Abstract
Objectives: Hyperprolactinaemia (HPRL) is a classical side effect of antipsychotic drugs primarily attributed to blockade of dopamine D2 subtype receptors in the pituitary gland. Although dopamine is considered the primary factor inhibiting prolactin release, the activity of prolactin-producing lactotrophs is also regulated by the secretagogues thyrotrophin releasing hormone, vasoactive intestinal polypeptide and serotonin (5-hydroxytryptamine; 5-HT).
Methods: We describe the association between HPRL and a set of 29 SNPs from 5-HT receptor genes HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, HTR3B and HTR6 in a population of 446 Caucasians (221 males/225 females) with a clinical diagnosis of schizophrenia (according to ICD-10: F20) who were treated with classical and/or atypical antipsychotic drugs.
Results: None of the studied autosomal markers were found to be associated with HPRL. However, a significant association was established between various HTR2C polymorphisms and HPRL.
Conclusions: This study revealed an association between HPRL and X-chromosome haplotypes comprised of the rs569959 and rs17326429 polymorphisms.
Introduction
Regular therapy for schizophrenia includes maintenance antipsychotic treatment, which improves the long-term prognosis of the disease and contributes to its transition into remission (Tandon Citation2011; Bruijnzeel et al. Citation2014). Schizophrenic patients often require this therapy their entire lives (Miyamoto et al. Citation2005). Unfortunately, antipsychotics also have a spectrum of side effects, including metabolic, endocrine, cardiovascular and movement disorders (Staller Citation2006; Hasan et al. Citation2012, Citation2013; Lally and MacCabe Citation2015). Low efficacy of therapy and intolerable side effects are the main causes of non-compliance and discontinuation of treatment in more than 70% of patients with schizophrenia, often resulting in the relapse of psychosis (Miyamoto et al. Citation2005). One of the common side effects of these drugs is hyperprolactinaemia (HPRL; Ajmal et al. Citation2014; Peuskens et al. Citation2014). Prolactin (PRL; Latin pro = for; lac, gen. lactis = milk) secretion is under the permanent inhibitory control of dopamine (Fitzgerald and Dinan Citation2008; Peuskens et al. Citation2014), and antipsychotic drugs are potent dopamine receptor blockers. However, the vulnerability to developing this side effect may also depend on the functionality of 5-hydroxytryptamine (5-HT) receptors. First, the relationship with certain genetic variants should be studied, as this may indicate dysfunction of its biochemical product.
PRL, also called lactotrophin hormone, is a 199-amino acid polypeptide hormone synthesised and secreted in a pulsatile manner (∼10 peaks per day in young adults) by the lactotroph cells of the anterior lobe of the pituitary gland (i.e., the adenohypophysis) (Fitzgerald and Dinan Citation2008; Peuskens et al. Citation2014). These cells comprise 20–50% of the cellular population of the gland (Fitzgerald and Dinan Citation2008; Peuskens et al. Citation2014). Dopamine is the most important factor inhibiting hypothalamic PRL release (Fitzgerald and Dinan Citation2008; Peuskens et al. Citation2014), exerting tonic inhibition via two main pathways: the tuberoinfundibular dopaminergic (so-called TIDA) system and the tuberohypophysial tract (Fitzgerald and Dinan Citation2008; Peuskens et al. Citation2014). The TIDA system consists of a population of dopaminergic neurons in the arcuate nucleus of the hypothalamus and is the most important pathway regulating PRL release in humans. These dopaminergic neurons release dopamine into the perivascular spaces of the medial eminence, where it is subsequently transported via long portal vessels to the anterior lobe of the pituitary. Dopamine binds to the dopamine D2 receptors (DRD2s) on the membranes of lactotroph cells. DRD2 stimulation inhibits transcription of PRL, the synthesis and release of PRL, and lactotroph proliferation (Fitzgerald and Dinan Citation2008; Peuskens et al. Citation2014). Depending on their location within the pituitary, lactotrophs exhibit marked heterogeneity in their response to dopamine; those located in the inner zone are more responsive to dopamine and those in the outer zone are more responsive to thyrotrophin-releasing hormone (TRH) (Fitzgerald and Dinan Citation2008).
At least three physiologically active substances play a role in promoting PRL release from pituitary cells: TRH, vasoactive intestinal polypeptide (VIP) and serotonin (also known as 5-HT) (Apfelbaum Citation1998). However, 5-HT stimulates PRL secretion through a complex, multi-level action on both the hypothalamus and the pituitary gland (Jørgensen Citation2007; Fitzgerald and Dinan Citation2008). Several studies have substantiated the role of serotonergic raphe neurons mediating this effect through the hypothalamus (Jørgensen Citation2007). In particular, serotonergic terminals stimulating the paraventricular nucleus of the hypothalamus are thought to play a key role in PRL secretion by stimulating oxytocin or VIP release (Fitzgerald and Dinan Citation2008). However, evidence suggests that dopaminergic TIDA cells can be inhibited by the stimulation of GABAergic interneurons (Mirkes and Bethea Citation2001; Fitzgerald and Dinan Citation2008), which in turn can be activated by 5-HT2 receptors (HTR2s) (Loonen and Ivanova Citation2016). Whether 5-HT reacts with lactotrophic pituitary cells directly is uncertain (Jørgensen Citation2007). Initial studies failed to show a direct stimulatory effect of 5-HT on PRL release from pituitary tissue in vitro (Lamberts and MacLeod Citation1978; Garthwaite and Hagen Citation1979). However, later studies showed that stimulation of HTR2 affects PRL release by acting directly at the pituitary gland level (Meltzer et al. Citation1983; Apfelbaum Citation1987; Balsa et al. Citation1998). Stimulation of HTR2 not only promoted PRL release via the local autoparacrine action of VIP, but also directly by activating HTR2s on lactotrophs (Apfelbaum Citation1998). Data concerning HTRs expressed in the pituitary gland and their functions are very limited. De Souza (Citation1986) described the presence of 5-HT2 binding sites in all three lobes of the rat pituitary gland. However, the density in the anterior lobe was much lower than the density in the intermediate and posterior lobes. To the best of our knowledge, no data are available of the contribution of HTR2A relative to that of HTR2C in the pituitary gland. Nevertheless, although a direct pro-secretory effect on anterior pituitary cells has not been established, 5-HT is generally considered to have an indirect modulator effect on PRL secretion, with the hypothalamus as its predominant site of action, as several 5-HT receptor types have been found to play a role in PRL secretion (Jørgensen Citation2007).
The pharmacological effects of 5-HT are mediated through HTR binding (Pandey et al. Citation1995; Filip and Bader Citation2009). These HTRs are classified into seven families (HTR1 to HTR7) and at least 14 different subtypes. Except for the HTR3, which is ionotropic, all of these receptors belong to G protein-coupled receptors (Pandey et al. Citation1995; Filip and Bader Citation2009). Irrespective of their localisation, the involvement of HTR1A, HTR2A, HTR2C and HTR3 in the serotonergic-induced PRL response is well documented, and HTR1B, HTR5A and HTR7 are possibly involved (Jørgensen Citation2007). Activation of HTR1A causes neuronal hyperpolarization due to the activation of G protein-coupled K+ channels. These inhibitory receptors are localised as autoreceptors on the soma and dendrites of 5-HT neurons in the raphe nuclei or as postsynaptic receptors in several limbic areas. HTR1A is located on human chromosome 5q11.1-q13 (Filip and Bader Citation2009). Stimulation of HTR1A results in PRL release, which can be blocked with selective HTR1A antagonists (Jørgensen et al. Citation2001; Kühn et al. Citation2002). The HTR2 family consists of three subtypes: HTR2A, HTR2B (embryological) and HTR2C. The 5-HT2A and the 5-HT2C (formerly termed 5-HT1C) receptors have similar cerebral distribution and function (Leysen Citation2004). HTR2s couple to Gq/11 and the phosphoinositol hydrolysis signal transduction system to stimulate inositol 1,4,5-trisphosphate accumulation and intracellular Ca2+ release (Filip and Bader Citation2009). These receptors spontaneously signal for cellular effector mechanisms in the absence of ligands, and HTR2C may have higher constitutive activity than HTR2A (Aloyo et al. Citation2009). In this situation, a ligand binding to the receptor may also act as an inverse agonist, changing the activity of the receptor in the opposite direction instead of increasing or blocking activity. Whether HTR2A or HTR2C is the most important receptor mediating the PRL response is still unclear, but both appear to contribute (Jørgensen Citation2007). HTR2A is located on human chromosome 13q14-q21 and HTR2C on human chromosome Xq24 (Filip and Bader Citation2009). The HTR3 family is an ion channel assembled as a pentamer of several subunits. Molecular composition of the HTR3 family includes multiple isoforms (5-HT3A, 5-HT3B, 5-HT3C, 5-HT3D and 5-HT3E) that are products of different genes located on human chromosome 11 (HTR3A and HTR3B: 11q23.1-q23.2) due to a local duplication event, or on human chromosome 3 (HTR3C, HTR3D and HTR3E: 3q27.1). To determine the functional properties of HTR3, the heteromeric combination of 5-HT3A and 5-HT3B subunits is necessary (Filip and Bader Citation2009). The brain HTR3s mediate rapid neuronal depolarisation and excitation in several areas due to a transient inward current, resulting from the opening of non-selective cation channels (Na+ and Ca2+ influx, K+ efflux). Evidence suggests that both peripheral and central HTR3s are involved in the serotonergic-induced PRL response. However, the involvement of other HTRs has not been clarified. The human genes HTR1B and HTR6 are located on human chromosomes 6 (6q13) and 1 (1p35-p36) (Filip and Bader Citation2009).
Several authors have studied the functional consequences of specific polymorphisms in HTR2A and HTR2C after challenging the system with (indirect) 5-HT agonists, such as meta-chlorophenylpiperazine (m-CPP), fenfluramine or citalopram and measuring the PRL response (Kühn et al. Citation2002; Reist et al. Citation2004; Bruce et al. Citation2005; Corregiari et al. Citation2012). Kühn et al. (Citation2002) observed that Cys23Ser HTR2C (rs6318) exhibits a slightly stronger, but not significantly different, PRL response to the m-CPP challenge. According to Reist et al. (Citation2004), T102C HTR2A (rs6313), but not His452Tyr HTR2A (rs6314), has an effect on changes in the PRL level after challenging with fenfluramine, though the difference is not significant. Women with bulimia nervosa who are GG homozygous at -1438G/A HTR2A (rs6311) have a blunted PRL response following m-CPP compared to controls (Bruce et al. Citation2005). Corregiari et al. (Citation2012) measured G861C HTR1B (rs6296), T102C HTR2A (rs6313) and C516T HTR2A (rs6305) in patients with obsessive–compulsive disorders. Only patients who were CC homozygous at G681C HTR1B (rs6296) had a different PRL response to acute intravenous challenge with citalopram.
Here, we present new data on the association between a set of 29 polymorphisms in 5-HT receptor genes (HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, HTR3B, HTR6) and HPRL in antipsychotic drug-treated patients with schizophrenia from Siberia, Russian Federation.
Patients and methods
Patients
The work described in this article was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki 1975, revised in Fortaleza, Brazil, 2013) for experiments involving humans. The patients in this study were recruited from three psychiatric hospitals in Tomsk, Kemerovo and Chita oblasts in Siberia. Written informed consent was obtained from each patient after obtaining approval for the study (protocol N63/7.2014) from the Local Bioethics Committee of the Mental Health Research Institute. None of the participants had a compromised capacity/ability to consent; thus, consent from the next of kin was not necessary and not recommended by the local ethics committee. The inclusion criteria were a clinical diagnosis of schizophrenia according to ICD-10 (F20) and age 18–75 years old. Exclusion criteria were non-Caucasian physical appearance (e.g., Mongoloid, Buryats or Khakassians), pregnancy or relevant gynecological and endocrine (thyroid) disorders, relevant pharmacological withdrawal symptoms or organic brain disorders (e.g., epilepsy, Parkinson’s disease). The Positive and Negative Syndrome Scale for Schizophrenia was used to assess the leading clinical symptoms. In our study the leading positive/negative symptoms were observed in 69/31% of patients accordingly. The paranoid-hallucinatory and delusional disorders dominated among the productive disorders. The simplex syndrome with emotional and volitional and associative reduction dominated among the negative disorders. A total of 191 patients were treated with conventional antipsychotics in oral and/or long-acting formulations. The most common conventional antipsychotic was haloperidol, which was used in 110 patients, but other treatments included oral chlorpromazine (CPZ), chlorprothixene, trifluoperazin and zuclopenthixol, and/or long-acting formulations of haloperidol-, zuclopenthixol- and flupenthixol-decanoate. A total of 176 patients were treated with atypical antipsychotics: risperidone, clozapine, quetiapine, olanzapine, amisulpride, paliperidone and sertindole. Different combinations of classical and atypical drugs were used by 79 patients. To compare antipsychotic medications, all dosages were converted into CPZ equivalents (CPZeq; Andreasen et al. Citation2010). Blood samples were taken 8 h after overnight fasting in tubes containing EDTA for DNA extraction and in tubes with CAT (clot activator) to obtain serum (BD Vacutainer). Blood with EDTA was stored in several aliquots at −20 °C until DNA isolation. Blood samples with CAT were centrifuged for 30 min at 1,500 rpm at 4 °C to obtain serum.
Hormone analysis
The PRL concentration was measured in serum using the AccuBind ELISA Microwells kit (Monobind, Lake Forest, CA). In this microplate immunoenzymatic assay, the ELISA has a sensitivity of 0.004 ng/well. This is equivalent to a sample containing 0.150 ng/ml PRL. The upper limits for normal PRL concentration were set at ≤20 ng/ml for men and ≤25 ng/ml for non-pregnant, non-nursing women (Kelly et al. Citation2013). This corresponds to the criteria for HPRL applied by Peuskens et al. (Citation2014).
DNA analysis
DNA was isolated from the leukocytes in whole peripheral blood from patients with mental disorders using the standard phenol-chloroform micro method (Ivanova et al. Citation2012). The mandatory condition was pre-freezing the blood.
Genotyping was carried out for HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, HTR3B and HTR6 on the MassARRAY Analyzer 4 (Agena Bioscience) using the set SEQUENOM Consumables iPLEX Gold 384. DNA sample preparation for SEQUENOM MassARRAY Analyzer 4 includes several steps: a standard PCR reaction to obtain the amplification products, a shrimp alkaline phosphatase reaction to neutralise the unincorporated dNTPs in the amplification products, the PCR iPLEX Gold extension reaction, and then placing the samples on a special chip (SpectroCHIP array) using Nanodispenser RS1000 prior to loading them into the analyser.
We selected a subset of 29 SNPs from the following 5-HT receptor genes: HTR1A, HTR1B, HTR2A, HTR2C, HTR3A, HTR3B and HTR6 (see Supplementary Table available online).
Statistical analysis
The Mann–Whitney test (MWT) was used to compare qualitative traits and χ2 test for categorical traits. Hardy–Weinberg equilibrium (HWE) was checked using the χ2 test except for SNPs in the X-chromosomal HTR2C. We analysed associations between the polymorphisms and HPRL using logistic regression models including HPRL as a dependent variable and polymorphisms as the predictors. Age, sex, duration of the disease, smoking, leading symptomatology and the CPZeq dose were used as covariates. Three genotypic models were analysed: log-additive, recessive and dominant models (Li and Ji Citation2005). First, we tested only the additive models, and only if they were significant did we test recessive and dominant models to verify the phenotypic effect of the allele.
The significance level for the study was estimated using methodology described in Li and Ji (Citation2005). This approach is based on the calculation of the effective number of truly independent tests estimated by the matrix of pair-wise correlations between SNPs. Using this approach, we estimated that 12 SNPs were independent, leading to the significance level of 0.0042 with Bonferroni correction for multiple testing. Therefore, the models with P < .0042 were considered significant.
All calculations were performed in the R v.3.1.3 statistical environment using basic R functions and the SNPassoc v.1.9-2 and haplo.ccs v.1.3.1 packages (González et al. Citation2007; French et al. Citation2012).
Results
The total sample consisted of 446 patients (221 males/225 females). The characteristics of studied patients are presented in . The women were significantly older (P = 2.6 × 108, MWT) than the men. Among the women, 86 were >50 years of age. Women suffered from the disease for a significantly longer (P = .0002, MWT) period of time. Significantly (P = 2.2 × 1016, χ2) more men were tobacco smokers. The median daily dose of antipsychotic was 500 mg CPZeq (quartiles 280; 750) in men and 320 mg CPZeq (quartiles 200; 750) in women (P = .0002, MWT). A total of 227 patients suffered from HPRL (98 males/129 females) according to the predefined criteria (Kelly et al. Citation2013; Peuskens et al. Citation2014).
Table 1. Characteristics of the studied patients.
From the list of 29 SNPs in 5-HT receptors, we excluded those with a minor allele frequency <5%. This left 24 SNPs for the analysis of associations with HPRL. Among these 24 SNPs, five had >5% missing genotypes: rs130058 (HTR1B), rs1928040 (HTR2A), rs6314 (HTR2A), rs9316233 (HTR2A) and rs1805054 (HTR3A). No deviation from HWE was found for any of the SNPs except rs1176744 (P = .002, χ2). Taking into account that we did not test a population sample, we kept this SNP in the analysis.
In logistic regression models adjusted for the described covariates, none of the studied autosomal markers were associated with HPRL. However, a significant association was established between HPRL and the X-chromosome polymorphism rs569959, and to a lesser extent rs17326429, which was most pronounced for the log-additive () and dominant models. A trend of association was found between rs3813929, rs6318, rs4911871 and rs5946189, but these P values failed to reach the threshold of 0.0042 (with Bonferroni correction for multiple testing). In all cases, the log-additive and dominant models were most significant.
Figure 1. The –log10 P values for log-additive models of the association between polymorphisms in serotonin receptor genes and hyperprolactinaemia in patients with schizophrenia. For the rs6314 polymorphism, the log-additive model could not be calculated due to the messiness of homozygotes for the rare allele.
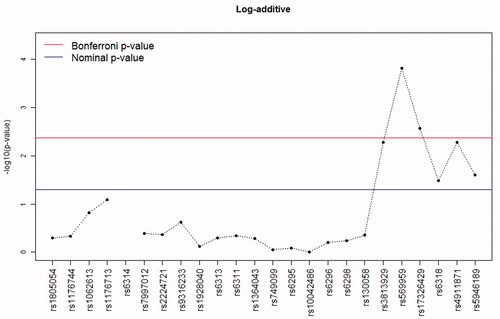
Taking into account the hemizygotic status of X-chromosomal markers for men, the analysis of these markers was carried out separately in men and women. For men, only the log-additive model was estimated. Although most of the markers were nominally significant (P < .05) in men and/or women, only the rs569959 in men reached the significance threshold accepted in this study. The fact that other SNPs did not achieve significance is likely explained by the reduced power of this sex-specific analysis.
Because atypical antipsychotics may act as inverse agonists on biochemically normally functioning HTR2Cs (Aloyo et al. Citation2009; Loonen and Ivanova Citation2016), we also analysed the association in 191 patients (91 males/100 females) who were only using first-generation antipsychotics (FGAs). None of the markers were significantly associated with HPRL in this group, probably due to the small sample size, and the odds ratio was not invariably higher when users of second-generation antipsychotics (SGAs) were excluded ().
Table 2. Odds ratios (95% confidence interval) for the association between HPRL and X-chromosome markers applying the log-additive model in users of both first- and second-generation antipsychotics (FGA & SGA) compared to patients only using classical antipsychotics.
Based on the results of the analysis of separate SNPs, we analysed the association between HPRL and haplotypes of X-chromosome SNPs. The analysis was carried out in the total sample, as well as in men and women separately. The most pronounced association with HPRL was identified for the TGAGGT haplotype. Also, a trend of association was observed for the CGGCAC haplotype (). In both cases, the G allele of rs569959 was present in the haplotype, which was significantly associated with HPRL in the analysis of separate SNPs. The most significant association was found for a combination of the rs569959*G and rs17326429*A alleles; the latter also exhibited a trend of association with HPRL with a P value very close to the threshold of significance.
Table 3. Analysis of the association between HPRL and X-chromosome haplotypes in the total sample.
Discussion
We studied the association between polymorphisms of relevant 5-HT receptor genes and antipsychotic drug-induced HPRL in white patients with schizophrenia from Siberia. We excluded patients with physiological or pathological conditions that may affect PRL secretion and corrected for variables related to PRL secretion and/or that may determine antipsychotic drug load. The assessment of a possible association is considered the first step in discovering the possible functional consequences of genetic variations. Our results indicate that genetic variants of HTR2C may have functional consequences on the modulation of PRL secretion. The largest effect was observed with rs569959, and to a lesser extent rs17326429. A smaller association with HPRL was found for rs3813929, rs6318, rs2911871 and rs5946189. When men and women were studied separately, only rs569959 in men reached the nominal significance threshold. This finding is probably related to the X-bound character of HTR2C (i.e., men are hemizygotes) and loss of statistical power. Interestingly, the association between Cys23Ser HTR2C (rs6318) and HPRL is smaller than that of rs569959, as Cys23Ser HTR2C is known to have certain functional consequences with respect to HPRL (Kühn et al. Citation2002). Therefore, the PRL response following a challenge of this receptor with a (selective) full and inverse HTR2C agonist in A/G rs569959 carriers would be interesting to study.
The strength of our study was the magnitude of the patient numbers, but our study also has several limitations. We studied both pre- and post-menopausal women with schizophrenia and used a fixed criterion for HPRL. However, varying this criterion did not significantly alter the results. Another limitation is the heterogeneity of antipsychotic drugs used. Antipsychotics differ with respect to their affinity for the P-gp transporter and, as the pituitary gland is outside the blood–brain barrier, this may affect the relative influence on central and peripheral receptors (Moons et al. Citation2011). This is the subject of a future study by our group.
Though HPRL is primarily attributed to blockade of DRD2 in the pituitary gland, the secretion of PRL is also modulated by 5-HT. The influence of 5-HT is probably mediated, in part, by affecting dopamine release from TIDA cells into the primary portal hypophyseal circulation (Mirkes and Bethea Citation2001; Fitzgerald and Dinan Citation2008). However, the affinity of dopamine for hypophyseal DRD2 receptors is low compared to most SGAs (Loonen and Ivanova Citation2016). Therefore, promoting dopamine release is an unlikely mechanism underlying the association of SGAs with less HPRL than FGAs. The involvement of the HTR1A, HTR2A, HTR2C and HTR3 in the serotonergic-induced PRL response is well documented (Jørgensen Citation2007). Apart from quetiapine and amisulpride, all SGAs are potent HTR2A antagonists (Meltzer and Massey Citation2011; Loonen and Ivanova Citation2016). However, their HTR2C/HTR2A affinity ratios vary greatly (Loonen and Ivanova Citation2016). Aripiprazole (Davies et al. Citation2004), clozapine (in vivo) (Celada et al. Citation2013), lurasidone (Woo et al. Citation2013), quetiapine (McIntyre et al. Citation2007) and ziprasidone (Stahl and Shayegan Citation2003) are partial 5-HT1A agonists. Some SGAs, including asenapine, clozapine, olanzapine and sertindole, are relatively potent 5-HT6 receptor antagonists and others, including amisulpride, asenapine, clozapine, amisulpride, lurasidone and risperidone, have high affinity for 5-HT7 receptors. These effects of SGAs may distort a possible relationship with genetic variations in receptor activity. Therefore, we excluded all users of SGAs from the analysed patient population in one of our analyses. Although the associations failed to reach the necessary significance level due to the limited sample size, the type of association was hardly affected. Thus, it is very unlikely that the absence of associations for variations in autosomal 5-HT receptor genes is due to camouflage by receptor binding caused by the current drug treatment.
In conclusion, of the investigated genes only the X-bound HTR2C exhibited a relationship with HPRL in antipsychotic drug-treated patients with schizophrenia from Siberia. In particular, polymorphism rs569959 and, to a lesser extent, rs17326429 was associated with HPRL. In the analysis of the association between HPRL and haplotypes of X-chromosome SNPs, the most significant association was found for a combination of the rs569959*G and rs17326429*A alleles. Our results are not invalidated by the binding potential of the antipsychotic drug used by the patients, and we found no clear evidence that the studied HTR2C variants correspond to a lack of constitutive activity of this receptor.
Supplementary_Table.pdf
Download PDF (92 KB)Acknowledgments
This work would not have been possible without the kind assistance of Dr P. van der Vlies, Genome Analysis Facility, Department of Genetics (Head: Prof. Dr C. Wijmenga), University Medical Centre Groningen (UMCG), Groningen, the Netherlands. The manuscript was edited and proofread by San Francisco Edit (www.sfedit.net).
Disclosure statement
The authors declare no conflict of interest.
Funding
The research project described in this paper was supported by the Russian Science Foundation, 10.13039/501100006769 [Grant 14-35-00023] (S.I., D.O., M.F., O.F., A.B., I.P. and A.S.: study protocol, collecting clinical data, DNA isolation, genotyping the samples, statistical analysis) and by A.J.M.L. through the Groningen Centre of Drug Research Fund of the University of Groningen.
References
- Ajmal A, Joffe H, Nachtigall LB. 2014. Psychotropic-induced hyperprolactinemia: a clinical review. Psychosomatics. 55:29–36.
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. 2009. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther. 121:160–173.
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 67:255–262.
- Apfelbaum ME. 1987. Effect of serotonin on basal and TRH-induced release of prolactin from rat pituitary glands in vitro. Acta Endocrinol (Copenh). 114:565–571.
- Apfelbaum ME. 1998. Role of vasoactive intestinal peptide and 5-HT2 receptor subtype in serotonin stimulation of basal and thyrotropin-releasing-hormone-induced prolactin release in vitro from rat pituitary cells. Neuroendocrinology. 67:45–50.
- Balsa JA, Sánchez-Franco F, Pazos F, Lara JI, Lorenzo MJ, Maldonado G, Cacicedo L. 1998. Direct action of serotonin on prolactin, growth hormone, corticotropin and luteinizing hormone release in cocultures of anterior and posterior pituitary lobes: autocrine and/or paracrine action of vasoactive intestinal peptide. Neuroendocrinology. 68:326–333.
- Bruce KR, Steiger H, Joober R, Ng Ying Kin NM, Israel M, Young SN. 2005. Association of the promoter polymorphism -1438G/A of the 5-HT2A receptor gene with behavioral impulsiveness and serotonin function in women with bulimia nervosa. Am J Med Genet B Neuropsychiatr Genet. 137B:40–44.
- Bruijnzeel D, Suryadevara U, Tandon R. 2014. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 11:3–7.
- Celada P, Bortolozzi A, Artigas F. 2013. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 27:703–716.
- Corregiari FM, Bernik M, Cordeiro Q, Vallada H. 2012. Endophenotypes and serotonergic polymorphisms associated with treatment response in obsessive-compulsive disorder. Clinics (Sao Paulo). 67:335–340.
- Davies MA, Sheffler DJ, Roth BL. 2004. Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 10:317–336.
- De Souza EB. 1986. Serotonin and dopamine receptors in the rat pituitary gland: autoradiographic identification, characterization, and localization. Endocrinology. 119:1534–1542.
- Filip M, Bader M. 2009. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 61:761–777.
- Fitzgerald P, Dinan TG. 2008. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol (Oxford). 22:12–19.
- French B, Lumley T, Cappola TP, Mitra N. 2012. Non-iterative, regression-based estimation of haplotype associations with censored survival outcomes. Stat Appl Genet Mol Biol. 11:Article 4. doi:10.1515/1544-6115.1764.
- Garthwaite TL, Hagen TC. 1979. Evidence that serotonin stimulates a prolactin-releasing factor in the rat. Neuroendocrinology. 29:215–220.
- González JR, Armengol L, Solé X, Guinó E, Mercader JM, Estivill X, Moreno V.2007. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 23:644–645.
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia. 2012. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 13:318–378.
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ; WFSBP Task force on Treatment Guidelines for Schizophrenia. 2013. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 14:2–44.
- Ivanova SA, Loonen AJ, Pechlivanoglou P, Freidin MB, Al Hadithy AF, Rudikov EV, Zhukova IA, Govorin NV, Sorokina VA, Fedorenko OY, et al. 2012. NMDA receptor genotypes associated with the vulnerability to develop dyskinesia. Transl Psychiatry. 2:e67.
- Jørgensen H, Kjaer A, Warberg J, Knigge U. 2001. Differential effect of serotonin 5-HT(1A) receptor antagonists on the secretion of corticotropin and prolactin. Neuroendocrinology. 73:322–333.
- Jørgensen HS. 2007. Studies on the neuroendocrine role of serotonin. Dan Med Bull. 54:266–288.
- Kelly DL, Wehring HJ, Earl AK, Sullivan KM, Dickerson FB, Feldman S, McMahon RP, Buchanan RW, Warfel D, Keller WR, et al. 2013. Treating symptomatic hyperprolactinemia in women with schizophrenia: presentation of the ongoing DAAMSEL clinical trial (Dopamine partial Agonist, Aripiprazole, for the Management of Symptomatic ELevated prolactin). BMC Psychiatry. 13:214.
- Kühn KU, Quednow BB, Bagli M, Meyer K, Feuchtl A, Westheide J, Frahnert C, Maier W, Rao ML. 2002. Allelic variants of the serotonin(2C) receptor and neuroendocrinological responses to the serotonin(2C) receptor agonist m-chlorophenylpiperazine in healthy male volunteers. Pharmacopsychiatry. 35:226–320.
- Lally J, MacCabe JH. 2015. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 114:169–179.
- Lamberts SW, MacLeod RM. 1978. Effects of cyproheptadine on prolactin synthesis and release by normal and suppressed pituitary glands and by dispersed pituitary tumor cells. Endocrinology. 103:1710–1717.
- Leysen JE., 2004. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 3:11–26.
- Li J, Ji L. 2005. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb). 95:221–227.
- Loonen AJ, Ivanova SA. 2016. Role of 5-HT2C receptors in dyskinesia. Int J Pharm Pharm Sci. 8:5–10.
- McIntyre RS, Soczynska JK, Woldeyohannes HO, Alsuwaidan M, Konarski JZ. 2007. A preclinical and clinical rationale for quetiapine in mood syndromes. Expert Opin Pharmacother. 8:1211–1209.
- Meltzer HY, Massey BW. 2011. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 11:59–67.
- Meltzer HY, Simonovic M, Gudelsky GA. 1983. Effects of pirenperone and ketanserin on rat prolactin secretion in vivo and in vitro. Eur J Pharmacol. 92:83–89.
- Mirkes SJ, Bethea CL. 2001. Oestrogen progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. J Neuroendocrinol. 13:182–192.
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. 2005. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 10:79–104.
- Moons T, de Roo M, Claes S, Dom G. 2011. Relationship between P-glycoprotein and second-generation antipsychotics. Pharmacogenomics. 12:1193–1211.
- Pandey SC, Davis JM, Pandey GN. 1995. Phosphoinositide system-linked serotonin receptor subtypes and their pharmacological properties and clinical correlates. J Psychiatry Neurosci. 20:215–225.
- Peuskens J, Pani L, Detraux J, De Hert M. 2014. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 28:421–453.
- Reist C, Mazzanti C, Vu R, Fujimoto K, Goldman D. 2004. Inter-relationships of intermediate phenotypes for serotonin function, impulsivity, and a 5-HT2A candidate allele: His452Tyr. Mol Psychiatry. 9:871–878.
- Stahl SM, Shayegan DK. 2003. The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry. 64:6–12.
- Staller J. 2006. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol. 16:317–326.
- Tandon R. 2011. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 72:4–8.
- Woo YS, Wang HR, Bahk WM. 2013. Lurasidone as a potential therapy for bipolar disorder. Neuropsychiatr Dis Treat. 9:1521–1519.
