Four categories of evidence were used:
Level A
Good research-based evidence to support the recommendation. This level is achieved if research-based evidence for efficacy has been derived from at least three moderately large, positive, randomized controlled (double-blind) studies (RCT). At least one of these three studies must moreover be a well-conducted, placebo-controlled study.
Level B
Fair research-based evidence to support the recommendation. This includes evidence of efficacy from at least two moderately large randomized, double-blind studies (this can be either at least two comparator studies or one comparator-controlled and one placebo-controlled study) or from one moderately large randomized, double-blind study (placebo-controlled or comparator-controlled) and at least one prospective, moderately large (sample size of ≥ 50 participants), open-label, naturalistic study.
Level C
Minimal research-based evidence to support the recommendation. This level is achieved if one randomized, double-blind study with a comparator treatment and one prospective, open-label study/case series (with a sample size of ≥ 10 participants) showed efficacy, or at least two prospective, open-label study/case series (with a sample size of ≥ 10 participants) showed efficacy.
Level D
Expert opinion-based (from authors and members of the WFSBP Task Force on Unipolar Depression) supported by at least one prospective, open-label study/case series (sample size ≥10 participants).
No level of evidence
Expert opinion for general treatment procedures and principles.
1.5 Epidemiology and course of major depressive disorder
Major depressive disorder (MDD) is a severe mood disorder associated with significant morbidity and mortality affecting individuals of all ages and races. The recent worldwide Global Burden of Disease (GBD) study of the World Health Organization (WHO) has shown variations by country and region, but patterns and trends for depressive disorders are remarkably similar worldwide (Murray and Lopez Citation1997a,Citationb). MDD is characterized by single or recurrent major depressive episodes (MDEs). The essential feature of a major depressive episode is a period of at least 2 weeks of depressed mood with abnormalities of neurovegetative function (appetite, weight loss, sleep disturbances), psychomotor activity (e.g., loss of energy and interests, agitation or retardation), cognition (feelings of worthlessness, hopelessness or inappropriate guilt), as well as anxiety and suicidal ideation (). MDD has a median lifetime prevalence of 16.1% (range 4.4–18) (Wittchen Citation2000; Waraich et al. Citation2004). It occurs in about 5–10% of the adult population during any 1-year period of time, with women at higher risk than men (the ratio is approximately 2:1) (Regier et al. Citation1993; Kessler et al. Citation1994; Picinelli and Gomez-Homen Citation1997; Ialongo et al. Citation2004).
Table I. Classification and criteria of major depressive disorder (DSM-IV) and depressive episode (ICD-10)
At least 10% of all patients presenting in the primary care setting suffer from depression (Üstün and Sartorius Citation1995; Backenstrass et al. Citation2006), with about 50% presenting with primarily or only somatic symptoms (Fisch Citation1987). Of all primary care patients with depressive symptomatology, around 25% classify as having MDD, 30% as having minor depression and 45% present with non-specific depressive symptoms. Seen together, the latter two groups could constitute a subthreshold depression (Backenstrass et al. Citation2006). Even severely depressed patients are commonly seen in primary care, frequently thinking that because of the severity of their symptoms they might be suffering from a somatic illness.
MDD can begin at any age, even in childhood and adolescence, but there are two peaks in the 20s and 40s (Angst and Preisig Citation1995; American Psychiatric Association Citation2000). The mean age of onset of MDD has been estimated around the age of 30 (Wittchen Citation2000).
Untreated, a typical major depressive episode lasts about 6 months or more (Angst and Preisig Citation1995; Solomon et al. Citation1997; American Psychiatric Association Citation2000; Wang Citation2004). Modern pharmacotherapy can alleviate suffering during acute episodes, and placebo-controlled trials show that response and remission occur faster in actively treated groups. MDD is a recurrent disorder and 50–85% of the patients who experience an episode will eventually have another (Keller et al. Citation1986; Mueller et al. Citation1999).
The prognosis for a depressive episode is good, and most patients return to normal functioning when the episode is over. However, in 20–30% of cases remission is incomplete, with some depressive symptoms persisting chronically (Angst Citation1986; Keller et al. Citation1986; Scott Citation1988; Paykel Citation1994; Judd et al. Citation1998; Bauer et al 2002b). MDD is associated with considerable morbidity and mortality, and for many patients an initial episode of depression evolves into a recurrent and debilitating chronic illness with significant and pervasive impairments in psychosocial functioning (Klerman and Weissman Citation1992; Mintz et al. Citation1992; Hirschfeld et al. Citation2000; Judd et al. Citation2000; Bromberger Citation2004; Melartin et al. Citation2004; Papakostas Citation2004). Studies on the effects of depression on health-related quality of life demonstrate detrements equal to or greater than those for patients with chronic medical illnesses such as ischemic heart disease or diabetes mellitus (Wells et al. Citation1989b; AHCPR Citation1999; Unützer et al. Citation2000a). The symptoms in depressive people with co-morbid medical illnesses tend to show less improvement, and these patients show a higher relapse rate during treatment (Iosifescu et al. Citation2004).
The most serious consequence of MDD is suicide. A recent meta-analysis showed that while the lifetime prevalence of suicide for the general population is under 0.5%, that of patients with affective disorders ranges from 2.2% for mixed inpatient/outpatient populations to 8.6% for those who have been hospitalized for suicidality (Bostwick and Pankratz Citation2000). Depression also substantially increases the risk of death by cardiovascular disease (Wulsin et al. Citation1999).
The Global Burden of Disease Study estimated that unipolar major depression is the fourth largest contributor to the global burden of disease (premature mortality and disability). With the addition of suicide, the burden of unipolar major depression increased by nearly 40% (Murray and Lopez Citation1997a). By the year 2020, unipolar MDD is projected to be the second largest contributor to the global burden of disease after heart disease (Murray and Lopez Citation1997b).
In addition to the personal suffering of individuals and their families, depression imposes significant costs on society (Brunello et al. Citation1995; Thase Citation2001; Fava et al. Citation2003a; Greenberg et al. Citation2003; McIntyre and O'Donovan Citation2004), even more so when not properly diagnosed or undertreated (Wells et al. Citation1989a; Üstün and Sartorius 1995; Unützer et al. Citation2000b, Young et al. Citation2001).
1.6 Indications and goals of treatment for major depressive disorder
Antidepressant treatment should be considered for patients who meet diagnostic criteria for depressive episode (ICD-10) or major depressive disorder (DSM-IV) (see ). Guidelines differ with respect to the recommendation of antidepressants in mild depressive episodes or depression in primary care (Depression: management of depression in primary and secondary care – NICE Guideline 2004; Practice guideline for the treatment of patients with major depressive disorder, APA 2000). Depending on individual characteristics and/or requests of the patient antidepressant treatment might be indicated, otherwise psycho- and sociotherapeutic approaches alone may be sufficient.
Current diagnostic criteria in both classification systems represent a clinical and historical consensus on the most prominent and important symptoms and signs of depressive illness ().
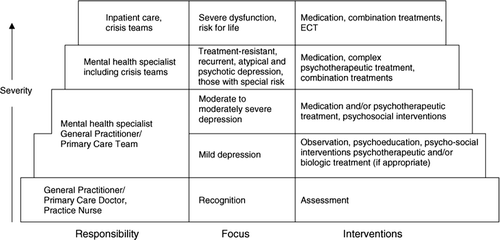
Before initiating treatment, the general practitioner should take the patient's preferences and previous treatment experiences into consideration. Especially if a patient exhibits psychotic features (e.g., delusions) or suicidality or if the depression occurs in the context of a bipolar illness, treatment by a specialist or inpatient treatment is indicated. See for a stepped-care model. Early recognition of bipolar disorder is especially important since treatment approaches differ substantially from those for unipolar depression and early appropriate treatment positively influences long-term outcome. Bipolar patients are over-represented in treatment-resistant patients falsely diagnosed with unipolar depressive disorder and antidepressant treatment alone seems to have unstabilizing impact on the course of the disease (Ghaemi Citation2002). Apart from asking the patient and his family about manic or hypomanic symptoms, screening instruments could help to identify those patients (e.g., Mood Disorders Questionnaire, MDQ, Hirschfeld Citation2000; Hypomanic Checklist, Angst et al. Citation2003, Citation2005).
Figure 1. Stepped-care model (adapted version, original idea from NICE guideline (Citation2004).
The treatment of major depressive disorder requires conceptualization of acute, intermediate and long-term goals. Kupfer and colleagues (Kupfer Citation1993) have developed a model for the typical course of a major depressive episode including the risk of recurrence and corresponding structured treatment approach. In this model, the three phases of treatment correspond to three stages of the illness: (1) acute therapy, (2) continuation therapy, and (3) maintenance therapy (see ).
Figure 2. Phases of disease and treatment (adapted version, original from Kupfer (Citation1991)).
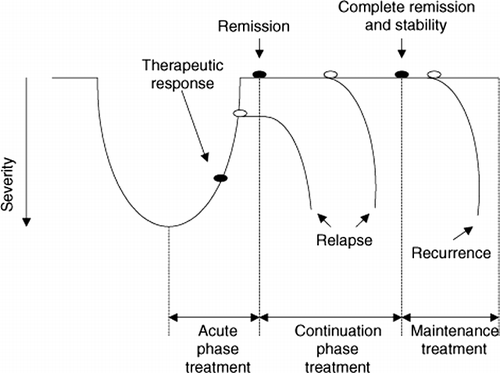
The acute phase of therapy is the time period from the initiation of treatment to remission, which is the primary therapeutic goal (Frank et al. Citation1991; Kupfer Citation1993). The continuation phase follows the acute phase to preserve and stabilize the remission. It is the phase in which the treatment is extended for a period of time in order to prevent a return of the depression. If the depressive syndrome returns during the continuation therapy, a relapse has occurred. When the patient has been asymptomatic for approximately 6 months, a recovery from the episode has occurred. The recovery may be confirmed by continued absence of depressive symptoms after the cessation of medication. Recovery applies only to individual episodes of the illness and does not imply the patient would be free of recurrences (Bauer and Helmchen Citation2000; Möller et al. Citation2003). Maintenance (prophylactic) treatment is aimed at preventing a new episode of depression and suicide.
2 Acute-phase treatment of major depressive disorder
These guidelines become applicable at the point when (1) the diagnosis of a major depressive episode has been made by a physician according to one of the two established classification systems, the International Classification of Diseases (ICD-10, World Health Organization 1992) or the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, American Psychiatric Association Citation1994a () and when the practitioner has thoroughly considered: (2) any concurrent psychiatric disorders (mania, schizoaffective disorders, alcohol or substance abuse/dependence, anxiety disorders, eating disorders, personality disorders) and somatic disorders (e.g., endocrine, neurological, autoimmune, infectious disorders, carcinomas), as well as (3) any other factors (e.g., non-psychiatric medications or psychosocial stress factors) that might contribute to a depressive syndrome or interfere with treatment. It is the task of the physician to make the initial assessment of depression, encompassing a thorough somatic examination.
The most common treatments for major depressive disorder will be reviewed below, with a focus on somatic treatment interventions. Components of psychiatric management and general “psychotherapeutic support” (American Psychiatric Association Citation2000) should be initiated and continued throughout the entire treatment. These components include: determining the treatment plan and treatment setting; establishing and maintaining a therapeutic alliance; monitoring and reassessing psychiatric status (including the patient's risk for suicide); reassessing the adequacy of diagnosis; monitoring the patient's treatment response, side effects and general medical condition, and enhancing treatment adherence by providing education to patients and families (American Psychiatric Association Citation2000). During the acute treatment phase, weekly or bi-weekly visits are recommended where feasible. During the continuation phase, the frequency of visits may vary, but a frequency of one visit every 1–2 months is recommended.
2.1 Antidepressants
The development of antidepressant medications is one of the most important achievements in the treatment of major depression. Many different types have been introduced to the pharmacotherapeutic armamentarium. At least 38 different antidepressants are available worldwide, but market availability varies considerably from country to country ().
Table II. Antidepressants: Mode of action and commonly used doses.
The “newer” antidepressants were developed with a view to reduced side effects, the classes of antidepressants currently available differ little in their antidepressant efficacy, all producing treatment responses of 50–75%.
The selection of a particular antidepressant for the individual patient therefore depends on various factors (adapted from AHCPR Citation1993): patient's prior experience with medication (positive/negative response), concurrent medical conditions that may be worsened by selected antidepressants (e.g., metabolic syndrome), concomitant use of nonpsychiatric medications that may lead to negative drug–drug interactions (see ), a drug's short- and long-term side effects (those side effects which affect quality of life are critical for patients’ satisfaction and compliance), physician's experience with the medication, patients’ history of adherence to medication, history of first-degree relatives responding to a medication, patient preferences, and the cost and availability of specific antidepressants.
2.1.1 Classification and efficacy
Unfortunately, the classification of antidepressants used in clinical practice does not always reflect a systematic approach. Traditionally, antidepressant medications have been grouped into the following main categories: tricyclic antidepressants (TCA), tetracyclic antidepressants (both are non-selective serotonin and norepinephrine reuptake inhibitors), selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (NRIs), selective serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOI) (including irreversible MAOIs and reversible inhibitors of monoamine oxidase A [RIMA]), and “other” antidepressants.Footnote2
The “older” antidepressants have, in numerous placebo-controlled studies, proved effective in treating major depressive disorder. They include the tricyclics, tetracyclics and irreversible MAO inhibitors (all classes Level A) (Khan et al. Citation2000; Storosum et al. Citation2001; Fiedorowicz and Swartz Citation2004). Similarly, numerous double-blind controlled trials have demonstrated superior efficacy of the SSRIs compared to placebo (Level A) (AHCPR Citation1999; Bech et al. Citation2000; Khan et al. Citation2000; Mace and Taylor Citation2000). In addition, the efficacy of SNRIs compared to placebo has been demonstrated in numerous double-blind controlled trails (Level A) (Entsuah et al. Citation2001; Hirschfeld and Vornik Citation2004). For mirtazapine the efficacy compared to placebo is also well documented (Level A) (Bech Citation2001). Recently agomelatine, a melatonergic antidepressant, has also shown antidepressant efficacy in trials versus placebo (Kennedy and Emsley Citation2006*).
The “older” (irreversible) MAO inhibitors (e.g., tranylcypromine and phenelzine) are not considered first-line treatments. Although their efficacy is comparable to tricyclic antidepressants, they entail the risk of potentially fatal hypertensive crisis or serotonin syndrome (see below) in patients who eat foods containing tyramine (e.g., aged cheese, aged or cured meats, soy sauce and soy bean condiments, salted fish, and red wine; see manufacturer‘s warning notices) or use certain medications (Level B) (American Psychiatric Association Citation2000).
2.1.2 Comparative efficacy and tolerability
The numerous tricyclics do not differ among themselves in terms of efficacy, but do show different side effect profiles (Level A) () (Hotopf et al. Citation1997). With respect to SSRIs, a meta-analysis has shown no significant difference in efficacy between the individual compounds (Level A) (Edwards and Anderson Citation1999).
Table III. Side effect profiles of antidepressants.a
In general, there are no clinically significant differences in efficacy and effectiveness between tricyclic antidepressants and SSRIs (Level A) (Möller et al. Citation1994; American Psychiatric Association Citation2000; Anderson 2000; Bech et al. Citation2000; Geddes et al. Citation2001). One meta-analysis of 102 RCTs did show evidence that TCAs may be slightly more effective than SSRIs in hospitalized patients and severely ill patients (Level A) (American Psychiatric Association Citation2000; Anderson 2000; see also Danish University Antidepressant Group Citation1986 Group Citation1990). However, another meta-analysis of fewer RCTs using a different methodology found that the advantage of TCAs over SSRIs did not reach statistical significance (Geddes et al. Citation2001).
SSRIs are generally tolerated better than TCAs and show lower rates of treatment discontinuation (Level A) (Simon et al Citation1996; AHCPR Citation1999; Anderson 2000; Bech et al. Citation2000; Peretti et al. Citation2000; see also the review by Vaswani et al. Citation2003). SSRIs are safer and have higher tolerability profiles than tricyclic and tetracyclic antidepressants, causing fewer anticholinergic side effects and cardiovascular toxicities (Level A) (Mace and Taylor Citation2000; Peretti et al. Citation2000; Ray et al. Citation2004). SSRIs and other “newer antidepressants” are therefore first-choice medications for mild (when appropriate) to moderate depression, particularly in the outpatient and primary care setting, and in patients with cardiovascular diseases (Kasper Citation1997; Shores et al. Citation1998; Sauer et al. Citation2001; Alvarez and Pickworth Citation2003).
Regarding the “newer” antidepressants, recent meta-analyses have suggested that the SNRI venlafaxine is more effective than SSRIs (Einarson et al. Citation1999; Anderson Citation2001; Thase et al. Citation2001; Stahl et al. Citation2002) and other antidepressants (Smith et al. Citation2002).
For severe depression, TCAs, SSRIs and SNRIs can be recommended, as well as ECT, if appropriate (see 2.3) (Level B).
The NRI reboxetine is not different in efficacy from the SSRI fluoxetine but accompanied by less study drop-outs (meta-analysis, Cipriani et al. Citation2005). Compared to another SSRI (citalopram) reboxetine showed comparable efficacy and less resulting sexual dysfunction (the drop-out rate was higher for reboxetine, possibly due to missing dose titration in the beginning, Langworth et al. Citation2006).
Side effects vary between classes of antidepressants and to some extent between individual agents (). An agent may have a side effects profile which makes it particularly suitable for patients with specific concurrent nonpsychiatric medical conditions. For patients with coronary artery disease, for example, drugs that do not lower blood pressure or are not associated with changes in cardiac conduction (e.g., bupropion, SSRIs, mianserin) are preferable. Among the tricyclics, the secondary amines (e.g., desipramine, nortriptyline) have fewer side effects than do the tertiary amines (e.g., amitriptyline, imipramine).
The most frequent side effects of TCAs and tetracyclics are: anticholinergic/antimuscarinergic (dry mouth, constipation, blurred vision, urinary hesitation, and tachycardia), cardiovascular (α-adrenergic blockade, orthostatic hypotension, brady arrhythmias, tachycardia), antihistaminergic (sedation, weight gain), and neurological (mild myoclonus, seizures in overdoses, delirium in elderly patients) (). TCAs and tetracyclics should therefore not be used in patients with moderate to severe cardiovascular disorders (Shores et al. Citation1998), narrow-angle glaucoma, prostatic hypertrophy, cognitive impairment, seizures or delirium.
The most frequent side effects of SSRIs are: gastrointestinal (nausea, vomiting and diarrhea), activation/restlessness (exacerbation of restlessness, agitation, sleep disturbances), sexual dysfunction (loss of erectile or ejaculatory function in men, loss of libido and anorgasmia in both genders) and neurologic (exacerbation of migraine headaches and tension headaches) (). The use of SSRIs is contraindicated in combination or shortly before/after treatment with MAO inhibitors because of the risk of serotonin syndrome. The most frequent clinical features of the serotonin syndrome are changes in mental status, restlessness, myoclonus, hyperreflexia, shivering, abdomial pain, diarrhoea and tremor (Sternbach Citation1995; Finfgeld Citation2004). The serotonin syndrome is most commonly the result of the interaction between irreversible MAO inhibitors and SSRIs but can also occur with other serotonergic agents (e.g., clomipramine, l-tryptophan, fenfluramine, buspirone, venlafaxine, milnacipran, nefadozone, and trazodone).
Side effects with SNRI (venalfaxine, milnacipran and duloxetine) resemble those of SSRIs. Blood pressure should be monitored for possible elevation. With mirtazapine weight gain may be induced.
Antidepressants differ with regard to the sexual side effects which incur from their usage (Ferguson Citation2001; Montejo et al. Citation2001; Montgomery et al. Citation2002; Damsa et al. Citation2004). TCAs, SSRIs and venlafaxine more likely result in sexual dysfunction than duloxetine and reboxetine (Werneke et al. Citation2006). For managing antidepressant sexual side effects, see Zajecka (Citation2001) and Worthington and Peters (Citation2003).
The degree of benefit attained from adequate treatment appears to increase proportionally with the severity of depression (Angst and Stassen Citation1994). For mild depressive episodes, the benefit of treatment with antidepressants is uncertain; education, support and problem solving are treatment alternatives (Level B) (Anderson et al. Citation2000; Depression: management of depression in primary and secondary care – NICE Guideline 2004).
2.1.3 Suicidality
Suicide is a major risk in patients with major depression. It should be assessed from the start and reviewed regularly over the course of treatment. Factors alerting the general practitioner to a patient at high risk of suicide are: affective illness, poor impulse control, age and gender (males between age 20 and 30 and over age 50 years and especially very old males; and females between age 40 and 60), history of previous suicide attempt (being the most relevant factor), family history of suicidal behaviour, positive family history of early-onset affective disorder, substance abuse (particularly alcohol abuse), marital status (single, divorced or widowed), sudden change in socioeconomic status (loss of job, financial problems, undesired retirement), and lack of support (Blumenthal Citation1990; Appleby Citation1992; Nordstrom et al. Citation1995a,Citationb; Angst Citation1999b; Bostwick and Pankratz Citation2000; Möller Citation2003). If the patient has suicidal thoughts or intent, close surveillance and specialist treatment are necessary and admission to a psychiatric ward is recommended (). Hospital admittance without patient consent may be necessary. Immediate and intensive care should be initiated and should include intensive pharmacotherapy and psychotherapy addressing psychosocial factors. There is no specific, acutely acting “anti-suicidal” medication.
Many clinicians have successfully added antipsychotics or benzodiazepines (Furukawa et al. Citation2001) to the treatment regimen. (For information on treatment recommendations for MDD with psychotic features [delusional depression], see 2.6.1 [Antipsychotics]). For patients considered likely to take an overdose, it is recommended to prescribe only one week's supply of potentially lethal antidepressants (e.g., the TCAs or irreversible MAO inhibitors) at a time, and the antidepressant chosen should be one which is relatively safe if it is indeed taken in overdose (; AHCPR Citation1993).
Epidemiological studies revealed a reduction of the frequency of suicides and increased prescriptions of antidepressants within the last decades. In contrast, there is a debate on whether certain antidepressants, or antidepressants in general, potentially increase the risk of suicidal behaviour. Clinical conditions as co-morbid personality disorders and inadequate treatment of bipolar depression may be of importance within this context.
Some data suggest that treatment with SSRIs, potentially with other antidepressant drug classes too, the risk of suicidality (preferentially suicidal attempts) maybe increased in some patients (Möller Citation2006). This risk might be pronounced in the initial phase of treatment (Jick et al. Citation2004). Simon and colleagues showed that the risk of suicide is highest in the month before starting an antidepressant, much lower in the first week of treatment, thereafter dropping to even lower, stable rates (data from computerized health plan records of 65,000 patients with depression, Simon et al. Citation2006). Khan et al. compared the incidence of suicide and suicide attempts with several of the “newer” antidepressants and placebo and did not find statistically significant differences (Khan et al. Citation2000).
However, the concerns mentioned above have induced official warnings (e.g., by the US Food and Drug Administration Citation2005), especially for child and adolescent psychiatry as here for most antidepressants efficacy has not been demonstrated. In medical decision making, the potential risk should be carefully balanced with the benefit of antidepressive treatment. Consideration of the individual disease history including risk factors for suicidal behaviour and close observation of the patient (e.g., every week in the first weeks of treatment) are recommended when starting antidepressant treatment.
2.1.4 Evaluating the efficacy of the initial treatment
The general practitioner can evaluate the efficacy of the initial treatment by defining a time period in that context and then performing a reasonable assessment of the patient's response to the antidepressant. This may include (apart from clinical global impression) the use of patient self-rating scales (e.g., Beck Depression Inventory [BDI, Beck Citation1961], or the nine-item module of the Patient Health Questionnaire [PHQ-9; Spitzer et al. Citation1999]) and/or observer-ratings scales (e.g., Hamilton Rating Scale for Depression [HRSD; Hamilton Citation1960] or the Montgomery–Asberg Depression Rating Scale [MADRS]; Montgomery and Asberg Citation1979) (Rush and Kupfer Citation2001).
Criteria recommended for clarifying the relevant terms of treatment response are:
nonresponse:≤25% decrease in symptom severity compared to baseline
partial response: 26–49% decrease in symptom severity compared to baseline
response:≥50% decrease in symptom severity compared to baseline
response with residual symptoms: response with partial remission
remission: absence of symptoms defined by absolute scale score (sometimes called full response or full remission, for example a HRSD score of ≤7).
2.1.5 When to declare initial treatment failure
When to discontinue treatment with an antidepressant should be decided by the physician and the patient in collaboration, since the right moment for a change in the treatment plan is crucial. Changing the treatment strategy too early may lead to false conclusions, e.g., that the medication is ineffective, and discourage the patient. In contrast, persisting over a too long period without any response may lead to unnecessary prolongation of the patients’ suffering and duration of the episode.
The general consensus is that if the patient does not show any improvement after 2–4 weeks of treatment with an antidepressant dose at the upper level of the standard dose (), it becomes less likely that he/she will respond to this particular medication later. If the patient is showing a partial response after 2–4 weeks it becomes more likely that the patient will respond after 8–12 weeks of therapy (Stassen et al. Citation1996; Szegedi et al. Citation2003). There is some evidence that older adults may take longer to show a response to “older” antidepressant medications (up to 12 weeks, Katona Citation1994). However, recent studies with SSRI suggest, that here older patients may not routinely require longer time to show response (see Sackeim et al. Citation2005).
2.1.6 Diagnostic reassessment and optimizing antidepressant medication
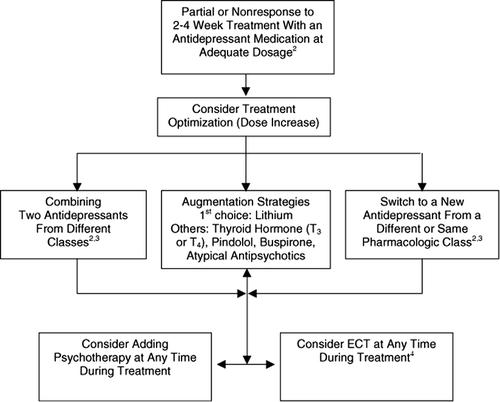
Before considering a switch in the treatment strategy, the first step should be a reappraisal of the diagnosis and adherence to the current treatment regimen. It may be important to take pharmacokinetic factors that affect plasma levels of antidepressants into consideration. If available, plasma levels of tricyclic antidepressants can be helpful in evaluating the adequacy of the dosage and the need for dose adjustment (see below and ). A review of the findings from physical examinations and laboratory results is wise to avoid overlooking coexisting general medical conditions, poorly controlled pain, non-psychiatric medications or hidden substance abuse that may underlie or be associated with the depressive episode. Persistent psychosocial stressors are also considered as a reason for non-response to treatment. Reevaluation of the adequacy of the medication dose is also advised. Often an optimization of the treatment can be achieved by increasing the dose of the antidepressant (). This strategy is particularly useful for patients receiving TCAs, but there is less evidence to support it in SSRI-treated patients (Baker et al. Citation2003). Licht and Qvitzau even found a lower response rate with a substantial dose increase of sertraline than with staying at the same (moderate) dose for another 5 weeks (Licht and Qvitzau Citation2002). For a review see Adli et al. (Citation2005)).
Figure 3. Flow Chart: Therapeutic options in partial and nonresponders1 to initial treatment with an antidepressant in major depressive disorder. 1Partial response: 26–49% decrease in baseline symptom severity; nonresponse:≤25% decrease in baseline symptom severity. 2See . 3Caution with combining irreversible MAO inhibitors (see 2.1.8.2). 4For indications see 2.3.
2.1.7 Therapeutic drug monitoring
Therapeutic drug monitoring (TDM) involves measuring the plasma concentration of a drug to ascertain whether concentrations are above, below or within an optimal therapeutic range. Other indications for TDM are to determine absorption and compliance with medication ingestion. Unlike with some tricyclical antidepressants, there is no clear relationship between clinical efficacy and plasma concentration of SSRIs, nor any threshold that defines toxic concentrations; therefore, routine monitoring of SSRI plasma levels cannot be recommended (see also Adli et al. Citation2005). Plasma concentrations of antidepressants vary considerably among patients treated with similar dosages (Hiemke and Härtter Citation2000; Kent Citation2000).
2.1.8 Treatment options for the partial and non-responding patient
Regardless of the initial choice of antidepressant, at least 30% of depressions will not respond sufficiently to treatment (Thase and Rush Citation1995; Tranter et al. Citation2002; Nelson Citation2003). Various alternative treatment strategies have been proposed for these non- or partially responsive depressions (Amsterdam Citation1991; Nolen et al. Citation1994; Marangell Citation2001; Shelton Citation2003; Pridmore and Turnier-Shea Citation2004). The major types of strategies employed after reviewing correctness of diagnosis and sufficiency of drug dosing and compliance, are (1) switching to another antidepressant from a different pharmacological class (e.g., from a SSRI to a TCA), (2) switching to another antidepressant within the same pharmacological class (e.g., from a SSRI to another SSRI), (3) combining two antidepressants from different classes (e.g., a TCA plus a SSRI), (4) augmenting the antidepressant with other agents (e.g., lithium or thyroid hormone) to enhance antidepressant efficacy, and (5) combining the antidepressant with a psychotherapeutic intervention. These strategies have been examined in a variety of agents and combinations; however, most studies have not been subjected to rigorous scientific investigation or have included small study groups.
Currently, no clear consensus exists on which strategy should be favoured for the non-responding patient, since to date no rigorous trial with a randomized, double-blind design has been conducted to answer this question (Crismon et al. Citation1999; Lam et al. Citation2004). Finally, some authors have argued in favour of augmentation strategies, e.g., lithium, because they have been repeatedly investigated in placebo-controlled trials.
2.1.8.1 Strategy 1: Switching to another antidepressant from a different class
The advantage of a switch to another antidepressant class is that it minimizes polypharmacy, which helps prevent toxicity and negative drug–drug interactions, it may lead to fewer or more tolerable side effects and can, therefore, improve patient compliance (Reynaert-Dupuis et al. Citation2002; Thase et al. Citation2002; Fava et al. Citation2003b).
Switching from an SSRI to, e.g., novel dual-acting antidepressants, selective norepinephrine or noradrenergic/dopaminergic agents, tricyclic anti-depressants or mianserin appears legitimate (see Ruhe et al. Citation2006 for a systematic review).
Disadvantages in such a switch are the loss of partial efficacy by switching from initial antidepressant and the relatively long period until the second agent can develop its antidepressive activity (delayed onset compared to augmentation or combination).
With longer use of most antidepressants step-down discontinuation within a period of 1–2 weeks is recommended rather than abrupt discontinuation, for this may cause withdrawal symptoms. Switching from or to an irreversible MAO inhibitor should be performed with caution and with a 2-week wash-out period between the two drugs (5 weeks with switch from fluoxetine) (Level B).
2.1.8.2 Strategy 2 Switching to another antidepressant from the same class
This has especially been demonstrated in a series of open-label studies showing that patients not responsive to one SSRI have a 40–70% chance of responding to a second SSRI (Level C) (Thase and Rush Citation1997). Another study has shown response rates from 50 to 60% when switching to another SSRI (Howland and Thase Citation1999).
2.1.8.3 Strategy 3: Combining two antidepressants from different classes
Reasons in support of such combination treatment include avoidance of loss of partial response with a monotherapy and less fear of worsening of depressive symptoms when a partially effective medication is discontinued. Disadvantages of this strategy are the increased risk of drug–drug interactions, potentiation of side effects and drug costs. Although often applied in clinical practice, few controlled data in support of the utility and efficacy of this strategy exist (Level C, applies to all combinations, Licht and Qvitzau Citation2002; De Battista et al. Citation2003). Combining irreversible MAO inhibitors with SSRIs and other antidepressants which act on the serotonergic system (e.g., clomipramine, venlafaxine) must be strictly avoided due to potentially fatal interactions (serotonin syndrome). Similarly, combinations of an SSRI with l-tryptophan must be avoided. See systematic review on combination by Dodd et al. (Citation2005)).
In the STAR*D trial, the second-generation antidepressant bupropion or the anxiolytic buspirone added to the SSRI citalopram in patients that did not respond sufficiently to citalopram alone resulted in remission rates of about 30% in each group (Trivedi et al. Citation2006; for design issues of this multisite, prospective, sequentially randomized trial in psychiatric depressed outpatients, see Rush et al. Citation2004).
2.1.8.4 Strategy 4: Augmentation of antidepressants
This type of augmentation therapy involves adding a second drug other than an antidepressant to the treatment regimen when no response or only partial response has been achieved, with the goal of enhancing treatment. One advantage of augmentation is that it eliminates the period of transition between one antidepressant to another and builds on the partial response. Consequently, when they work, augmentation strategies can have a rapid effect. Secondly, augmentation is of benefit for patients who have had some response and may be reluctant to risk losing that improvement.
Numerous augmentation strategies have been described for use in treatment-resistant depression. Of these, lithium is the most important and best-documented, with more than 27 open studies and 10 placebo-controlled trials in the acute treatment phase of unipolar and bipolar depression (Level A, Review: Fawcett Citation2003; Bauer et al. Citation2006). Adding lithium to ongoing antidepressant treatment is thus recommended as the first choice of an augmentation strategy (Bauer and Döpfmer Citation1999). Lithium has been found to augment the therapeutic effects of a broad spectrum of antidepressants including TCAs (Joffe et al. Citation1993; Katona et al. Citation1995) and SSRIs (Katona et al. Citation1995; Baumann et al. Citation1996; Zullino and Baumann Citation2001). A meta-analysis including 10 prospective studies provided firm evidence that lithium augmentation is superior to placebo in unipolar major depression, with response rates on average of 41.2% in the lithium group and 14.4% in the placebo group (Crossley and Bauer, Citationin press). Lithium augmentation should be administered for 2–4 weeks in order to allow assessment of the patient's response. The recommended lithium doses (about 20–30 mmol/day) characteristically achieve serum lithium levels of 0.6–0.8 mmol/l (Bschor et al. Citation2003). In the case of response, lithium augmentation should be continued for at least 12 months (Bauer et al. Citation2000; Bschor et al. Citation2002).
Studies assessing the effects of thyroid hormones in treatment-resistant depression have largely focused on T3 as the augmenting thyroid hormone. Numerous case series and at least 13 prospective trials (nine open and four controlled double-blind studies) have evaluated the use of T3; most studies administered 25–37.5 µg/day T3 to potentiate the response to TCAs in non-responders (Level B) (Joffe et al. Citation1993; Altshuler et al. Citation2001). However, not all controlled double-blind studies yielded significant results in favour of T3. A subsequent meta-analysis did not find consistent results in favour of T3 augmentation (Aronson et al. Citation1996). A small number of open studies have reported response rates of about 50% for treatment-resistant depressed patients using higher, supraphysiological doses of l-thyroxine (T4) (Level D) (Bauer et al. Citation1998, Citation2005). Thyroid hormones should be administered with caution because of potential unwanted effects.
Another more recent strategy is to combine antidepressants with an atypical antipsychotic. Mainly open studies and case series show favourable outcomes with combination and augmentation (Ostroff and Nelson Citation1999; Barbee et al. Citation2004; Worthington et al. Citation2005). Only one 8-week double-blind controlled trial showed significantly greater improvement with the combination of olanzapine and fluoxetin than with either drug alone (Shelton et al. Citation2001b). See Shelton et al. (Citation2005) and Corya et al. (Citation2006) for further double blind RCTs.
2.2 Herbal remedies
For patients who are reluctant to take traditional antidepressants, herbal remedies provide an alternative. There is evidence from a substantial number of controlled trials that suggests that extracts from the plant Hypericum perforatum (popularly called St. John's wort) are more effective than placebo for the short-term treatment of mild to moderate depressive disorders (Level A) (Linde and Mulrow Citation2001 (Cochrane Review); update Linde et al. Citation2005). Compared to tricyclic antidepressants and SSRIs, there seems to be no significant difference in treatment response (Linde et al. Citation2005). However, a recent placebo-controlled multi-centre trial found no benefits of St. John's wort compared to placebo treatment of patients with moderate to severe major depression (Shelton et al. Citation2001a). Thus, from the available data, St. John's wort cannot be recommended for the treatment of severe depression (Wernecke et al. Citation2004).
The standard administered dose of hypericum (St. John's wort) is 600–900 mg/day. Adverse side effects appear to occur less frequently with St. John's wort compared to tricyclic antidepressants (Kim et al. Citation1999). As yet, little information is available on the herb's medium to long-term efficacy and side effects (AHCPR Citation1999; Linde and Mulrow Citation2001). Health care providers should keep in mind that there is evidence that hypericum can interact with a number of prescription drugs (for example, it can decrease blood levels of TCAs and antiretroviral medications used in the treatment of HIV infection, Izzo Citation2004). In addition, there have been concerns about the purity and variable potency of the herbal remedies.
2.3 Electroconvulsive therapy
Among the indications for electroconvulsive therapy (ECT) as a first-line treatment are: severe major depression with psychotic features, severe major depression with psychomotor retardation, “true” treatment-resistant major depression (see 6.3), refusal of food intake or in other special situations when rapid relief from depression is required (e.g., in severe suicidality or in pregnancy) (American Psychiatric Association Citation2000). In these cases, referral to specialist psychiatric care and, in most cases, inpatient treatment, should be considered. ECT in treatment-resistant depression was shown in a randomized study to yield significantly more reduction in scores of mood-rating scales compared to paroxetine (Folkerts et al. Citation1997). In a meta-analysis, ECT was more effective than antidepressant treatment (both compared to TCAs and MAOIs, Pagnin et al. Citation2004). In general, maintenance treatment is needed, either with medication or ECT.
2.4 Psychotherapy
Though not the main focus of this guideline, psychotherapy plays an important part in the management of depressed patients in primary care. It should be considered as an initial treatment modality for patients with mild depression. Further, psychotherapy is recommended in combination with antidepressants for patients with moderate to severe forms of depression and for patients who have had partial responses to antidepressant medications or who have had problems with adherence to antidepressants (Rush and Thase Citation1999).
Brief, structured psychotherapy sessions have been shown to be effective in the acute-phase treatment of major depression (Frank et al. Citation2000) and in preventing relapse in the continuation-phase treatment (Jarrett et al. Citation2001). The best studied psychotherapies efficacious for depression include: cognitive behavioural therapy (CBT) (Rush et al. Citation1977; Beck et al Citation1979; Dobson Citation1989; Gaffan et al Citation1995; Blackburn and Moore Citation1997; Glogauen et al Citation1998; DeRubeis et al Citation1999; Hollon et al. Citation1992; Petersen et al. Citation2004), behavioural therapy (Rehm Citation1979; Bellack and Hersen Citation1983; Lewinsohn and Clarke Citation1984; Nezu Citation1986; AHCPR Citation1993; Jarrett et al Citation1994), interpersonal therapy (IPT) (Klerman et al Citation1984; Elkin et al Citation1989; Schulberg et al Citation1996; Markowitz Citation2003) and the cognitive behavioural analysis system of psychotherapy (CBASP) (McCullough Citation2000, Citation2003). Several of these forms of psychotherapy also appear effective in elderly depressed patients (Hautzinger and Welz Citation2004; for a systematic review see Hollon et al. Citation2005). There is less empirical evidence for the efficacy of other types of psychotherapy (for example psychodynamic psychotherapy); however, this does not imply that such treatments are not effective.
Combination of antidepressants and psychotherapy is more effective than the isolated prescription of pharmacotherapy (de Jonghe Citation2001; Burnand et al. Citation2002; Jindal and Thase Citation2003; see Keller et al. Citation2000 for efficacy in chronic depression). However, one study has shown no advantage of combining antidepressants with psychotherapy (de Jonghe et al. Citation2004).
Problem-solving treatment (PST) has been shown in one randomized controlled trial to be an effective treatment for depressive disorders in primary care (Mynors-Wallis et al. Citation2000). In reducing depressive symptoms in elderly people, PST is also an effective treatment option (Alexopoulus Citation2003). PST can be delivered by non-specialists after training and is, therefore, a cost-effective alternative to formal psychotherapy. It is, however, often not available at all or not promptly enough in the primary care settings of many countries.
When evaluating study data which compare psychotherapeutic and pharmacological treatment, potential bias due to the expectations of the patients and ineffective blinding, as well as insufficient power, have to be considered.
2.5 Light therapy
Seasonal affective disorder (SAD) is a distinct subtype of recurrent major depression that manifests in a seasonal pattern (Rosenthal et al. Citation1984; American Psychiatric Association Citation1994a). It is estimated that about 5–10% of the general population, predominantly women, are affected (Kasper et al. Citation1989; Rosen et al. Citation1990). “Winter” depression is the most common type of SAD in which patients experience symptoms of clinical depression during the fall and winter, with full remission during the spring and summer seasons.
The first choice treatment of SAD is light therapy where administration is possible and compliance is given (Level A). SSRIs seem to be equally effective but take longer to improve symptoms and induce more side effects (Lam et al. Citation1995; Ruhrmann et al. Citation1998; Lee and Chan Citation1999; Thompson Citation2002). For detailed reviews, see Golden et al. (Citation2005)) and Pjrek et al. (Citation2005)).
The preferred device for light therapy is a fluorescent light box (which provides white, fluorescent light with ultraviolet wavelengths filtered out) that produces light intensities greater than 2,500 lux. The starting “dose” for light therapy is 10,000 lux for 30–40 min/day, administered each morning for a 2–4-week period. Alternatively, light boxes emitting 2,500 lux require 2 h of exposure per day (Lam and Levitt Citation1999). Correct positioning (seated close enough to the light box, i.e. no more than 50–80 cm apart, eyes opened) is important. Patients usually show improvement within 1 week, but it can take up to 4 weeks for the full response to be achieved. If a light box is not available, “natural light treatment” may be administered in patients with SAD by a daily 1-hour outdoor morning walk for two or more weeks (Wirz-Justice et al. Citation1996; Levitt et al Citation2002).
There are no absolute contraindications to light therapy and no evidence that it is associated with ocular or retinal damage. However, patients with ocular risk factors should have a pretreatment ophthalmological consultation. The common side effects of light therapy reported by patients in clinical trials include eye strain or visual disturbances, headache, agitation, nausea, sedation and, very rarely, hypomania or mania. These side effects are generally mild and transient and resolve with time or with reduction of the light dosage (Lam and Levitt Citation1999).
A meta-analysis of studies assessing the efficacy of light therapy in nonseasonal depression did not find an overall statistically significant difference in treatment response compared to control treatment. However, high-quality studies and studies applying morning treatment did show superiority of bright light therapy (Tuunainen et al. Citation2004 (Cochrane Review)).
2.6 Adjunctive therapy
Interventions intended to provide complementary effects are referred to as adjunctive therapies (Thase et al. Citation1998). Pharmacological as well as non-pharmacological adjunctive therapies have been suggested for the treatment of major depression (Marangell Citation2000). Included below is a review of antipsychotics, tranquilizers/anxiolytics, sleep deprivation and exercise training. Many of these treatments may help to reduce anxiety/insomnia and psychotic symptoms until full recovery is achieved.
2.6.1 Antipsychotics
Major depressive disorder may be associated with delusions and/or hallucinations (American Psychiatric Association Citation2000). Patients with psychotic depression have a considerably better response rate to the combination of an antidepressant plus an antipsychotic than to treatment with either component alone (Level A) (Spiker et al. Citation1985; Rothschild et al. Citation1993; Thase Citation2002; Rothschild Citation2003; Shelton Citation2003; Klein et al. Citation2004). In these patients, it is recommended to combine an antidepressant with an antipsychotic medication when treatment is initiated (Level A). In a recent meta-analysis combining two studies (Spiker et al. Citation1985; Mulsant et al. 2001) the combination of a tricyclic antidepressant with a classical antipsychotic was more efficient than the tricyclic alone; however, the difference did not reach statistical significance (Wijkstra et al. Citation2006). The newer, “atypical” antipsychotics (e.g., amisulpride, aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone) may be preferred over the classic antipsychotics (e.g., chlorpromazine, fluphenazine, haloperidol) or clozapine, due to their lower risk of extrapyramidal symptoms (Ostroff and Nelson Citation1999; Corya et al. Citation2003; Barbee et al 2004; Masand Citation2004). However, the higher risk of metabolic syndrome with atypical antipsychotics should be considered.
There are no controlled data that have compared the “newer” with the “older” antipsychotics in psychotic depression. Usually antipsychotics are administered to depressed patients at lower doses than those used in schizophrenia.
2.6.2 Tranquilizers/anxiolytics
Although tranquilizers (especially benzodiazepines) are frequently used as adjunctive medication in clinical practice worldwide, it is believed by many experts that benzodiazepines in general do not considerably affect the state of mood. Yet a review reported rates for co-administration of an antidepressant and a tranquilizer to be between 30 and 60% of depressed patients in most countries (Furukawa et al. Citation2001; Valenstein et al. Citation2004). The reason for this widespread use is most likely the fast onset of action that reduces anxiety, agitation and insomnia in many patients, and the high rate (between 33 and 85% across studies) of anxiety co-morbidity among patients with major depression. In a systematic review, Furukawa et al. (Citation2001) showed that patients with combination treatment of antidepressants and anxiolytics were more likely to show response at 1 and 4 weeks than patients with antidepressant treatment only (although the difference was not longer significant at 6–8 weeks). Benefits of adding anxiolytics have to be balanced against risk of dependence and proneness to accidents.
In each individual patient the potential benefits of adjunctive treatment with benzodiazepines must be carefully balanced against possible harm (including sedation, psychomotor and cognitive impairment, memory loss, potentiation of other central nervous system depressants and treatment-emergent depression, development of dependence and discontinuation syndromes). Predisposed individuals are at greater risk for developing dependency and tolerance; thus, benzodiazepines should not be administered to patients with a history of or current alcohol or drug abuse/dependence. It is also recommended that the duration of benzodiazepine administration in depressed patients be restricted to a maximum period of approximately 4 weeks.
2.6.3 Sleep deprivation
Total or partial sleep deprivation (SD) may be the only antidepressant intervention with marked beneficial same-day effects providing transient amelioration of depression in about 60% of patients (Level A) (Kuhs and Tölle Citation1991; Wirz-Justice and Van den Hoofdakker Citation1999; Giedke et al. Citation2003). It is an attractive adjunctive treatment for major depression because it works rapidly, is noninvasive, inexpensive and well tolerated by the majority of patients. However, most patients who do respond subsequently relapse after one night of sleep (Wu and Bunney Citation1990; Giedke and Schwarzler Citation2002). Usually, the antidepressant effect can be replicated by repeated total sleep deprivation (Level B) (Wiegand et al. Citation2001) or by combining sleep deprivation with subsequent phase advance of the sleep period (Level D) (Riemann et al. Citation1999). Bright light therapy has been shown to stabilize the antidepressant effect of partial sleep deprivation (Neumeister et al. Citation1996).
2.6.4 Exercise training
Studies of healthy young people have shown that physical activity may have positive effects on mood. Open studies of short-term effects of an adjunctive daily aerobic exercise program suggested relatively rapid (after 14 days) mood improvements in patients with major depression (Dimeo et al. Citation2001). A critical review on this treatment option discussed the potential mechanism of action of exercise (Brosse et al. Citation2002). The effectiveness of this treatment strategy could not be analysed in a meta-analysis because of a lack of good quality research (Lawler and Hopker Citation2001). Recently, in a randomized, placebo-controlled study, significant antidepressant effects of walking could be shown in 38 depressed patients (Knubben et al. Citation2006).
2.7 Other treatment options
Rapid transcranial magnetic stimulation (rTMS) works via direct stimulation of brain areas through an electromagnetic coil placed near the scalp. Studies evaluating the efficacy of rTMS are heterogeneus regarding the frequency and location of stimulation and show inconsistent results. A recent meta-analysis showed a small benefit compared to sham treatment immediately after 2-week treatment trials (Martin et al. Citation2003).
Vagus nerve stimulation (VNS) stimulates the brain indirectly via the vagus nerve (cranial nerve X). A generator about the size of a pocket watch is implanted subcutaneously into the left chest wall and is connected to bipolar electrodes that are attached to the left vagus nerve within the neck. Theoretically, activation of the vagus nerve may improve mood via its ascending projections to the amygdala and other limbic structures known to influence emotion and mood (George et al. Citation2000). There are two small open studies showing response rates of about 30% (Sackeim et al. Citation2001; Marangell et al. Citation2002). Data of a sham-controlled trial were published after the date of literature search, essentially showing no difference in response after 10 weeks but increasing response rates for VNS over the year (George et al. Citation2005; Rush et al. Citation2005).
3 Continuation-phase treatment of major depressive disorder
The objective of continuation treatment is to decrease the likelihood of relapse in the vulnerable period following symptomatic recovery from depression (i.e. to prevent a return of the current episode of depression) (AHCPR Citation1993). The continuation phase of treatment is generally considered to be the 6-month period of time immediately following full remission. However, some authors recommend continued treatment for up to 9 months (Reimherr et al. Citation1998; Hirschfeld Citation2001; Rush and Kupfer Citation2001). In general, patients with a history of long previous episodes are candidates for continuation-phase treatment of more than 9 months (Rush and Kupfer Citation2001). Because residual symptoms (partial remission) are strong predictors of subsequent early relapse, it is recommended to continue treatment until such symptoms have subsided (Paykel et al. Citation1995).
In placebo-controlled continuation therapy trials, relapse rates ranged from 31 to 80% for those patients who received placebo, compared with only 0 to 31% of those who received active medication (Prien and Kupfer Citation1986; Prien Citation1990; Geddes et al. Citation2003). It is recommended that the same antidepressant successfully used to achieve relief in the acute-phase therapy be continued at the same dose during the continuation phase (Level A) (Thase Citation1999; Rush and Kupfer Citation2001). If no relapse occurs during continuation therapy, a gradual discontinuation of the antidepressant medication is recommended (Rosenbaum et al. Citation1998). Patients should be carefully monitored during and immediately after discontinuation to ensure the stability of the remission (American Psychiatric Association Citation2000). If tapering off results in a return of symptoms, the medication in the original dose should be continued for at least another 6 months before attempting discontinuation again. After a successful course of acute-phase lithium augmentation, combined treatment using an antidepressant and lithium is suggested to be better than the combination of an antidepressant and placebo in the continuation-phase (Bauer et al. Citation2000; Bschor et al. Citation2002).
4 Maintenance-phase treatment of major depressive disorder
4.1 General treatment principles of maintenance treatment
4.1.1 Goals and indications
The goals of long-term maintenance (prophylactic) treatment are to prevent a recurrence, suicide and development of chronicity. A recurrence is an episode of depression that appears after a completely asymptomatic period (remission) has been achieved for a 6-month period (recovery) (Frank et al. Citation1991; Kupfer Citation1993; Keller Citation2002). Consideration of the patient's course of illness and treatment history is essential for the implementation of maintenance phase therapy. Even though no definite recommendation can be given as to when prophylactic therapy should be initiated, it is clearly indicated in situations associated with a high risk of recurrence (Brunello et al. Citation1995; Dawson et al. Citation1998; Angst Citation1999a; Hirschfeld Citation2001; Paykel Citation2001) (). Patient preference, severity of functional impairments and side effects experienced during the continuation phase also play a role in determining whether or not maintenance treatment should be implemented (AHCPR Citation1993; American Psychiatric Association Citation2000).
Table IV. Factors associated with increased risk for recurrence in major depressive disorder.
4.1.2 Treatment implementation
Key elements of long-term treatment of recurrent depressive disorders include: (1) psychoeducation, (2) pharmacotherapy and (3) adherence monitoring. Because maintenance treatment requires medication compliance, education and a close therapeutic alliance with patients and their families are essential (Kupfer Citation1993). Strategies to prepare patients and their families for maintenance treatment should include the following topics: typical course of the illness, treatment options, medication effects and side effects, use of (daily) self-reporting instruments to track mood and early warning signs of relapse or recurrence, long-term perspectives and projected end of treatment. It is also important to inform the patient that several different treatments may be necessary before the best individual treatment is identified.
The frequency of visits may range from monthly visits to every 3–6 months in stable patients and involve (brief) psychiatric evaluation and medication monitoring (e.g., side effect assessment, medication blood levels). In unstable patients, more frequent visits are required. If the patient develops other medical conditions while on maintenance treatment, potential drug–drug interactions should be considered by the treating physician (see ). Patients/families should be also educated to inform the treating physician when and if signs of depression reoccur.
Table V. Possible interaction of antidepressants with comedications.
4.2 Pharmacotherapy of maintenance treatment
4.2.1 Evidence of efficacy
Pharmacotherapy, especially antidepressant medications and lithium, is the most studied treatment modality in the long-term maintenance treatment of recurrent unipolar depression. The majority of controlled trials investigating these medications in maintenance treatment demonstrated efficacy for relapse prevention (AHCPR Citation1993, Citation1999; Solomon and Bauer Citation1993; Davis et al. Citation1999; Hirschfeld Citation2001).
The first choice medications for the treatment of unipolar depression are either the antidepressant with which remission was achieved in the acute/continuation phase or lithium (NIMH Consensus Development Conference Citation1985; AHCPR Citation1993; Prien and Kocsis Citation1995; American Psychiatric Association Citation2000; Paykel Citation2001). Likely reasons why antidepressants may be preferred to lithium are that patients are usually treated with antidepressants during the acute/continuation phase and they usually prefer to use medication that does not require regular monitoring by blood tests. Most importantly, the choice of which medication to use depends on how individual patients respond to and tolerate treatment with antidepressants and lithium (Schou Citation1997). Patients’ preference and experience with maintenance treatment should also be considered in the choice of the medication.
4.2.1.1 Antidepressants
Many patients receive antidepressants during the acute and continuation phase. Further, to prevent recurrence of depression the best treatment recommendation is to continue the antidepressant medication that was effective during the acute and continuation phase of treatment at the same dose during the maintenance phase (Level B) (Frank et al. Citation1993; Franchini et al. Citation1998; for a systematic review with meta-analysis, see Geddes et al. Citation2003).
Even mild to moderate side effects during maintenance treatment may lead to noncompliance with the consequence of symptom worsening and increased risk of recurrence. Using medications with a more favourable side effect profile than the tricyclic antidepressants may facilitate patient compliance with pharmacotherapy. The “newer” antidepressants are associated with fewer long-term side effects than are the older tricyclics and tetracyclics (AHCPR Citation1993, Citation1999; American Psychiatric Association Citation2000; Peretti et al. Citation2000; Masand and Gupta Citation2002).
4.2.1.2 Lithium
The use of lithium as maintenance therapy for unipolar recurrent depression is well established (Level A) (Goodwin and Jamison Citation1990; Schou Citation1997; Dunner Citation1998; Coppen Citation2000; Paykel Citation2001; Fawcett Citation2003). Two meta-analyses found evidence that lithium is more effective than placebo in preventing recurrence of unipolar depressive illness (Souza and Goodwin Citation1991; Burgess et al. Citation2003), but only in one were the results statistically significant (Souza and Goodwin Citation1991). Over the past decade, evidence has accumulated from retrospective and prospective studies that long-term lithium prophylaxis may reduce suicide risk and even normalize the high mortality rate (Level C) (Coppen et al. Citation1990; Müller-Oerlinghausen Citation1992, Citation1994; Tondo et al. Citation1997; Schou Citation2000; Coryell et al. Citation2001; Tondo et al. Citation2001; Goodwin et al. Citation2003).
Serum lithium levels of 0.5–0.8 mmol/l (mEq/l) measured 12 h after last lithium intake are usually recommended for maintenance treatment (Schou Citation1989). In patients 60 years of age or younger these recommended serum levels are generally achieved with a daily dose of about 12–30 mmol (about 10–20 mmol for Asian patients), and about 6–12 mmol in older patients (see also Birch et al. Citation1993). There is no difference in efficacy whether lithium tablets are administered once or twice per day. Some patients find a single daily dose facilitates long-term treatment compliance and reduces side effects. In general, extended release forms of lithium are better tolerated.
One advantage of maintenance therapy with lithium is the long and worldwide experience with this agent. Specialized lithium clinics for the prophylactic long-term treatment of patients with affective disorders have been established for more than 30 years in many countries and have provided longitudinal assessments of the side effects of lithium treatment (Schou Citation1997). Side effects of lithium treatment are usually dose dependent and can often be prevented or relieved by a moderate reduction in dosage (American Psychiatric Association Citation1994b). Side effects may include: hand tremor (counteracted by a β-blocker), goitre and hypothyroidism (counteracted by additional administration of l-thyroxine [l-T4] to achieve euthyroid status), lowered renal concentrating ability and polyuria and/or polydipsia (warning against dehydration, possibly reduction of dosage), weight gain (requiring moderate dieting and excercise), gastrointestinal problems such as nausea, dyspepsia, loose stools (managed by administering lithium with meals or switching lithium preparations or dose reduction) and in few cases memory impairment/mental slowness (counteracted by dose reduction) (Birch et al. Citation1993; American Psychiatric Association Citation1994b). A small percentage of patients treated with lithium may develop rising creatinine concentrations after 10 years or more of treatment. However, in patients treated with 15 or more years of lithium therapy, affection of both glomerular and tubular function seems to be more common (Bendz et al. Citation1994).
During the long-term use of lithium, regular laboratory monitoring of serum lithium levels (three to four times per year and more frequently if clinically required, e.g., in early stages of treatment, with older patients or after clinical changes have become apparent), thyroid function (e.g., TSH level) and renal function (creatinine) (once or twice a year) is recommended (Birch et al. Citation1993; American Psychiatric Association Citation1994b; Schou Citation1997; Kleiner et al. Citation1999). The purpose of measuring serum lithium levels is to ensure that high serum lithium levels are detected and lowered and to ensure that steps are taken to prevent abnormally low serum levels. It is also important to educate the patients and their families on the warning signs of lithium toxicity.
A relatively small number of studies have directly compared different medications for maintenance treatment in recurrent unipolar depression (Solomon and Bauer Citation1993). A meta-analysis of studies comparing lithium with other antidepressants showed no conclusive advantage for lithium in the prophylaxis of unipolar illness (Souza and Goodwin Citation1991). Although the evidence for prophylactic efficacy of carbamazepine in unipolar depression is limited, results indicate that carbamazepine may be an alternative for those patients who do not tolerate or respond to maintenance treatment with lithium or antidepressants (Level C) ().
Figure 4. Flow chart: therapeutic options for maintenance treatment of major depressive disorder. CBZ, carbamazepine; MT, maintenance treatment; Li, lithium. *Maintenance electroconvulsive therapy (ECT) is an option for patients responding to ECT in the acute-phase treatment or who fail two or more maintenance medication treatments. **Additional course of psychotherapy may also be considered.
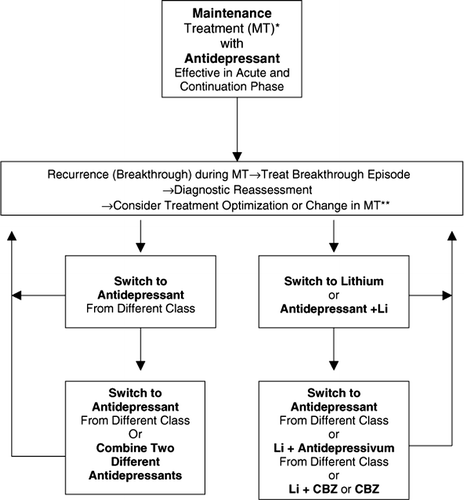
4.2.2 Treatment of symptomatic worsening and recurrence
Brief, mild depressive symptoms (“blips”) frequently occur during maintenance treatment. They are self-limited and, in contrast to recurrences, do not require specific interventions or a change in the maintenance treatment plan. Psychiatric management (e.g., dose adjustment, reassurance) and additional short-term treatment with a benzodiazepine or hypnotic medication to treat insomnia and/or anxiety or an adjunctive course of psychotherapy to help address specific psychosocial stressors may be useful (Rush Citation1999).
During a prodromal phase of a full-blown recurrence many patients display a somewhat predictable pattern of symptoms. When a patient suffers a recurrence of a depressive episode despite ongoing maintenance treatment (breakthrough episode), physicians face a considerable challenge. Early intervention can shorten the length of the episode (Kupfer et al. Citation1989). The “differential diagnosis” of a recurrence includes evaluation of occult substance abuse, occult physical illness (e.g., thyroid dysfunction), nonadherence to medication and the possibility of adverse life events (Rush Citation1999). Patients experiencing a new depressive episode while taking a mood stabilizer or an antidepressant may benefit from treatment optimization (e.g., increase of serum level to the upper end of the therapeutic level, addition of thyroid hormone if thyroid function is low – particularly in lithium-treated patients – additional psychotherapeutic interventions and visits). If the patient does not improve with treatment optimization, another round of adequate acute phase treatment should be initiated followed by continuation treatment (see above).
4.2.3 Maintenance treatment options for prophylaxis-resistant depression
There is growing recognition that prophylactic treatment of affective disorders may be inadequately performed in a substantial proportion of patients. The maintenance treatment of patients who experience recurrences during prophylactic treatment with standard agents, e.g., lithium or antidepressants, is one of the most challenging issues in the treatment of these disorders. However, little data from formal studies is available to guide physicians in the maintenance treatment of patients suffering from recurrences (Bauer and Helmchen Citation2000). An algorithm including some options for the maintenance treatment of patients with MDD is presented in . The possible treatment options include combining an antidepressant with lithium, combining lithium plus carbamazepine, combining two different antidepressants and ECT (Level D).
4.3 Duration and discontinuation of maintenance treatment
The optimal time to discontinue long-term medication is difficult to predict. Substantial evidence from a controlled 5-year study shows that patients who receive active full-dose medication for at least 5 years benefit the most from continued prophylaxis (Level B) (Kupfer et al. Citation1992). For some patients, maintenance treatment is required over a long period of time (e.g., a decade) and for others indefinitely (Rush and Kupfer Citation2001). Three years of maintenance therapy is appropriate almost as a routine for patients with recurrences, particularly where an episode prior to the present one has occurred in the last 5 years or where remission has been difficult to achieve. Maintenance for over 5 years or indefinitely is recommended for those patients at greater risk, particularly where two or three attempts to withdraw medication have been followed by another episode within a year. In clinical practice, antidepressants should always be tapered off slowly over a 4–6-month period after long-term maintenance therapy to allow the early detection of emerging symptoms and to minimize the risk of discontinuation syndromes. Discontinuation symptoms after abrupt antidepressant cessation have been reported for all drug classes. They are in general mild and brief but can nevertheless be distressing for the patient. They include apart from a high risk for early recurrences (Viguera et al. Citation1998), e.g., with SSRIs and SNRIs dizziness, ataxia, gastrointestinal and flu-like symptoms as well as sleep disturbances. With lithium, the risk for recurrences after discontinuation seems to be also mainly influenced by the abruptness of the cessation. There is a higher risk for immediate new manic and depressive episodes after lithium discontinuation (Cavanagh et al. Citation2004), although there is continuing debate about whether this applies to unipolar depressive patients and whether discontinuation might result in decrease in efficacy (see also MacQueen and Joffe Citation2004). A special discontinuation syndrome has so far not been clearly shown (Schou Citation1998).
During the period of discontinuation, the patient should be closely monitored. To identify those in whom a relapse is likely after the discontinuation is completed, the monitoring should continue for the next few months (e.g., particularly for the next 6 months, which appear to be a period of high risk for recurrence; Rush and Kupfer Citation2001). If the full depressive episode recurs during or after discontinuation, the full therapeutic dosage should be promptly readministered (AHCPR Citation1993). Regardless of the reason for the point in time at which long-term pharmacotherapy is discontinued, the patient should be educated about the risk of recurrence and its early warning signs.
4.4 Switching from unipolar depression to bipolar disorder
A change of diagnosis over time from unipolar depression to bipolar disorder has been described in approximately 10–20% of patients (Angst et al. Citation1978; Akiskal et al. Citation1995; Solomon et al. Citation1997). Antidepressants, particularly tricyclics, can precipitate mania in some patients with apparent unipolar depression (Altshuler et al. Citation1995; Parker and Parker Citation2003). If a switch to mania occurs during the maintenance phase treatment in unipolar depression, rapid tapering of the antidepressant and concomitant treatment of the manic episode is essential (for more information on the treatment of mania, see WFSBP Guidelines for the Treatment of Bipolar Disorder).
4.5 Psychotherapy
These guidelines focus on biological (somatic) treatments; therefore, psychotherapeutic treatments alone or in combination with pharmacotherapy will only be mentioned briefly and no levels of evidence are provided. Instead, references for further reading are given.
Maintenance psychotherapy as the sole treatment to prevent recurrence has received less study and at this point is not recommended as a first-line treatment unless the patient does not wish to or cannot take medication for some reason (e.g., pregnancy) (AHCPR Citation1993). It is, however, a treatment option for some patients. Preliminary data suggest that cognitive behavioural therapy (CBT) may be an effective treatment for preventing recurrence (Teasdale et al Citation2000; Jarrett et al. Citation2001; Vos et al. Citation2004), including in patients who had been successfully treated with antidepressant drugs (Fava et al. Citation1998). There is some indication that patients with residual depression benefit from CBT for preventing recurrence (Fava et al. Citation1998; Paykel et al. Citation1999). Maintenance interpersonal psychotherapy (IPT-M) has also been suggested (Frank et al. Citation2000; Browne et al Citation2002). In addition, a cognitive-behavioural analysis system of psychotherapy may provide an alternative maintenance treatment for chronic depression (Klein et al. Citation2004).
5 Treatment of chronic depressive disorders
The most striking characteristics of chronic depressive disorders are undertreatment and social impairment. Patients with chronic depression are often not or inadequately treated (Keller et al. Citation1995).
5.1 Dysthymic disorder
ICD-10 defines dysthymia broadly as a chronic depression of mood which does not currently fulfill the criteria for recurrent depressive disorder in terms of either severity or duration of individual episodes (WHO Citation1991). Similarly, DSM-IV characterizes dysthymic disorder as a chronic mild depressive syndrome that has been present for two years or longer (American Psychiatric Association Citation1994a). Individuals with dysthymia frequently have a superimposed major depressive disorder (“double depression”), and those patients are less likely to have a complete recovery compared to patients with major depressive disorder without dysthymia (American Psychiatric Association Citation2000). However, patients with “double depression” who are treated during a major depressive episode also benefit with respect to their dysthymia (Akiskal Citation1994; Kocsis Citation2003).
Dysthymia is a relatively common disorder with a median point prevalence of 2.1% across studies worldwide (Wittchen Citation2000; Waraich et al. Citation2004). The lifetime prevalence has been estimated between 3.1% (Weissman et al. Citation1988) and 6.4% (Kessler et al. Citation1994). There is epidemiological evidence of high co-morbidity (75%) with other psychiatric disorders, such as major depression, anxiety disorders and substance abuse (Klein and Santiago Citation2003). Dysthymia (and subthreshold and minor depression) are also common in older adults (Murphy et al. Citation2002).
5.2 Pharmacotherapy of dysthymic disorder
Although data from controlled studies with antidepressants are still limited, a comprehensive review has confirmed efficacy of various antidepressants in dysthymic disorder (Level A) (World Psychiatric Association Dysthymia Working Group Citation1995). A recent meta-analysis of 15 randomized controlled trials that used various drugs (mostly antidepressants, TCAs, SSRIs and MAO inhibitors) versus placebo showed that drugs are more effective than placebo, with no difference between and within classes of drugs (De Lima and Moncrieff Citation2000; De Lima and Hotopf Citation2003). Although the optimal length of pharmacotherapy in dysthymia has not been studied in a controlled design, a course of treatment with an antidepressant for at least 2–3 years is recommended as in MDD. Better tolerability and side effect profiles compared to the “older” antidepressants (e.g., TCAs) make the SSRIs and other “newer” antidepressants the prime candidates for the long-term treatment of dysthymia (Level A). Amisulpride, a second-generation antipsychotic, showed similar efficacy to the SSRI paroxetine (Rocca et al. Citation2002) and a higher efficacy than sertraline (Montgomery Citation2002). Recommended antidepressant doses for dysthymia are similar to those given for acute treatment of a major depressive episode.
There is less evidence regarding the efficacy of antidepressants in dysthymia in older patients. One recent trial suggested that fluoxetine has only limited efficacy compared to placebo and that further research for prediction of response in elderly dysthymic patients is needed (Devanand et al. Citation2005). An open label trial of venlafaxine suggested a high response rate, although only 18 of 23 patients completed the 12-week treatment (Devanand et al. Citation2004).
6 Treatment in special circumstances
Under special circumstances the treatment of depression must be modified. These circumstances include depression co-occurring with other psychiatric conditions (anxiety disorders, substance abuse or dependencies), depression in children and adolescents, depression in older adults (where appropriate), depression due to a general medical condition and depression during pregnancy and breast-feeding. In these cases a psychiatric specialist should be involved (see WFSBP guidelines by Bauer et al. Citation2002a).
6.1 Comorbidity of depression with other psychiatric disorders
6.1.1 Co-morbid anxiety disorders
Up to 30% of unipolar depressive patients suffer from additional anxiety disorders including panic disorder and posttraumatic stress disorder (PTSD) (Wittchen et al. Citation1999). SSRIs and dual antidepressants (Fawcett und Barkin Citation1998; Rudolph et al. Citation1998) but also TCAs and MAO inhibitors can be used effectively. The initial dose should be low (e.g., 5 mg fluoxetine or 10 mg paroxetine) and a gradual increase up to the therapeutic dose should be adapted to the occurrence of side effects. CBT is also effective in treating anxiety disorders co-ocurring with depression. Anxiolytics should only be used in the first few days of the episode when severe anxiety is present.
6.1.2 Substance abuse or dependence
Co-morbid substance abuse or dependence is highly prevalent in depression (in 30–60% of patients with abuse/dependence mood and anxiety disorders occur and approximately one-third of patients with mood disorders additionally report times of substance abuse/dependence in their history; Regier et al. Citation1993; Scott et al. Citation1998). In primary mood disorders, it is very important to treat both disorders, as co-morbid substance abuse impairs treatment adherence and effectiveness. In some cases treating the substance abuse first should be considered as this could result in decrease of depressive symptomatology. Be aware of possible interactions of antidepressants and, e.g., methadone, where respiratory insufficiency and sedation may occur.
Substance-induced mood symptoms only occur in intoxication and withdrawal (whereas primary depression usually precedes abuse and can be present in abstinence too; American Psychiatric Association 1994). Here, antidepressants should be given with caution because of a higher risk of unwanted effects. Tailored cognitive psychotherapy proved effective (Scott et al. Citation1998).
6.2 Treatment of depression in older adults
MDD in late life is more prevalent than previously reported. Underrecognized and undertreated MDD in late life is associated with a poor prognosis (Cole et al Citation1999; Katona Citation2000; Steffens et al Citation2000; Whyte et al. Citation2004). There are particular challenges in treating older people with MDD effectively and safely. Changes in physiology associated with advancing age produce clinically significant differences in drug metabolism and pharmacokinetics in older versus younger adult patients (Rabheru Citation2004). Older adults are also more likely to require and receive treatment for co-morbid illnesses, which increases the potential for serious pharmaco-dynamic and pharmacokinetic drug–drug interactions (Preskorn Citation1993; Dunner Citation2003).
There is relatively little data on the use of antidepressants in older patients, especially in the very old (>75 years) and in those with significant medical co-morbidity, dementia or neurological problems (Flint Citation1998; Roose and Suthers Citation1998; Roose et al. Citation2004). Compared to placebo, a systematic review reported TCAs, SSRIs, and mirtazapine to be more effective in patients older than 55 years (Taylor and Doraiswamy Citation2004). Three meta-analyses of different antidepressant classes in older depressed patients (age >55 or >60) did not show significant differences in antidepressant class with regard to efficacy or tolerability (Mittmann et al Citation1997; McCusker et al Citation1998; Gerson et al Citation1999). Nortriptyline, a secondary amine tricyclic compound, has been the most systematically studied antidepressant in the elderly (Level A) (Flint Citation1998; Roose and Suthers Citation1998; Reynolds et al. Citation2001).
The efficacy and safety of SSRIs in older depressed patients have been evaluated in a number of clinical trials of sertraline, paroxetine and fluoxetine (Level A) (Dunner et al Citation1992; Tollefson et al Citation1995; Mulsant et al 1998; Roose and Suthers Citation1998; Bondareff et al Citation2000; Muijsers et al. Citation2002). Venlafaxine and reboxetine have also been shown to be effective in comparative double-blind trials (Katona et al Citation1999; Staab and Evans Citation2000) (Level B). Additionally, a meta-analysis found efficacy for moclobemide in geriatric patients (Level A) (Angst and Stabl Citation1992).
Compared to young adults, response to antidepressant treatment may be slower in older adults (although this only holds for “older” antidepressants [Katona Citation1994], whereas with SSRIs older patients may not routinely require longer time to show response [see Sackeim et al. Citation2005]). Older patients are suggested to be characterized by a higher relapse rate during continuation-phase treatment (Reynolds et al Citation1996). There is evidence which suggests that older patients may continue to benefit from active continuation-phase treatment. One placebo-controlled trial tested the SSRI citalopram (Klysner et al. Citation2002) (Level D).
For mainentance treatment there is evidence for efficacy in preventing recurrence compared to placebo for dothiepin (Old Age Depression Interest Group Citation1993), phenelzine (Georgotas et al. Citation1989), citalopram (Klysner et al. Citation2002), and lithium administered in addition to antidepressant treatment (Wilkinson et al. Citation2003) (Level B).
Cardiovascular side effects are a particular concern in older adults. In a trial comparing paroxetine with nortriptyline use for treatment of depressed patients with ischaemic heart disease, in which a sizable proportion of the patients were older than 60, the two drugs were equally effective for depression, but nortriptyline was associated with a significantly higher rate of serious cardiac adverse effects (Roose et al Citation1998). Anticholinergic adverse events (e.g., cognitive impairment, constipation, urinary retention) are another important issue in the older population (). Due to the equal efficacy of the various classes of antidepressants, choice of medication is determined by comparing side effect profiles. Because older patients are more prone to orthostatic hypotension and more sensitive to other adverse events such as cardiovascular and anticholinergic effects, SSRIs and the other/newer antidepressants are generally preferred to TCAs (Level A) (Katona Citation2000; Wilson and Mottram Citation2004). Older patients are typically started on a lower oral dose than younger adult patients, but it may be necessary to titrate doses for effectiveness. Higher plasma concentrations for a given dose are generally found in older compared to younger individuals (Anderson et al. Citation2000; American Psychiatric Association Citation2000) and doses may need to be adjusted particularly in patients with impaired renal or liver function. For a guideline on the management of late-life depression under primary care conditions see Baldwin et al. (Citation2003).
For treatment of dysthymia in older patients see 5.2.
6.3 Treatment-resistant depression
There is no universally accepted definition of treatment resistance. However, if the patient did not respond (according to validated psychometric scales) to at least two treatment trials with different drug classes (given at a dosage equivalent to 150 mg of tricyclics for a period of 4–6 weeks) treatment resistance is most likely. Since this condition seriously impairs patients, referral to a specialist is recommended (see 2.1.8 for options of treatment optimization; additional psychotherapy or, in severe cases, ECT).
6.3.1 Treatment-resistant depression in older adults
Treatment-resistant major depression is a common clinical problem in older depressed patients, reported to affect up to one-third of this population. Unidentified co-morbid medical or psychiatric conditions and misdiagnosis often contribute to treatment resistance. Atypical depressive symptoms such as somatic and cognitive symptoms, and co-morbid medical conditions that can themselves produce depressive symptoms, often make it difficult to accurately assess antidepressant response in this age group (Mulsant and Pollock Citation1998; Katona Citation2000). Options for treatment-resistant depression in older adults involve reconsideration of the diagnosis, optimizing treatment and use of alternative therapeutic approaches, including switching to another agent, combination therapy and electroconvulsive therapy.
Although there are fewer data than for young adults that support the use of lithium augmentation, lithium seems to be an effective augmenting agent in the treatment of depression in older adults (Level C) (Kushnir Citation1986; Katona and Finch Citation1991; Zimmer et al Citation1991; Uehlinger et al Citation1995). However, the use of lithium is more problematic in older adults due to less efficient clearance and interaction with concomitant medications (Sproule et al Citation2000). Regular clinical examination and regular blood level monitoring, aimed at keeping serum lithium levels within the range of 0.4–0.8 mmol/l (mEq/l), enable most older adults to continue lithium treatment safely (Katona and Finch Citation1991).
6.4 Treatment of depression in children and adolescents
A substantial proportion of patients experience their first episode of major depressive disorder during early childhood, prior to puberty or adolescence (Birmaher et al. Citation1998). Early-onset MDD is similar in many ways to MDD in adults, but it is a particularly serious form of affective disorder due to the high recurrence rate present at a critical developmental period. Although in these cases referral to a child and adolescent psychiatrist is recommended, a short overview of treatment options is given.
Antidepressants may prove useful in some cases and are especially recommended for patients with severe depression and psychosis (Birmaher et al 1998). Almost all double-blind controlled trials reported no significant difference in efficacy between TCAs and placebo. The therapeutic role of TCAs (particularly desipramine and imipramine) for children and adolescents must be seriously weighed against lethality of overdose, the possibility of sudden unexplained death (possibly related to cardiac conduction problems; Wilens et al Citation1996), and the availability of medications which are safer and easier to monitor (Geller et al. Citation1999). SSRIs appear to have superior efficacy compared to placebo in children and adolescents (Level B) (for fluoxetine, see Emslie et al Citation1997; for paroxetine, see Keller et al. Citation2001). The “newer” antidepressants have not yet been studied in RCTs, small open-label studies of venlafaxine (Mandoki et al Citation1997) and nefazodone (Goodnick et al. Citation2000) have shown some promising results (Level D). ECT may be considered for acutely suicidal, psychotic or treatment-resistant depression (Thorpe et al. Citation2001).
As mentioned above, there is discussion regarding an increased risk for suicidal behaviour (including suicidal ideation and attempts) with SSRIs (especially paroxetine, discrepant results for citalopram and sertraline) and newer antidepressants (e.g., venlafaxine) in children leading to official statements of the US Food and Drug Administration (FDA Citation2005) and the European Medicines Agency (EMEA Citation2005). Therefore, comprehensive information must be given both to patients and parents/legal guardian. If the patient is treated effectively with SSRIs, careful follow-up should be provided. In the case of only partial or non-response, gradual dose reduction under close observation is an option. Abrupt discontinuation of the medication must be avoided.
Given the high rate of relapse of depression, continuation therapy is recommended for all children and adolescents for at least 6 months. As with adults, antidepressants should be continued at the same dose used to attain remission of acute symptoms. At the end of the continuation phase, for patients who do not require maintenance treatment, medications should be discontinued gradually over a period of at least 6 weeks.
6.5 Treatment during pregnancy and breast-feeding
Major depressive disorder occurring during pregnancy is a difficult therapeutic problem (American Psychiatric Association Citation2000). In contrast to mood stabilizers (lithium, carbamazepine, and valproate), which do have some teratogenicity, antidepressants (TCAs, SSRIs) do not seem to infer increased risk of organ dysgenesis (Altshuler et al. Citation1996, Citation2001) and do not increase risk of intrauterine death or major birth defects (Wisner et al 1999). The (neuro-)development of children whose mothers took TCAs or fluoxetine during gestation did not differ from that of controls (Nulman and Koren Citation1996; Nulman et al. Citation1997). Direct drug effects and transient withdrawal symptoms (e.g., jitteriness, tachypnea) occurred in some infants whose mothers were treated with antidepressants near term (Wisner et al. Citation1999). For “newer” antidepressants, only little information is available. MAO inhibitors are contraindicated during pregnancy due to possible hypertensive crisis. Use of antidepressants during pregnancy is appropriate in many clinical situations, and should include thoughtful weighing of risk of prenatal exposure versus risk of relapse of the mother following drug discontinuation (risk–benefit decision making). Psychotherapy and ECT should be considered as important treatment alternatives. Close monitoring and interventions for patients with identified risks (e.g., poor weight gain) are recommended (Wisner et al. Citation1999).
Following childbirth, many women are at high risk for the onset or recurrence of a mood disorder. The transient 7–10-day depressive syndrome referred to as “postpartum blues” typically does not meet the criteria for major depressive disorder and does not require medication (American Psychiatric Association Citation2000). The term “postpartum depression” refers to a major depressive episode occurring within 4 weeks of delivery. Studies have shown a consistent incidence of depression in 10–15% of mothers in the early weeks after delivery (Hoffbrand et al. Citation2001). Women with a history of MDD have a 25–50% risk of a postpartum depressive episode. Since many women needing antidepressant treatment may wish to breast-feed their infants, several recent studies have identified antidepressants that can be safely used during nursing (Level C) (Wisner et al. Citation1996; Burt et al. Citation2001; Hoffbrand et al. Citation2001). The agents most scrutinized in breast-feeding women are paroxetine, sertraline, fluoxetine, clomipramine and nortriptyline (Stowe et al. Citation2000; Hendrick et al. Citation2001). When a psychotropic medication is administered, the infant should be monitored daily by the mother for changes in sleep, feeding patterns and behaviour. The mother should alert the physician if there is any reason for concern.
Update of Guideline
The Guideline will be updated in 2009 to review recommendations and integrate evidence from ongoing trials.
Disclosure statement
The preparation of these WFSBP guidelines has not been financially supported by any commercial organization.
The draft version of the guidelines was sent to all Presidents of the various national societies of biological psychiatry that are members of the WFSBP; our thanks go to those Presidents who sent us their comments on the guidelines.
Notes
1Note: It is emphasized that a graded efficacy evaluation has its limitations. The strength of a recommendation reflects the scientific evidence on which it is based and not necessarily its importance. Levels of recommendation only apply to treatment and not to other aspects.
2Note: Abbreviations used for antidepressant groups vary in the literature. For example, selective norepinephrine reuptake inhibitors are abbreviated as NRI or SNRI, selective serotonin and norepinephrine reuptake inhibitors as SNRI or SSNRI.
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
*Published after the date of systematic literature search
References
- Adli M, Baethge C, Heinz A, Langlitz N, Bauer M. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci 2005; 255(6)387–400
- AHCPR (Agency for Health Care Policy and Research). 1993. Depression Guidelines Panel. Depression in Primary Care: Clinical Practice Guideline No. 5. AHCPR pub. No. 93-0550. Rockville, MD.
- AHCPR (Agency for Health Care Policy and Research). 1999. Evidence Report on Treatment of Depression: Newer Pharmacotherapies. San Antonio Evidence-Based Practice Center. Washington, DC, AHCPR, Evidence-Based Practice Centers. AHCPR pub. No. 99-E014.
- Akiskal HS. Dysthymic and cyclothymic depressions: therapeutic considerations. J Clin Psychiatry 1994; 55(Suppl 4)46–52
- Akiskal HS, Benazzi F. Psychopathologic correlates of suicidal ideation in major depressive outpatients: is it all due to unrecognized (bipolar) depressive mixed states?. Psychopathology 2005; 38(5)273–280
- Akiskal HS, Maser JD, Zeller PJ, et al. Switching from ‘unipolar’ to bipolar II. An 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry 1995; 52: 114–123
- Alexopoulos GS, Raue P, Arean P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry 2003; 11: 46–52
- Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry 1995; 152: 1130–1138
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5)592–606
- Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001; 158: 1617–1622
- Alvarez W, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy 2003; 23: 754–771
- American Psychiatric Association. 1994a. Diagnostic and statistical manual of mental disorders. 4th revision (DSM-IV). Washington, DC: American Psychiatric Press.
- American Psychiatric Association. 1994b. Practice guideline for the treatment of patients with bipolar disorder. Am J Psychiatry 151((Suppl 12)):1–36.
- American Psychiatric Association. 2000. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 157((Suppl)):1–45.
- Amsterdam JD, editor. 1991. Advances in neuropsychiatry and psychopharmacology. Vol 2. Refractory depression. New York: Raven Press.
- Anderson IM, Nutt DJ, Deakin JF. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. British Association for Psychopharmacology. J Psychopharmacol 2000; 14: 3–20
- Anderson IM. Meta-analytical studies on new antidepressants. Br Med Bull 2001; 57: 161–178
- Angst J. The course of affective disorders. Psychopathology 1986; 19(Suppl 2)47–52
- Angst J. Major depression in 1998: are we providing optimal therapy?. J Clin Psychiatry 1999a; 60(Suppl 6)5–9
- Angst J. Suicide risk in patients with major depressive disorder. J Clin Psychiatry 1999b; 60(Suppl 2)57–62
- Angst J, Preisig M. Course of a clinical cohort of unipolar, bipolar and schizoaffective patients. Results of a prospective study from 1959 to 1985. Schweiz Arch Neurol Psychiatr 1995; 146: 5–16
- Angst J, Stabl M. Efficacy of moclobemide in different patient groups: a meta-analysis of studies. Psychopharmacology (Berlin) 1992; 106(Suppl)S109–113
- Angst J, Stassen HH. 1994. Methodische Aspekte von Studien zur antidepressiven Wirksamkeit. In: Spezielle Aspekte der antidepressiven Therapie. Neuere Ergebnisse zu Moclobemid. München: MMV Medizin Verlag GmbH. p S147–166.
- Angst J, Felder W, Frey R, Stassen HH. The course of affective disorders. I. Change of diagnosis ofmonopolar, unipolar, and bipolar illness. Arch Psychiatr Nervenkr 1978; 226: 57–64
- Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rossler W. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord 2003; 73(1–2)133–146
- Angst J, Adolfsson R, Benazzi F, et al. The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J Affect Disord 2005; 88(2)217–233
- Appleby L. Suicide in psychiatric patients: risk and prevention. Br J Psychiatry 1992; 161: 749–58
- Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996; 53(9)842–848
- Backenstrass M, Frank A, Joest K, Hingmann S, Mundt C, Kronmuller KT. A comparative study of nonspecific depressive symptoms and minor depression regarding functional impairment and associated characteristics in primary care. Comp Psychiatry 2006; 47(1)35–41
- Baker CB, Tweedie R, Duval S, Woods SW. Evidence that the SSRI dose response in treating major depression should be reassessed: a meta-analysis. Depress Anxiety 2003; 17: 1–9
- Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry 2003; 64(Suppl 5)44–52
- Baldwin RC, Anderson D, Black S, et al, Faculty of Old Age Psychiatry Working Group, Royal College of Psychiatrists. 2003. Guideline for the management of late-life depression in primary care. Int J Geriatr Psychiatry 18:829–838.
- Barbee JG, Conrad EJ, Jamhour NJ. The effectiveness of olanzapine, risperidone, quetiapine, and ziprasidone as augmentation agents in treatment-resistant major depressive disorder. J Clin Psychiatry 2004; 65: 975–981
- Bauer M, Döpfmer S. Lithium augmentation in treatment-resistant depression – A meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999; 19: 427–434
- Bauer M, Helmchen H. General principles of the treatment of depressive and manic disorders. Contemporary Psychiatry. Vol 3, H Helmchen, F Henn, H Lauter, N Sartorius. Springer, Heidelberg 2000; 305–316
- Bauer M, Hellweg R, Gräf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998; 18: 444–455
- Bauer M, Bschor T, Kunz D, Berghöfer A, Ströhle S, Müller-Oerlinghausen B. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry 2000; 157: 1429–1435
- Bauer M, Whybrow PC, Angst J, Versiani M, Möller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for biological treatment of unipolar depressive disorders, part 1: acute and continuation treatment of major depressive disorder. World J Biol Psychiatry 2002a; 3: 5–43
- Bauer M, Whybrow PC, Angst J, Versiani M, Möller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for biological treatment of unipolar depressive disorders, part 2: maintenance treatment of major depressive disorder and treatment of chronic depressive disorders and subthreshold depressions. World J Biol Psychiatry 2002b; 3: 69–86
- Bauer M, London ED, Rasgon N, et al. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in women with bipolar depression. Mol Psychiatry 2005; 10: 456–469
- Bauer M, Crossley NA, Gerber S, Bschor T. The acute antidepressive effects of lithium: from monotherapy to augmentation. Lithium in neuropsychiatry – The comprehensive guide, M Bauer, P Grof, Müller-Oerlinghausen. Informa Healthcare, Abingdon 2006; 109–128
- Baumann P. Pharmacokinetic-pharmacodynamic relationship of the selective serotonin reuptake inhibitors. Clin Pharmacokinet 1996; 31: 444–469
- Beasley CM, Jr, Dornseif BE, Bosomworth JC, et al. Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. Br Med J 1991; 303(6804)685–692
- Bech P. Meta-analysis of placebo-controlled trails with mirtazapine using the core items of the Hamilton Depression scale as evidence of a pure antidepressive effect in the short-term treatment of major depression. Int J Neuropsychopharmacol 2001; 4: 337–345
- Bech P, Cialdella P, Haugh MC, et al. Meta-analysis of randomised controlled trials of fluoxetine v. placebo and tricyclic antidepressants in the short-term treatment of major depression. Br J Psychiatry 2000; 176: 421–428
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guilford, New York 1979
- Bellack AS, Hersen M. A comparison of social-skills training, pharmacotherapy, and psychotherapy for depression. Behav Res Ther 1983; 21: 101–107
- Bendz H, Aurell M, Balldin J, Mathe AA, Sjodin I. Kidney damage in long-term lithium patients: a cross-sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant 1994; 9: 1250–1254
- Benkert O, Hippius H. Kompendium der Psychiatrischen Pharmakotherapie. 5. Überarbeitete Auflage. Springer, Berlin, Heidelberg 2005
- Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical handbook of psychotropic drugs. Hogrefe & Huber Publishers, Seattle 1996
- Birch NJ, Grof P, Hullin RP, Kehoe RF, Schou M, Srinivasan DP. Lithium prophylaxis: proposed guidelines for good clinical practice. Lithium 1993; 4: 225–230
- Birmaher B, Brent DA, Benson RS. Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 1998; 37: 1234–1238
- Blackburn IM, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. Br J Psychiatry 1997; 171: 328–334
- Blumenthal SJ. Youth suicide: risk factors, assessment, and treatment of adolescent and young adult suicidal patients. Psychiatr Clin North Am 1990; 13: 511–556
- Bocchetta A, Ardau R, Burrai C, Chillotti C, Quesada G, Del Zompo M. Suicidal behavior on and off lithium prophylaxis in a group of patients with prior suicide attempts. J Clin Psychopharmacol 1998; 18: 384–389
- Bondareff W, Alpert M, Friedhoff AJ, Richter E, Clary CM, Batzar E. Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry 2000; 157: 729–736
- Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry 2000; 157: 1925–1932
- Bromberger T. A psychosocial understanding of depression in women: for the primary care physician. J Am Med Womens Assoc 2004; 59: 198–206
- Brøsen K. Differences in interactions of SSRIs. Int Clin Psychopharmacol 1998; 13(Suppl 5)S45–7
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32: 741–760
- Browne G, Steiner M, Roberts J, et al. Sertraline and/or interpersonal psychotherapy for patients with dysthymic disorder in primary care: 6-month comparison with longitudinal 2-year follow-up of effectiveness and costs. J Affect Disord 2002; 68: 317–330
- Brunello N, Burrows GD, Jönsson CPB, et al. Critical issues in the treatment of affective disorders. Depression 1995; 3: 187–198
- Bschor T, Berghöfer A, Ströhle A, et al. How long should the lithium augmentation strategy be maintained? A 1-year follow up of a placebo-controlled study in unipolar refractory major depression. J Clin Psychopharmacol 2002; 22: 427–430
- Bschor T, Lewitzka U, Sasse J, Adli M, Köberle U, Bauer M. Lithium augmentation in treatment resisant depression: clinical evidence, serotonergic and endocrine mechanisms. Pharmacopsychiatry 2003; 36(Suppl 3)S230–234
- Burgess S, Geddes J, Hawton K, Townsend E, Jamison K, Goodwin G 2003. Lithium for maintenance treatment of mood disorders (Cochrane Review). In: The Cochrane Library, Issue 3. Oxford: Update Software.
- Burnand Y, Andreoli A, Kolatte E, Venturini A, Rosset N. Psychodynamic psychotherapy and clomipramine in the treatment of major depression. Psychiatr Serv 2002; 53: 585–590
- Burt VK, Suri R, Altshuler LL, Stowe ZN, Hendrick V, Muntean E. The use of psychotropic medications during breast-feeding. Am J Psychiatry 2001; 158: 1001–1009
- Canadian Psychiatric Association and the Canadian Network for Mood and Anxiety Treatments (CANMAT). 2000. Clinical Guidelines for the Treatment of Depressive Disorders. Can J Psychiatry 46((Suppl 1)):1–90S.
- Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow-up. Acta Psychiatr Scand 2004; 109(2)91–95
- Cipriani A, Brambilla P, Furukawa T, et al 2005. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews, Issue 4.
- Cole MG, Bellavance F, Asmaâ M. Prognosis of depression in elderly community and primary care populations: a systematic review and meta-analysis. Am J Psychiatry 1999; 156: 1182–1189
- Coppen A, Standish-Barry H, Bailey J, Houston G, Silcocks P, Hermon C. Long-term lithium and mortality. Lancet 1990; 335: 1347
- Coppen A. Lithium in unipolar depression and the prevention of suicide. J Clin Psychiatry 2000; 61(Suppl 9)52–56
- Corya SA, Andersen SW, Detke HC, et al. Long-term antidepressant efficacy of olanzapine/fluoxetine combination: a 76-week open-label study. J Clin Psychiatry 2003; 64: 1349–1356
- Corya SA, Williamson D, Sanger TM, Briggs SD, Case M, Tollefson G. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety 2006; 23(6)364–372
- Coryell W, Arndt S, Turvey C, et al. Lithium and suicidal behavior in major affective disorder: a case-controlled study. Acta Psychiatr Scand 2001; 104: 193–197
- Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry 1999; 60: 142–156
- Crossley NA, Bauer M Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized placebo-controlled trials. J Clin Psychiatry, in press.
- Damsa C, Bumb A, Bianchi-Demicheli F, et al. “Dopamine-dependent” side effects of selective serotonin reuptake inhibitors: a clinical review. J Clin Psychiatry 2004; 65: 1064–1068
- Danish University Antidepressant Group. 1986. Citalopram: clinical effect profile in comparison with clomipramine. A controlled multicenter study. Psychopharmacology (Berlin) 90:131–138.
- Danish University Antidepressant Group. 1990. Paroxetine: a selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study. J Affect Disord 18((4)):289–299.
- Davis JM, Janicak PG, Hogan DM. Mood stabilizers in the prevention of recurrent affective disorders: a meta-analysis. Acta Psychiatr Scand 1999; 100: 406–417
- Dawson R, Lavori PW, Coryell WH, Endicott J, Keller MB. Maintenance strategies for unipolar depression: an observational study of levels of treatment and recurrence. J Affect Disord 1998; 49: 31–44
- De Battista C, Solvason HB, Poirier J, Kendrick E, Schatzberg AF. A prospective trail of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol 2003; 23: 27–30
- De Lima MS, Moncrieff J. Drugs versus placebo for dysthymia. Cochrane Database Syst Rev 2000 2000; 4: CD001130
- De Lima MS, Hotopf M. A comparison of active drugs for the treatment of dysthymia. Cochrane Database Syst Rev 2000;3 2003; 3: CD00404
- De Jonghe F, Kool S, van Aalst G, Dekker J, Peen J. Combining psychotherapy and antidepressants in the treatment of depression. J Affect Disord 2001; 64: 217–229
- De Jonghe F, Hendricksen M, van Aalst G, et al. Psychotherapy alone and combined with pharmacotherapy in the treatment of depression. Br J Psychiatry 2004; 185: 37–45
- DeRubeis RJ, Gelfand LA, Tang TZ, Simons AD. Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry 1999; 156: 1007–1013
- Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde, DGPPN. 2000. Praxisleitlinien in Psychiatrie und Psychotherapie. In: Gaebel W, Falkai P, editors. Band 5. Behandlungsleitlinie Affektive Erkrankungen. Darmstadt: Steinkopff
- Devanand DP, Juszczak N, Nobler MS, et al. An open treatment trial of venlafaxine for elderly patients with dysthymic disorder. J Geriatr Psychiatry Neurol 2004; 17(4)219–224
- Devanand DP, Nobler MS, Cheng J, et al. Randomized, double-blind, placebo-controlled trial of fluoxetine treatment for elderly patients with dysthymic disorder. Am J Geriatr Psychiatry 2005; 13(1)59–68
- Dimeo F, Bauer M, Varahram I, Proest G, Halter U. Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med 2001; 35: 114–117
- Dobson KS. A metaanalysis of the efficacy of cognitive therapy for depression. J Consult Clin Psychol 1989; 57: 414–419
- Dodd S, Horgan D, Malhi GS, Berk M. To combine or not to combine? A literature review of antidepressant combination therapy. J Affect Disord 2005; 89(1–3)1–11
- Dunner DL. Lithium carbonate: maintenance studies and consequences of withdrawal. J Clin Psychiatry 1998; 59(Suppl 6)48–55
- Dunner DL. Treatment considerations for depression in the elderly. CNS Spectr 2003; 12(Suppl 3)14–19
- Dunner DL, Cohn JB, Walshe T, et al. Two combined, multicenter double-blind studies of paroxetine and doxepine in geriatric patients with major depression. J Clin Psychiatry 1992; 53(Suppl)57–60
- Edwards JG, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs 1999; 57: 507–533
- Einarson TR, Arikian SR, Casciano J, Doyle JJ. Comparison of extended-release venlafaxine, selective serotonin reuptake inhibitors, and tricyclic antidepressants in the treatment of depression: a meta-analysis of randomized controlled trials. Clin Ther 1999; 21: 296–308
- Elkin I, Shea MT, Watkins JT, et al. NIMH Treatment of Depression Collaborative Research Program: general effectiveness of treatments. Arch Gen Psychiatry 1989; 46: 971–982
- European Medicines Agency (EMEA). 2005. [ http://www.emea.eu.int].
- Emslie GJ, Rush AJ, Weinberg WA, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54: 1031–1037
- Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry 2001; 62: 869–77
- Ernst CL, Goldberg JF. Antisuicide properties of psychotropic drugs: a critical review. Harv Rev Psychiatry 2004; 12: 14–41
- Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 1998; 55: 816–820
- Fava M, Rush AJ, Trivedi MH, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am 2003a; 26: 457–494
- Fava M, McGrath PJ, Sheu WP, Reboxetine Study Group. 2003b. Switching to reboxetine: an efficacy and safety study in patients with major depressive disorder unresponsive to fluoxetine. J Clin Psychopharmacol 23:365–369.
- Fawcett J, Barkin RL. A meta-analysis of eight randomized, double-blind, controlled clinical trials of mirtazapine for the treatment of patients with major depression and symptoms of anxiety. J Clin Psychiatry 1998; 59: 123–127
- Fawcett JA. Lithium combinations in acute and maintenance treatment of unipolar and bipolar depression. J Clin Psychiatry 2003; 64(Suppl 5)32–37
- Ferguson JM. The effects of antidepressants on sexual functioning in depressed patients: a review. J Clin Psychiatry 2001; 62(Suppl 3)22–34
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract 2004; 10: 239–248
- Finfgeld DL. Serotonin syndrome and the use of SSRIs. J Psychosoc Nurs Ment Health Serv 2004; 42: 16–20
- Fisch RZ. Masked depression: its interrelations with somatization, hypochondriasis and conversion. Int J Psychiatry Med 1987; 17: 367–379
- Flint AJ. Choosing appropriate antidepressant therapy in the elderly. A risk-benefit assessment of available agents. Drugs Aging 1998; 13: 269–280
- Folkerts HW, Michael N, Tolle R, Schonauer K, Mucke S, Schulze-Monking H. Electroconvulsive therapy vs. paroxetine in treatment-resistant depression – a randomized study. Acta Psychiatr Scand 1997; 96(5)334–342
- Franchini L, Gasperini M, Perez J, Smeraldi E, Zanardi R. Dose-response efficacy of paroxetine in preventing depressive recurrences: a randomized, double-blind study. J Clin Psychiatry 1998; 59: 229–232
- Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 1991; 48: 851–855
- Frank E, Kupfer DJ, Perel JM, et al. Comparison of full-dose versus half-dose pharmacotherapy in the maintenance treatment of recurrent depression. J Affect Disord 1993; 27: 139–145
- Frank E, Thase ME, Spanier C, Cyranowski JM, Siegel L. Psychotherapy of affective disorders. Contemporary psychiatry. Vol. 3, H Helmchen, F Henn, H Lauter, N Sartorius. Springer, Heidelberg 2000; 348–363
- Furukawa T, Streiner DL , Young LT. 2001 Antidepressant and benzodiazepine for major depression (Cochrane Review) Cochrane Database Syst Rev 2002 2001 CD001026
- Gaffan EA, Tsaousis I, Kemp-Wheeler SM. Researcher allegiance and meta-analysis: the case of cognitive therapy for depression. J Consult Clin Psychol 1995; 63: 966–980
- Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J. 2001. Selective serotonin reuptake inhibitors (SSRIs) for depression (Cochrane Review). In: The Cochrane Library, Issue 3, Oxford: Update Software.
- Geddes JR, Carney SM, Davis C, et al. Relapse prevention with antidepressant drug treatment in depressive disorder: a systematic review. Lancet 2003; 361: 653–661
- Geller B, Reising D, Leonard HL, Riddle MA, Walsh BT. Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999; 38: 513–516
- George MS, Sackeim HA, Rush AJ, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 2000; 47: 287–295
- George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry 2005; 58(5)364–73
- Georgotas A, McCue RE, Cooper TB. A placebo-controlled comparison of nortriptyline and phenelzine in maintenance therapy of elderly depressed patients. Arch Gen Psychiatry 1989; 46(9)783–786
- Gerson S, Belin TR, Kaufman A, Mintz J, Jarvik L. Pharmacological and psychological treatments for depressed older patients: a meta-analysis and overview of recent findings. Harv Rev Psychiatry 1999; 7: 1–28
- Ghaemi SN, Ko JY, Goodwin FK. “Cade's disease” and beyond: misdiagnosis, antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry 2002; 47(2)125–134
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev 2002; 6: 361–377
- Giedke H, Klingberg S, Schwarzler F, Schweinsberg M. Direct comparison of total sleep deprivation and late partial sleep deprivation in the treatment of major depression. J Affect Disord 2003; 76: 85–93
- Glogauen V, Cottraux J, Cucherat M, Blackburn IM. A metaanalysis of the effects of cognitive therapy in depressed patients. J Affect Disord 1998; 49: 59–72
- Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry 2005; 162(4)656–662
- Goodnick PJ, Jorge CA, Hunter T, Kumar AM. Nefazodone treatment of adolescent depression: an open-label study of response and biochemistry. Ann Clin Psychiatry 2000; 12: 97–100
- Goodwin FK, Jamison KR. Manic-depressive illness. Oxford University Press, New York 1990
- Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lthium and divalproex. J Am Med Assoc 2003; 290: 1467–1473
- Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the united states: how did it change between 1990 and 2000?. J Clin Psychiatry 2003; 64: 1465–1475
- Grunze H, Kasper S, Goodwin G, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of bipolar disorders. Part I: Treatment of bipolar depression. World J Biol Psychiatry 2002; 3(3)115–124
- Grunze H, Kasper S, Goodwin G, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders, Part II: Treatment of Mania. World J Biol Psychiatry 2003; 4(1)5–13
- Grunze H, Kasper S, Goodwin G, Bowden C, Moller HJ. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders, part III: maintenance treatment. World J Biol Psychiatry 2004; 5(3)120–135
- Healy D, Whitaker C. Antidepressants and suicide: risk-benefit conundrums. J Psychiatry Neurosci 2003; 28: 331–337
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62
- Hautzinger M, Welz S. Cognitive behavioral therapy for depressed older outpatients – a controlled, randomized trial]. Z Gerontol Geriatr 2004; 37(6)427–435
- Hendrick V, Fukuchi A, Altshuler LL, Widawsky M, Wertheimer A, Brunhuber M. Use of sertraline, paroxetine and fluvoxamine by nursing women. Br J Psychiatry 2001; 179: 163–166
- Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 2000; 85: 11–28
- Hirschfeld RM, Montgomery SA, Keller MB, et al. Social functioning in depression: a review. J Clin Psychiatry 2000; 61: 268–275
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000; 157(11)1873–185
- Hirschfeld RM. Clinical importance of long-term antidepressant treatment. Br J Psychiatry (Suppl) 2001; 42: S4–8
- Hirschfeld RM, Vornik LA. Newer antidepressants: review of efficacy and safety of escitalopram and duloxetine. J Clin Psychiatry 2004; 65(Suppl 4)46–52
- Hoffbrand S, Howard L, Crawley H 2001. Antidepressant treatment for post-natal depression (Cochrane Review). In: The Cochrane Library, Issue 3. Oxford: Update Software.
- Hollon SD, DeRubeis RJ, Evans MD, et al. Cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry 1992; 49: 774–781
- Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment?. J Clin Psychiatry 2005; 66(4)455–468
- Hotopf M, Hardy R, Lewis G. Discontinuation rates of SSRIs and tricyclic antidepressants: a meta-analysis and investigation of heterogeneity. Br J Psychiatry 1997; 170: 120–127
- Howland RH, Thase ME. What to do with SSRI nonresonders?. J Psych Pract 1999; 5: 216–223
- Ialongo N, McCreary BK, Pearson JL, et al. Major depressive disorder in a population of urban, African-American young adults: prevalence, correlates, comorbidity and unmet mental health service need. J Affect Disord 2004; 79: 127–136
- Iosifescu DV, Bankier B, Fava M. Impact of medical comorbid disease on antidepressant treatment of major depressive disorder. Curr Psychiatry Rep 2004; 6: 193–201
- Izzo AA. Drug interactions with St John′s wort (Hypericum perforatum): a review of clinical evidence. Int J Clin Pharmacol Ther 2004; 42: 139–148
- Jarrett RB, Rush AJ. Short term psychotherapy of depressive disorders: current status and future directions. Psychiatry 1994; 57: 115–132
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Arch Gen Psychiatry 2001; 58: 381–388
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. J Am Med Assoc 2004; 292(3)338–343
- Jindal RD, Thase ME. Integrating psychotherapy and pharmacotherapy to improve outcomes among patients with mood disorders. Psychiatr Serv 2003; 54: 1484–1490
- Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993; 50: 387–393
- Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry 1998; 55(8)694–700
- Judd LL, Akiskal HS, Zeller PJ, et al. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch Gen Psychiatry 2000; 57: 375–380
- Kasper S, Wehr TA, Bartko JJ, Gaist PA, Rosenthal NE. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Arch Gen Psychiatry 1989; 46: 823–833
- Kasper S. Efficacy of antidepressants in the treatment of severe depression: the place of mirtazapine. J Clin Psychopharmacol 1997; 17(Suppl 1)19–28
- Katona CL. Approaches to the management of depression in old age. Gerontology 1994; 40(Suppl 1)5–9
- Katona CL. Managing depression and anxiety in the elderly patient. Eur Neuropsychopharmacol 2000; 10(Suppl 4)S427–432
- Katona CL, Finch EJL. Lithium augmentation for refractory depression in old age. Advances in neuro-psychiatry and psychopharmacology. Vol 2: Refractory Depression, JD Amsterdam. Raven Press, New York 1991; 177–184
- Katona CL, Abou-Saleh MT, Harrison DA, et al. Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br J Psychiatry 1995; 166: 80–86
- Katona CL, Bercoff E, Chiu E, Tack P, Versiani M, Woelk H. Reboxetine versus imipramine in the treatment of elderly patients with depressive disorders: a double-blind randomised trial. J Affect Disord 1999; 55: 203–213
- Keller MB. Rationale and options for the long-term treatment of depression. Hum Psychopharmacol 2002; 17((Suppl 1))S43–46
- Keller MB, Lavori PW, Rice J, Coryell W, Hirschfeld RMA. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry 1986; 143: 24–28
- Keller MB, Harrison W, Fawcett JA, et al. Treatment of chronic depression with sertraline or imipramine: preliminary blinded response rates and high rates of undertreatment in the community. Psychopharmacol Bull 1995; 31: 205–212
- Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. New Engl J Med 2000; 342(20)1462–1470
- Keller MB, Ryan ND, Strober M, et al. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2001; 40: 762–772
- Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet 2000; 355: 911–918
- Kennedy SH, Emsley R. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol 2006; 16: 93–100
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry 1994; 51: 8–19
- Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration database. Arch Gen Psychiatry 2000; 57: 311–317
- Kim HL, Streltzer J, Goebert D. St. John's wort for depression: a meta-analysis of well-defined clinical trials. J Nerv Ment Dis 1999; 187: 532–58
- Klein DN, Santiago NJ. Dysthymia and chronic depression: introduction, classification, risk factors and course. J Clin Psychol 2003; 59: 807–816
- Klein DN, Santiago NJ, Vivian D, et al. Cognitive-behavioral analysis system of psychotherapy as a maintenance treatment for chronic depression. J Consult Clin Psychol 2004; 72: 681–688
- Kleiner J, Altshuler L, Hendrick V, Hershman JM. Lithium-induced subclinical hypothyroidism: review of the literature and guidelines for treatment. J Clin Psychiatry 1999; 60: 249–255
- Klerman GL, Weissmann MM, Rounsaville BJ, Chevron ES. Interpersonal psychotherapy of depression. Basic Books, New York 1984
- Klerman GL, Weissman MM. The course, morbidity, and costs of depression. Arch Gen Psychiatry 1992; 49: 831–834
- Klysner R, Bent-Hansen J, Hansen HL, et al. Efficacy of citalopram in the prevention of recurrent depression in elderly patients: placebo-controlled study of maintenance therapy. Br J Psychiatry 2002; 181: 29–35
- Knubben K, Reischies F, Adli M, Bauer M, Schlattmann P, Dimeo FC. A randomized, controlled study on the effects of a short-term endurance training programme in patients with major depression. Br J Sports Med 2006; 41(1)29–33
- Kocsis JH. Pharmacotherapy for chronic depression. J Clin Psychol 2003; 59: 885–892
- Kuhs H, Tölle R. Sleep deprivation therapy. Biol Psychiatry 1991; 29: 1129–1148
- Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry 1991; 52(Suppl)28–34
- Kupfer DJ. Managment of recurrent depression. J Clin Psychiatry 1993; 54(Suppl 2)29–33
- Kupfer DJ, Frank E, Perel JM. The advantage of early treatment intervention in recurrent depression. Arch Gen Psychiatry 1989; 46: 771–775
- Kupfer DJ, Frank E, Perel JM, Cornes C, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ. Five-year outcome for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1992; 49: 769–773
- Kushnir SL. Lithium-antidepressant combinations in the treatment of depressed, physically ill geriatric patients. Am J Psychiatry 1986; 143: 378–379
- Lam RW, Gorman CP, Michalon M, et al. Multicenter, placebo-controlled study of fluoxetine in seasonal affective disorder. Am J Psychiatry 1995; 152: 1765–1770
- Lam RW, Levitt AJ, editors. 1999. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. VancouverBC, Canada: Clinical & Academic Publishing.
- Lam RW, Hossie H, Solomons K, Yatham LN. Citalopram and bupropion-SR: combining versus switching in patients with treatment resistant depression. J Clin Psychiatry 2004; 65: 337–340
- Langworth S, Bodlund O, Agren H. Efficacy and tolerability of reboxetine compared with citalopram: a double-blind study in patients with major depressive disorder. J Clin Psychopharmacol 2006; 26(2)121–127
- Lawler DA, Hopker SW. The effecitiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trails. Br Med J 2001; 322: 1–8
- Lee TM, Chan CC. Dose-response relationship of phototherapy for seasonal affective disorder: a meta-analysis. Acta Psychiatr Scand 1999; 99: 315–323
- Lewinsohn PM, Clarke G. Group treatment of depressed individuals. The coping with depression course. Adv Behav Res Ther 1984; 6: 99–114
- Lewitt AJ, Lam RW, Levitan R. A comparison of open treatment of seasonal major and minor depression with light therapy. J Affect Disord 2002; 71: 243–248
- Licht RW, Qvitzau S. Treatment strategies in patients with major depression not responding to first-line sertraline treatment. A randomized study of extended duration of treatment, dose increase or mianserin augmentation. Psychopharmacology 2002; 161: 143–151
- Linde K, Mulrow CD 2001. St. John's wort for depression (Cochrane Review). In: The Cochrane Library, 1. Oxford: Update Software.
- Linde K, Mulrow CD Berner M,Egger M. 2005. St. John's wort for depression Cochrane Database Syst Rev 2): CD000448.
- Lotufo-Neto F, Trivedi M, Thase ME. Meta-analysis of the reversible inhibitors of monoamine oxidase type A moclobemide and brofaromine for the treatment of depression. Neuropsychopharmacology 1999; 20: 226–247
- Mace S, Taylor D. Selective serotonin reuptake inhibitors: a review of efficacy and tolerability in depression. Expert Opin Pharmacother 2000; 1: 917–933
- MacQueen G, Joffe RT. The clinical effects of lithium discontinuation: the debate continues. Acta Psychiatr Scand 2004; 109(2)81–82
- Mandoki MW, Tapia MR, Tapia MA, Sumner GS, Parker JL. Venlafaxine in the treatment of children and adolescents with major depression. Psychopharmacol Bull 1997; 33: 149–154
- Maragnell LB. Augmentation of standard depression therapy. Clin Ther (Suppl A) 2000; 22: A25–38
- Marangell LB. Switching antidepressants for treatment-resistant major depression. J Clin Psychiatry 2001; 62(Suppl 18)12–17
- Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 2002; 51(4)280–287
- Markowitz JC. Interpersonal psychotherapy for chronic depression. J Clin Psychol 2003; 59: 847–858
- Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V, Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis. Br J Psychiatry 2003; 182: 480–491
- Masand PS, Gupta S. Long-term side effects of newer generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry 2002; 14: 175–182
- Masand PS. Atypical antipsychotics in the treatment of affective symptoms: a review. Ann Clin Psychiatry 2004; 16: 3–13
- McCullough JP. Treatment for chronic depression: Cognitive behavioral analysis system of psychotherapy. Guilford Press, New York 2000
- McCullough JP, Jr. Treatment for chronic depression using cognitive-behavioral analysis system of psychotherapy (CBASP). J Clin Psychol 2003; 59: 833–846
- McCusker J, Cole M, Keller E, Bellavance F, Berard A. Effectiveness of treatments of depression in older ambulatory patients. Arch Intern Med 1998; 158: 705–712
- McIntyre RS, O′Donovan C. The human cost of not achieving full remission in depression. Can J Psychiatry 2004; 49(Suppl 1)10–16
- Melartin TK, Rytsala HJ, Leskala US, Lestela-Mielonen PS, Sokero TP, Isometsa ET. Severity and comorbidity predict episode duration and recurrence of DSM-IV major depressive disorder. J Clin Psychiatry 2004; 65: 810–819
- Mintz J, Mintz LI, Arruda MJ, Hwang SS. Treatments of depression and functional capacity to work. Arch Gen Psychiatry 1992; 49: 761–768
- Mittmann N, Herrmann N, Einarson TR, et al. The efficacy, safety and tolerability of antidepressants in late life depression: a meta-analysis. J Affect Disord 1997; 46: 191–217
- Möller HJ. Suicide, suicidality and suicide prevention in affective disorders. Acta Psychiatr Scand (Suppl) 2003; 418: 73–80
- Möller HJ. Is there evidence for negative effects of antidepressants on suicidality in depressive patients?. Eur Arch Psychiatry Clin Neurosci 2006; 256(8)476–496
- Möller HJ, Fuger J, Kasper S. Efficacy of new generation antidepressants: meta-analysis of imipramine-controlled studies. Pharmacopsychiatry 1994; 27: 215–223
- Möller HJ, Demyttenaere K, Sacchetti E, Rush AJ, Montgomery SA. Improving the chance of recovery from the short- and long-term consequences of depression. Int Clin Psychopharmacol 2003; 18: 219–225
- Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001; 62(Suppl 3)10–21
- Montgomery SA. Dopaminergic deficit and the role of amisulpride in the treatment of mood disorders. Int Clin Psychopharmacol 2002; 17(Suppl 4)S9–15
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389
- Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: a review of the evidence for drug-induced sexual dysfunction. J Affect Disord 2002; 69: 119–140
- Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry 1999; 156: 1000–1006
- Müller-Oerlinghausen B, Ahrens B, Grof E, et al. The effect of long-term lithium treatment on the mortality of patients with manic-depressive and schizoaffective illness. Acta Psychiatr Scand 1992; 86: 218–222
- Müller-Oerlinghausen B, Wolf T, Ahrens B, et al. Mortality during initial and during later lithium treatment: A collaborative study by the International Group for the Study of Lithium-treated Patients (IGSLI). Acta Psychiatr Scand 1994; 90: 295–297
- Müller-Oerlinghausen B, Berghöfer A, Ahrens B. The antisuicidal and mortality-reducing effect of lithium prophylaxis: consequences for guidelines in clinical psychiatry. Can J Psychiatry 2003; 48: 433–439
- Muijsers RB, Plosker GL, Noble S. Spotlight on sertraline in the managment of major depressive disorder in elderly patients. CNS Drugs 2002; 16: 789–794
- Mulsant BH, Pollock BG. Treatment-resistant depression in late life. J Geriatr Psychiatry Neurol 1998; 11: 186–193
- Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997a; 349: 1436–1442
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997b; 349: 1498–1504
- Mynors-Wallis LM, Gath DH, Day A, Baker F. Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care. Br Med J 2000; 320: 26–30
- Murphy JM, Nierenberg AA, Laird NM, Monson RR, Sobol AM, Leighton AH. Incidence of major depression: prediction from subthreshold categories in the Stirling County Study. J Affect Disord. 2002; 68(2–3)251–259
- Nelson JC. Managing treatment-resistant depression. J Clin Psychiatry 2003; 64(Suppl 1)5–12
- Neumeister A, Goessler R, Lucht M, Kapitany T, Bamas C, Kasper S. Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biol Psychiatry 1996; 39(1)16–21
- Nezu AM. Efficacy of social problem solving therapy for unipolar depression. J Consult Clin Psychol 1986; 54: 196–202
- NICE. Depression: management of depression in primary and secondary care NICE Guidance. 2004.
- Consensus Development Conference NIMH. Consensus Development Conference Statement. Mood disorders: pharmacologic prevention of recurrences. Am J Psychiatry 1985; 142: 469–476
- Nolen WA, Zohar J, Roose SP, Amsterdam JD, editors. 1994. Refractory depression: Current strategies and future directions. Chichester: J. Wiley & Sons.
- Nordstrom P, Asberg M, Aberg-Wistedt A, Nordin C. Attempted suicide predicts suicide risk in mood disorders. Acta Psychiatr Scand 1995a; 92: 345–350
- Nordstrom P, Samuelsson M, Asberg M. Survival analysis of suicide risk after attempted suicide. Acta Psychiatr Scand 1995b; 91: 336–340
- Nulman I, Koren G. The safety of fluoxetine during pregnancy and lactation. Teratology 1996; 53: 304–308
- Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. New Engl J Med 1997; 336: 258–262
- Old Age Depression Interest Group. 1993. How long should the elderly take antidepressants? A double-blind placebo-controlled study of continuation/prophylaxis therapy with dothiepin. Br J Psychiatry 162:175–182.
- Ostroff R, Nelson JC. Risperidone augmentation of SSRIs in major depression. J Clin Psychiatry 1999; 60: 256–259
- Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT 2004; 20(1)13–20
- Papakostas GI, Petersen T, Mahal Y, Mischoulon D, Nierenberg AA, Fava M. Quality of life assessments in major depressive disorder: a review of the literature. Gen Hosp Psychiatry 2004; 26: 13–17
- Parker G, Parker K. Which antidepressants flick the switch?. Aust NZ J Psychiatry 2003; 37: 464–468
- Paykel ES. Epidemiology of refractory depression. Refractory depression: Current strategies and future directions, WA Nolen, J Zohar, SP Roose, JD Amsterdam. J. Wiley & Sons, Chichester 1994; 3–17
- Paykel ES. Continuation and maintenance therapy in depression. Br Med Bull 2001; 57: 145–159
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995; 25: 1171–1180
- Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy. A controlled trial. Arch Gen Psychiatry 1999; 56: 829–835
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand 2000; 403(Suppl 2000)17–25
- Petersen T, Harley R, Papakostas GI, Montoya HD, Fava M, Alpert JE. Continuation cognitive-behavioral therapy maintains attributional style improvement in depressed patients responding acutely to fluoxetine. Psychol Med 2004; 34: 555–561
- Picinelli M, Gomez Homen F. Gender differences in the epidemiology of affective disorders and schizophrenia. World Health Organization, GenevaSwitzerland 1997
- Pjrek E, Winkler D, Kasper S. Pharmacotherapy of seasonal affective disorder. CNS Spectr 2005; 10(8)664–669
- Preskorn SH. Recent pharmacologic advances in antidepressant therapy for the elderly. Am J Med 1993; 94(Suppl 5A)2–12S
- Pridmore S, Turnier-Shea Y. Medication options in the treatment of treatment-resistant depression. Aust NZ J Psychiatry 2004; 38: 219–225
- Prien RF. Efficacy of continuation drug therapy of depression and anxiety: issues and methodologies. J Clin Psychopharmacol :S 1990; 10: 86–90S
- Prien RF, Kocsis JH. Long-term treatment of mood disorders. Psychopharmacology: The fourth generation of progress, EB Floyd, DJ Kupfer. Raven Press, New York 1995; 1067–1079
- Prien RF, Kupfer DJ. Continuation drug therapy for major depressive episodes: How long should it be maintained?. Am J Psychiatry 1986; 143: 18–23
- Rabheru K. Special issues in the management of depression in older patients. Can J Psychiatry 2004; 49(Suppl 1)41–50
- Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther 2004; 75: 234–241
- Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50: 85–94
- Rehm LP. Behavior Therapy for Depression. Academic Press, New York 1979
- Reimherr FW, Amsterdam JD, Quitkin FM, et al. Optimal length of continuation therapy in depression: a prospective assessment during long-term fluoxetine treatment. Am J Psychiatry 1998; 155(9)1247–1253
- Rudolph RL, Entsuah R, Chitra R. A meta-analysis of the effects of venlafaxine on anxiety associated with depression. J Clin Psychopharmacol 1998; 18: 136–144
- Reynaert-Dupuis C, Zdanowicz N, , for 600A-GAP-BE Study GroupLeyman S, Mignon A, Seghers S. 2002. Efficacy and tolerance of venlafaxine in depressed patients switched from prior antidepressant treatment. Primary Care Psychiatry 8:63–68.
- Reynolds CF 3rd, Frank E, Kupfer DJ, et al. Treatment outcome in recurrent major depression: a post hoc comparison of elderly (“young old”) and midlife patients. Am J Psychiatry 1996; 153(10)1288–1292
- Reynolds CF3rd, Alexopoulos GS, Katz IR, Lebowitz BD. Chronic depression in the elderly: approaches for prevention. Drugs Aging 18. 2001; 507–514
- Riemann D, Konig A, Hohagen F, et al. How to preserve the antidepressive effect of sleep deprivation: A comparison of sleep phase advance and sleep phase delay. Eur Arch Psychiatry Clin Neurosci 1999; 249: 231–237
- Rocca P, Fonzo V, Ravizza L, Rocca G, Scotta M, Zanalda E, Bogetto F. A comparison of paroxetine and amisulpride in the treatment of dysthymic disorder. J Affect Disord 2002; 70(3)313–317
- Roose SP, Suthers KM. Antidepressant response in late-life depression. J Clin Psychiatry 1998; 59(Suppl 10)4–8
- Roose SP, Laghrissi-Thode F, Kennedy JS, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. J Am Med Assoc 1998; 279: 287–291
- Roose SP, Sackeim HA, Krishnan KR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trail. Am J Psychiatry 161:2050–2059.
- Rosen LN, Targum SD, Terman M, et al. Prevalence of seasonal affective disorder at four latitudes. Psychiatry Res 1990; 31: 131–144
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry 1998; 44: 77–87
- Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 1984; 41: 72–80
- Rothschild AJ. Challenges in the treatment of depression with psychotic features. Biol. Psychiatry 2003; 53: 680–690
- Rothschild AJ, Samson JA, Bessette MP, Carter-Campbell JT. Efficacy of the combination of fluoxetine and perphenazine in the treatment of psychotic depression. J Clin Psychiatry 1993; 54: 338–342
- Ruhe HG, Huyser J, Swinkels JA, Schene AH. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry 2006; 67(12)1836–1855
- Ruhrmann S, Kasper S, Hawellek B, et al. Effects of fluoxetine versus bright light in the treatment of seasonal affective disorder. Psychol Med 1998; 28: 923–933
- Rush AJ. Strategies and tactics in the management of maintenance treatment for depressed patients. J Clin Psychiatry 1999; 60(Suppl 14)21–26
- Rush AJ, Kupfer DJ. Strategies and tactics in the treatment of depression. Treatment of psychiatric disorders3rd edn, GO Gabbard. American Psychiatric Publishing, Inc, Washington, DC 2001; 1417–1439
- Rush AJ, Thase ME. Psychotherapies for depressive disorders. WPA Series. Evidence and experience in psychiatry Volume 1, M Maj, N Sartorius. John Wiley & Sons, Depressive disorders. Chichester 1999; 161–206
- Rush AJ, Beck AT, Kovacs M, Hollon SD. Comparative efficacy of cognitive therapy and pharmacotherapy in the treatment of depressed outpatients. Cogn Ther Res 1977; 1: 17–37
- Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 2004; 25(1)119–142
- Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005; 58(5)347–354
- Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. J Am Med Assoc 2001; 285: 1299–1307
- Sackeim HA, Roose SP, Burt T. Optimal length of antidepressant trials in late-life depression. J Clin Psychopharmacol 2005; 25(4 Suppl 1))S34–37
- Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation 2001; 104(16)1894–1898
- Schou M. Lithium prophylaxis: myths and realities. Am J Psychiatry 1989; 146: 573–576
- Schou M. Forty years of lithium treatment. Arch Gen Psychiatry 1997; 54: 9–13
- Schou M. Has the time come to abandon prophylactic lithium treatment? A review for clinicians. Pharmacopsychiatry 1998; 31(6)210–215
- Schou M. Suicidal behavior and prophylactic lithium treatment of major mood disorders: a review of reviews. Suicide Life Threat Behav 2000; 30: 289–293
- Schulberg HC, Block MR, Madonia MJ, et al. Treating major depression in primary care practice: eight month clinical outcomes. Arch Gen Psychiatry 1996; 53: 913–919
- Scott J. Chronic depression. Br J Psychiatry 1988; 153: 287–297
- Scott J, Gilvarry E, Farrell M. Managing anxiety and depression in alcohol and drug dependence. Addict Behav 1998; 23: 919–931
- Shelton RC. The use of antidepressants in novel combination therapies. J Clin Psychiatry 2003; 64(Suppl 2)14–18
- Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St. John's Wort in major depression. A randomized controlled trial. J Am Med Assoc 2001a; 285: 1978–1986
- Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001b; 158: 131–134
- Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 2005; 66(10)1289–1297
- Shores MM, Pascualy M, Veith RC. Depression and heart disease: Treatment trials. Semin Clin Neuropsychiatry 1998; 3: 87–101
- Simon GE, VonKorff M, Heiligenstein JH, et al. 1996. Initial antidepressant choice in primary care. Effectiveness and cost of fluoxetine versus tricyclic antidepressants. J Am Med Assoc 275:(24): 1897–1902.
- Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry 2006; 163(1)41–47
- Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. Br J Psychiatry 2002; 180: 396–404
- Solomon DA, Bauer MS. Continuation and maintenance pharmacotherapy for unipolar and bipolar mood disorders. Psychiatr Clin North Am 1993; 16: 515–540
- Solomon DA, Keller MB, Leon AC, et al. Recovery from major depression. A 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry 1997; 54: 1001–1006
- Souza FGM, Goodwin GM. Lithium treatment and prophylaxis in unipolar depression: a meta-analysis. Br J Psychiatry 1991; 158: 666–675
- Spiker DG, Weiss JC, Dealy RS, Griffin SJ, Hanin I, Neil JF, Perel JM, Rossi AJ, Soloff PH. The pharmacological treatment of delusional depression. Am J Psychiatry 1985; 142: 430–436
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. J Am Med Assoc 1999; 282(18)1737–1744
- Sproule BA, Hardy BG, Shulman KI. Differential pharmaco-kinetics of lithium in elderly patients. Drugs Aging 2000; 16: 165–177
- Staab JP, Evans DL. Efficacy of venlafaxine in geriatric depression. Depress Anxiety 2000; 12(Suppl 1)63–68
- Stahl SM, Entsuah R, Rudolph RL. Comparative efficacy between venlafaxine and SSRIs: a pooled analysis of patients with depression. Biol Psychiatry 2002; 52: 1166–1174
- Stassen HH, Angst J, Delini-Stula A. Delayed onset of action of antidepressant drugs?. Survey of results of Zurich meta-analyses: Pharmacopsychiatry 1996; 29: 87–96
- Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000; 57: 601–607
- Sternbach H. The serotonin syndrome. Am J Psychiatry 1995; 148: 705–713
- Storosum JG, Elferink AJ, van Zwieten BJ, et al. Short-term efficacy of tricyclic antidepressants revisited: a meta-analytic study. Eur Neuropsychopharmacol 2001; 11: 173–180
- Stowe ZN, Cohen LS, Hostetter A, Ritchie JC, Owens MJ, Nemeroff CB. Paroxetine in human breast milk and nursing infants. Am J Psychiatry 2000; 157: 185–189
- Szegedi A, Muller MJ, Anghelescu I, Klawe C, Kohnen R, Benkert O. Early improvement under mirtazapine and paroxetine predicts later stable response and remission with high sensitivity in patients with major depression. J Clin Psychiatry 2003; 64: 413–420
- Taylor WD, Doraiswamy PM. A systematic review of antidepressant placebo-controlled trials for geriatric depression: limitations of current data and directions for the future. Neuropsychopharmacology 2004; 29(12)2285–2299
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000; 68: 615–623
- Thase ME. Redefining antidepressant efficacy toward long-term recovery. J Clin Psychiatry 1999; 60(Suppl 6)15–19
- Thase ME. The clinical, psychosocial, and pharmacoeconomic ramifications of remission. Am J Manage Care (Suppl) 2001; 11: S377–385
- Thase ME. What role do atypical antipsychotic drug have in treatment-resistant depression?. J Clin Psychiatry 2002; 63: 95–103
- Thase ME, Howland RH. Refractory depression: relevance of psychosocial factors and therapies. Psychiatr Ann 1994; 24: 232–240
- Thase ME, Rush AJ. Treatment-resistant depression. Psychopharmacology: The fourth generation of progress, FE Bloom, DJ Kupfer. Raven Press, New York 1995; 1081–1097
- Thase ME, Rush AJ. When at first you don't succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry 1997; 58(Suppl 13)23–29
- Thase ME, Howland RH, Friedman ES. Treating antidepressant nonresponders with augmentation strategies: an overview. J Clin Psychiatry 1998; 59(Suppl 5)5–12
- Thase ME, Entsuah A, Rudolph R. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry 2001; 178: 234–241
- Thase ME, Rush AJ, Howland RH, et al. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry 2002; 59: 233–239
- Thompson C 2002. Light therapy in the treatment of seasonal and non-seasonal affective disorders: a meta-analysis of randomized controlled trails. J Affect Disord 68 (abstract number S4.4):89.
- Thorpe L, Whitney DK, Kutcher SP, Kennedy SH, Depression Work Group. CANMAT. Clinical guidelines for the treatment of depressive disorders. VI. Special populations. Can J Psychiatry 2001; 46((Suppl 1))63–76
- Tollefson GD, Bosomworth JC, Heiligenstein JH, Potvin JH, Holman S. A double-blind, placebo-controlled clinical trial of fluoxetine in geriatric patients with major depression. The Fluoxetine Collaborative Study Group. Int Psychogeriatr 1995; 7: 89–104
- Tondo L, Jamison KR, Baldessarini RJ. Effect of lithium maintenance on suicidal behavior in major mood disorders. Ann NY Acad Sci 1997; 836: 339–351
- Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001; 104: 163–172
- Tranter R, O′Donovan C, Chandarana P, Kennedy S. Prevalence and outcome of partial remission in depression. J Psychiatry Neurosci 2002; 27: 241–247
- Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. New Engl J Med 2006; 354(12)1243–1252
- Tuunainen A, Kripke DF, Endo T. 2004. Light therapy for nonseasonal depression. Cochrane Database Syst Rev. CD 004050.
- Uehlinger C, Nil R, Amey B, Baumann P, Dufour H. Citalopram-lithium combination treatment of elderly depressed patients: a pilot study. Int J Ger Psychiatry 1995; 10: 281–287
- Unützer J, Patrick DL, Diehr P, Simon G, Grembowski D, Katon W. Quality adjusted life years in older adults with depressive symptoms and chronic medical disorders. Int Psychogeriatr 2000a; 12: 15–33
- Unützer J, Simon G, Belin TR, Datt M, Katon W, Patrick D. Care for depression in HMO patients aged 65 and older. J Am Geriatr Soc 2000b; 48: 871–878
- US Food and Drug Administration FDA. 2005 [ www.fda.gov/cder/drug/antidepressants/default.htm].
- üstün TB, Sartorius N. Mental Illness in general health care: an international study. Wiley, Chichester 1995
- Valenstein M, Taylor KK, Austin K, Kales HC, McCarthy JF, Blow FC. Benzodiazepine use among depressed patients treated in mental health settings. Am J Psychiatry 2004; 161: 654–661
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 85–102
- Viguera AC, Baldessarini RJ, Friedberg J. Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry 1998; 5(6)293–306
- Vos T, Haby MM, Barendregt JJ, Kruijshaar M, Corry J, Andrews G. The burden of major depression avoidable by longer-term treatment strategies. Arch Gen Psychiatry 2004; 61: 1097–1103
- Wang J. A longitudinal population-based study of treated and untreated major depression. Med Care 2004; 42: 543–550
- Waraich P, Goldner EM, Somers JM, Hsu L. Prevalence and incidence studies of mood disorders: a systematic review of literature. Can J Psychiatry 2004; 49: 124–138
- Weissman MM, Leaf PJ, Bruce ML, Florio L. The epidemiology of dysthymia in five communities: rates, risks, comorbidity, and treatment. Am J Psychiatry 1988; 145: 815–819
- Wells KB, Hays RD, Burnam MA, Rogers W, Greenfield S, Ware JE, Jr. Detection of depressive disorder for patients receiving prepaid or fee-for service care. Results from the Medical Outcomes Study. J Am Med Assoc 1989a; 262: 3298–3302
- Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. J Am Med Assoc 1989b; 262: 914–919
- Wernecke U, Horn O, Taylor DM. How effective is St John's wort? The evidence revisited. J Clin Psychiatry 2004; 65: 611–617
- Werneke U, Northey S, Bhugra D. Antidepressants and sexual dysfunction. Acta Psychiatr Scand 2006; 114(6)384–397
- Whyte EM, Dew MA, Gildengers A, et al. Time course of response to antidepressants in late-life major depression: therapeutic implications. Drugs Aging 2004; 21: 531–554
- Wiegand MH, Lauer CJ, Schreiber W. Patterns of response to repeated total sleep deprivations in depression. J Affect Disord 2001; 64: 257–260
- Wijkstra J, Lijmer J, Balk FJ, Geddes JR, Nolen WA. Pharmacological treatment for unipolar psychotic depression: Systematic review and meta-analysis. Br J Psychiatry 2006; 188: 410–415
- Wilens TE, Biederman J, Baldessarini RJ, et al. Cardiovascular effects of therapeutic doses of tricyclic antidepressants in children and adolescents. J Am Acad Child Adolesc Psychiatry 1996; 35: 1491–1501
- Wilkinson D, Holmes C, Woolford J, Stammers S, North J. Prophylactic therapy with lithium in elderly patients with unipolar major depression. Int J Geriatr Psychiatry 2003; 18(4)353–354
- Wilson K, Mottram P. A comparison of side effects of selective serotonin reuptake inhibitors and tricyclic antidepressants in older depressed patients: a meta-analysis. Int J Geriatr Psychiatry 2004; 19: 754–762
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go?. Biol Psychiatry 1999; 46: 445–453
- Wirz-Justice A, Graw P, Krauchi K, et al. Natural’ light treatment of seasonal affective disorder. J Affect Disord 1996; 37: 109–120
- Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry 1996; 153: 1132–1137
- Wisner KL, Gelenberg AJ, Leonard H, Zarin D, Frank E. Pharmacologic treatment of depression during pregnancy. J Am Med Assoc 1999; 282: 1264–1269
- Wittchen HU. Epidemiology of affective disorders. Contemporary psychiatry, H Helmchen, F Henn, H Lauter, N Sartorius. Springer, Heidelberg 2000; 3: 231–241
- Wittchen HU, Lieb R, Wunderlich U, Schuster P. Comorbidity in primary care: presentation and consequences. J Clin Psychiatry 1999; 60(Suppl 7)29–36
- World Health Organization. 1991. Tenth revision of the international classification of diseases, Chapter V (F): Mental and behavioural disorders. Geneva: World Health Organization.
- World Health Organization. 1992. The ICD-10 Classification of Mental and Behavioural Disorders – Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization.
- World Psychiatric Association Dysthymia Working Group. 1995. Dysthymia in clinical practice. Br J Psychiatry 166:174–183.
- Worthington JJ 3rd, Peters PM. Treatment of antidepressant-induced sexual dysfunction. Drugs Today (Barc) 2003; 39: 887–896
- Worthington JJ 3rd, Kinrys G, Wygant LE, Pollack MH. Aripiprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. Int Clin Psychopharmacol 2005; 20(1)9–11
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry 1990; 147: 14–21
- Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med 1999; 61: 6–17
- Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry 2001; 58: 55–61
- Zajecka J. Strategies for the treatment of antidepressant-related sexual dysfunction. J Clin Psychiatry 2001; 62(Suppl 3)35–43
- Zimmer B, Rosen J, Thornton JE, Perel JM, Reynolds CF 3rd. Adjunctive lithium carbonate in nortriptyline-resistant elderly depressed patients. J Clin Psychopharmacol 1991; 11: 254–256
- Zullino D, Baumann P. Lithium augmentation in depressive patients not responding to selective serotonin reuptake inhibitors. Pharmacopsychiatry 2001; 34: 119–127