Abstract
Objectives: To assess the efficacy and tolerability of lurasidone versus other atypical antipsychotic monotherapy agents in patients with bipolar depression, using a Bayesian network meta-analysis.
Methods: Fourteen randomised clinical trials (6221 patients) of lurasidone, quetiapine (extended release and immediate release), aripiprazole, olanzapine, and ziprasidone for bipolar depression were included. Efficacy assessments included change in the Montgomery–Åsberg Depression Rating Scale (MADRS), rates of response (≥50% improvement in MADRS) and remission (MADRS ≤12 at study endpoint), and change in the Clinical Global Impressions–Bipolar Disorder–Severity (CGI-BP-S) scale. Tolerability outcomes included weight, somnolence, extrapyramidal symptoms (EPS), and all-cause discontinuation. Changes from baseline or odds ratios (OR) with 95% credible intervals (CrI) were evaluated.
Results: Improvement in the MADRS associated with lurasidone treatment was significantly greater than placebo (–4.70, 95%CrI: –7.20, –2.21), aripiprazole (–3.62, 95%CrI: –7.04, –0.20), and ziprasidone (–3.38, 95%CrI: –6.68, –0.11), but not olanzapine (–0.15, 95%CrI: –3.12, 2.74) or quetiapine (0.10, 95%CrI: –2.68, 2.84). Results for improvement in the CGI-BP-S, and for response and remission were similar. Lurasidone was associated with less weight gain than olanzapine (–2.54 kg, 95%CrI: –3.42, –1.67) and quetiapine (–0.83kg, 95%CrI: –1.59, –0.08); and with lower rates of somnolence than quetiapine (OR: 0.33, 95%CrI: 0.11, 0.82) and ziprasidone (OR: 0.34, 95%CrI: 0.09, 0.93). No significant differences among atypical antipsychotic agents were observed in rates of discontinuation or in rates of EPS.
Conclusions: In this network meta-analysis, lurasidone was found to be more efficacious than aripiprazole and ziprasidone, and was associated with less weight gain than quetiapine and olanzapine and less somnolence than quetiapine and ziprasidone.
Introduction
In 2010, the World Health Organisation estimated that bipolar disorder affected 58.9 million people globally (Whiteford et al. Citation2015), with the lifetime prevalence rate estimated at 2.4% (Merikangas et al. Citation2011). Patients with bipolar disorder experience periods of mania and depression, as well as stable periods of euthymia (Murphy & Sahakian Citation2001). Symptomatic episodes associated with bipolar disorder are both disabling to patients and burdensome to caregivers and society, resulting in substantial productivity losses (Miller et al. Citation2014). During periods of even minor depression (i.e. presence of depressive symptoms below the threshold for major depressive disorder), patients’ psychosocial functioning is impaired and intensifies as depression severity increases (Judd et al. Citation2005). Accounting for roughly 67% of patients’ symptomatic time, depression contributes more significantly to productivity losses than mania (Miller et al. Citation2014). During depressive episodes of bipolar disorder, patients miss more days of work (Dilsaver et al. Citation1997; Simon et al. Citation2008), have a higher risk of suicide (Dilsaver et al. Citation1997; Hirschfeld Citation2004) and have more severe interpersonal relationship disruption (Hirschfeld Citation2004; Frye et al. Citation2014).
Historically, atypical antipsychotic treatments for bipolar disorder have been effective in reducing mania or preventing symptom relapse (Hirschfeld Citation2004; Frye et al. Citation2014). Lithium and lamotrigine are efficacious in treating manic episodes and maintaining stability between episodes, but are less effective in treating depressive episodes in bipolar disorder (Frye et al. Citation2014). Antidepressant monotherapy also has not been proven effective for bipolar depression, and may induce mania or rapid cycling (Yatham et al. Citation2013; Frye et al. Citation2014; Kemp Citation2014). Interestingly, olanzapine-fluoxetine (OFC), an atypical antipsychotic/selective serotonin reuptake inhibitor combination is approved for the treatment of bipolar depression by the US Food and Drug Administration (FDA) (Kemp Citation2014). Among all the available atypical antipsychotics, only lurasidone and quetiapine (extended release (XR) and immediate release (IR)) have been approved by the FDA as monotherapy to treat bipolar depression (Latuda Prescribing Information, 2012 Citation2013; Seroquel XR Prescribing Information, 2009 Citation2013). Lurasidone is the only atypical antipsychotic agent to have been approved by the FDA for the treatment of bipolar depression adjunctive with lithium or valproate (Franklin et al. Citation2015).
For atypical antipsychotics, newer guidelines recommend monotherapy with quetiapine (Yatham et al. Citation2013; NICE Citation2014; University of Florida Citation2015; Goodwin et al. Citation2016), olanzapine (NICE Citation2014; Goodwin et al. Citation2016) or lurasidone (University of Florida Citation2015; Goodwin et al. Citation2016) for first-line therapy. Lurasidone combined with lithium or valproate is also recommended for first-line treatment (University of Florida Citation2015), as is the combination of olanzapine and fluoxetine (OFC) (Yatham et al. Citation2013; NICE Citation2014), although the Florida Medicaid guideline restricted OFC to second-line therapy due to safety concerns (University of Florida Citation2015). Lurasidone monotherapy was recommended as second-line therapy in a Canadian guideline, which noted that lurasidone would be upgraded to first-line treatment in the next revision if clinical experience with this new treatment supports its efficacy (Yatham et al. Citation2013).
Consistent with treatment guidelines, previous conventional meta-analyses have found olanzapine and quetiapine, but not aripiprazole, to be more effective in acute bipolar depression than placebo (Cruz et al. Citation2010; Vieta et al. Citation2010). Recent network meta-analyses examined the effects of antipsychotics in bipolar depression and provided support for the efficacy of lurasidone, but not ziprasidone, in this patient population (NICE Citation2014; Taylor et al. Citation2014). These prior meta-analyses in patients with bipolar depression included a limited number of outcomes. Taylor et al. (Citation2014) investigated efficacy (using change from baseline in the Montgomery–Åsberg Depression Rating Scale (MADRS) or the Hamilton Depression Rating Scale) and one tolerability outcome (all-cause discontinuation) in their network meta-analysis (NMA) (Taylor et al. Citation2014). Common atypical antipsychotic-associated tolerability concerns such as extrapyramidal symptoms (EPS), somnolence and weight gain were not examined.
Materials and methods
Identification of trials
A systematic literature review searched for randomised controlled trials of atypical antipsychotic monotherapy in bipolar depression. Searches were run in the EMBASE, MEDLINE, PsycINFO, Cochrane Library and Google Scholar search engines from January 2014 through May 2015. Trials of OFC and lurasidone adjunctive with lithium or valproate were excluded from this study given that our aim was to evaluate the relative effects of antipsychotic agents used as monotherapy in bipolar depression and to enhance homogeneity and consistency assumptions of the included trials. Eligibility criteria for trial inclusion were designed using the Population, Intervention, Comparison, and Outcome framework (Schardt et al. Citation2007) and are detailed in . Only articles published in English were included. Abstracts and full articles were reviewed against the pre-specified eligibility criteria. In addition, studies from 1999 to 2013 that were included in the 2014 National Institute for Health and Care Excellence (NICE) systematic literature review of antipsychotics used for the management of bipolar depression were reviewed for inclusion (NICE Citation2014). Details of the systematic review are provided in the Appendix.
Table 1. Eligibility criteria for assessment of study inclusion.
NMA
The NMA was conducted using a Bayesian framework. The analysis followed guidance published in the NICE Decision Support Unit Technical Support Guidance (Schardt et al. Citation2007) and by the International Society for Pharmacoeconomics and Outcomes Research Task Force on Indirect Treatment Comparisons (Hoaglin et al. Citation2011; Jansen et al. Citation2011).
The primary efficacy outcome examined was the change from baseline in the MADRS total score. In addition, response (≥50% improvement in MADRS), remission (MADRS ≤12 at study endpoint) and change from baseline in Clinical Global Impressions–Bipolar Disorder–Severity (CGI-BP-S) score for depression, were also examined. Tolerability was assessed based on change in body weight (change from baseline in kg), somnolence (spontaneously reported adverse event), EPS and all-cause treatment discontinuation. Included studies used different imputation methods, such as last observation carried forward and mixed model repeated measures (MMRM) for continuous outcomes; wherever available MMRM-derived estimates were used.
Results are expressed as the difference in change from baseline between treatments for continuous outcomes and as odds ratios (ORs) for binary outcomes. In each case, results are reported as mean values with the associated standard deviation and 95% credible intervals (95% CrI). CrIs are the Bayesian analogue to confidence intervals. While formal significance testing was not conducted, findings were interpreted as statistically significant if the 95% CrI did not include zero for change scores or one for ORs.
NMA was conducted using WinBUGS version 1.4.3 (Lunn et al. Citation2000). Both fixed and random effects models were included in the analysis. Models were assessed based on the Deviance Information Criterion (DIC) and the posterior mean of the total residual deviance. The DIC is a Bayesian analogue to the Akaike Information Criterion that provides a measure of model fit that penalises model complexity with lower values of the DIC suggesting a more parsimonious model (Spiegelhalter et al. Citation2002). Details on the assessment of convergence, imputation of missing data and prior distributions can be found in the Appendix.
In the primary (i.e. base-case) analysis, multiple dose strengths of each atypical antipsychotic agent were pooled, as were quetiapine IR and quetiapine XR formulations, in line with previous network meta-analyses (NICE Citation2014, Taylor et al. Citation2014). Sensitivity analysis explored the impact of separating the evidence for the quetiapine IR and XR formulations.
Results
Study selection
The electronic database search of the systematic literature review identified a total of 2456 citations. After duplicate removal and abstract screening, 239 articles remained for full text review, of which 46 articles were considered for inclusion in evidence synthesis. Seven studies from the systematic literature review of the NICE were deemed eligible for inclusion. In total, 14 studies with 6221 patients were included in the NMA (see the PRISMA diagram in the online supplemental material for more details). No study assessing the efficacy of asenapine or risperidone that met the inclusion criteria was identified. The resulting network of evidence is shown in . The studies were all conducted between 2000 and 2012. All included randomised clinical trials compared active treatment to placebo. Study design and patient baseline characteristics from the final 14 studies are presented in .
Figure 1. Network of evidence. ARI: aripiprazole; LUR: lurasidone; OLA: olanzapine; PLO: placebo; QUE: quetiapine IR or XR; SLR: systematic literature review; ZIP: ziprasidone.
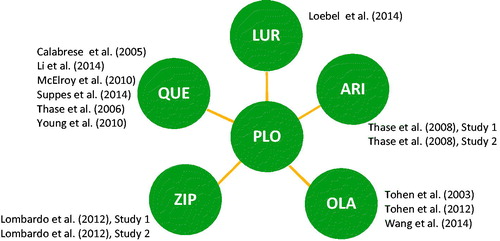
Table 2. Study design and patient baseline characteristics.
Study and patient characteristics
All of the studies included only patients with bipolar I, with the exception of the quetiapine studies, which included both patients with bipolar I and bipolar II (see ). The proportion of patients with bipolar I disorder in the quetiapine studies varied between 50 and 80.4%. Patients with psychotic features were explicitly excluded by Loebel et al. (Citation2014a) and Lombardo et al. (Citation2012), and included by Tohen et al. (Citation2003); however, most studies did not specify in the publication whether psychotic features were permitted. The age distribution across included studies was similar, varying between 35.5 and 42.2 years, except for the study by Wang et al. (Citation2014), which reported a mean age of 29.2 years. Baseline MADRS scores varied between 26.9 and 32.0, and baseline CGI-BP-S scores varied between 4.2 and 4.9. When reporting baseline body weight, most studies reported values between 75.5 and 88.8 kg, with the exception of the Wang et al. (Citation2014) study which reported a mean baseline weight of 63.9 kg. Duration of studies ranged between 6 and 8 weeks. The Li et al. (Citation2014) study, a published abstract, contained very little information about the patient population.
Assessment of effect modification
Studies included in the NMA were assessed for presence and extent of effect modification due to imbalances of effect modifiers across studies (heterogeneity) or across treatment comparisons (inconsistency) (Jansen & Naci Citation2013). The effect modifiers considered were the percentage of patients with bipolar I disorder, the gender distribution, mean age, mean baseline weight, mean baseline MADRS score and mean baseline CGI-BP-S score.
Pairwise meta-analyses were used to estimate the between-study heterogeneity for comparisons informed by multiple trials. In particular, the comparison of quetiapine versus placebo was assessed for the effect of including the Li et al. (Citation2014) study, which suggested no substantial between-study heterogeneity. Pairwise meta-analyses were also performed for the comparison of olanzapine versus placebo in order to assess the degree of heterogeneity attributed to the Wang et al. (Citation2014) study. Exclusion of the Wang et al. (Citation2014) study resulted in lower between-study heterogeneity for the majority of outcomes. The impact of removing the Wang et al. (Citation2014) study on meta-analysis results was explored in a sensitivity analysis. Since between-study heterogeneity was detected as part of assessing the evidence base, results from the random effects model are reported.
NMA results
The results for the efficacy outcomes from the NMA are presented in . Full results of the base-case and sensitivity analyses with quetiapine XR and IR as separate treatments are presented in the Appendix. For the primary efficacy measure, change from baseline in MADRS, lurasidone was associated with a statistically significantly greater improvement than placebo (–4.70, 95% CrI: –7.20, –2.21), aripiprazole (–3.62, 95% CrI: –7.04, –0.20), and ziprasidone (–3.38, 95% CrI: –6.68, –0.11), but similar improvement to olanzapine (–0.15, 95% CrI: –3.12, 2.74) and pooled quetiapine IR and XR (0.10, 95% CrI: –2.68, 2.84). For the other efficacy measures (response rate, remission rate, and change from baseline in CGI-BP-S), the same pattern of findings was observed ().
Table 3. Forest plots for base-case analysis of efficacy outcomes.
Results for the tolerability outcomes varied across each measure. Forest plots of the NMA on the tolerability outcomes of weight change and incidence of somnolence are presented in . Lurasidone was associated with less weight gain compared to pooled quetiapine IR and XR (mean difference: –0.83 kg, 95% CrI: –1.59, –0.08) and to olanzapine (mean difference: –2.54 kg, 95% CrI: –3.42, –1.67). Lurasidone was also associated with a lower incidence of somnolence compared to pooled quetiapine IR and XR (mean odds ratio: 0.33, 95% CrI: 0.11, 0.82) and compared to ziprasidone (mean odds ratio: 0.34, 95% CrI: 0.09, 0.93). Differences between active treatments in terms of EPS and all-cause discontinuation were not statistically significant.
Table 4. Forest plots for base-case analysis of tolerability outcomes.
Sensitivity analysis results
Sensitivity analysis explored the impact of dividing the quetiapine IR and XR formulations into separate nodes in the network of evidence. Results of the sensitivity analysis are presented in the Appendix and were found to be similar to those of the primary analysis, suggesting that lurasidone has comparable efficacy to both quetiapine formulations. For the change from baseline in MADRS, the estimated difference for lurasidone versus quetiapine IR (0.15, 95% CrI: –2.88, 3.09) and lurasidone versus quetiapine XR (–0.02, 95% CrI: –3.34, 3.37) both included zero in the CrI. In the sensitivity analysis of tolerability outcomes, lurasidone was associated with a statistically significant lower incidence of somnolence compared to both quetiapine IR (OR: 0.35, 95% CrI: 0.12, 0.86) and XR (OR: 0.24, 95% CrI: 0.05, 0.69). Weight change for lurasidone versus both quetiapine IR (–0.78 kg, 95% CrI: –1.59, 0.02) and quetiapine XR (–1.16 kg, 95% CrI: –2.41, 0.08), separately, were not significantly different in the sensitivity analyses as for the pooled analysis. This is likely due to the smaller sample sizes resulting from separating the formulations into distinct treatments.
A pairwise comparison of the lurasidone versus olanzapine studies indicated the presence of between-study heterogeneity, which was attributed to the study by Wang et al. (Citation2014). When the study by Wang et al. (Citation2014) was removed in a sensitivity analysis, the between-study heterogeneity was reduced and there was a statistically significantly higher response (OR: 1.73, 95% CrI: 1.05, 2.90) and a greater reduction in MADRS from baseline (–1.28, 95% Crl: –4.17, 1.59) for lurasidone. For other outcomes, results were consistent with those of the primary analysis (details are provided in the Appendix).
The use of fixed effects models and random effects models produced very similar results across all outcomes in the primary analysis as well as the sensitivity analysis. In addition, both fixed effects models and random effects models yielded similar DIC values throughout. Further details on the primary models and sensitivity analyses, including the DIC values, are available in the Appendix.
Discussion
This NMA, which examined atypical antipsychotic monotherapies for the treatment of patients with bipolar depression, found that lurasidone was significantly more efficacious than aripiprazole and ziprasidone for reducing depressive symptoms assessed by the MADRS and for improving overall severity assessed by the CGI-BP-S. While lurasidone was similar to olanzapine and quetiapine on the examined efficacy outcomes, it was associated with significantly less weight gain than olanzapine and quetiapine, in addition to significantly less somnolence than quetiapine and ziprasidone. Prior network meta-analyses have not examined these tolerability outcomes in patients with bipolar depression. This NMA suggests that lurasidone is an efficacious treatment option with a reduced risk of somnolence and weight gain for patients with bipolar depression.
Several prior meta-analyses have examined acute treatments for bipolar depression, and also reported that quetiapine and olanzapine were significantly more efficacious than placebo (Cruz et al. Citation2010; Vieta et al. Citation2010), while aripiprazole (Cruz et al. Citation2010; Vieta et al. Citation2010; NICE Citation2014; Taylor et al. Citation2014; Franklin et al. Citation2015) and ziprasidone were not (NICE Citation2014; Taylor et al. Citation2014). Given the recent advent of lurasidone into clinical practice, it was only included in more recent network meta-analyses and has been found to be significantly more efficacious than placebo (NICE Citation2014; Taylor et al. Citation2014). The NMA by Taylor et al. (Citation2014) included 29 trials (with 8331 participants) with all medications used in the treatment of bipolar depression including antidepressants, anticonvulsants, lithium and antipsychotics. However, only one tolerability outcome (all-cause discontinuation) was explored (Taylor et al. Citation2014). In contrast, our study included 14 trials (with 6221 participants) and provides a more comprehensive evaluation of lurasidone and other atypical antipsychotic monotherapies by including a wider range of efficacy and tolerability outcomes. Consistent with our findings, past research has found that lurasidone, quetiapine, and olanzapine are efficacious monotherapies for the treatment of bipolar depression, while aripiprazole and ziprasidone are not more efficacious than placebo (Cruz et al. Citation2010; Vieta et al. Citation2010; NICE Citation2014; Taylor et al. Citation2014). We note that lurasidone and quetiapine (both IR and XR) have been approved for the treatment of patients with bipolar depression in multiple countries; olanzapine has been approved for this population in Japan.
Relative to the other studies in this NMA, the Wang et al. (Citation2014) study was smaller (68 participants) and had a younger, Chinese population. When the Wang et al. (Citation2014) study was removed in the sensitivity analysis, the response rate of lurasidone was found to be statistically significantly higher than olanzapine. For the remaining outcomes, the findings of the sensitivity analyses were in agreement with the base-case analyses, with no changes in the magnitude of effects or statistical significance between comparators (see Appendix).
This is the first meta-analysis to examine tolerability outcomes based on common adverse events for atypical antipsychotics for patients with bipolar depression. Prior meta-analyses have examined individual study withdrawal rates as a measure of tolerability, but only the NMA by Taylor et al. (Citation2014) conducted indirect comparisons between lurasidone and other atypical antipsychotics (Vieta et al. Citation2010). Similar to the NMA by Taylor et al. (Citation2014), the current analysis found no significant differences in discontinuation rates between lurasidone and any of the other atypical antipsychotics when treating bipolar depression (Taylor et al. Citation2014). However, this NMA is the first to report that lurasidone was associated with significantly less weight gain than olanzapine and quetiapine as well as significantly less somnolence than quetiapine and ziprasidone in patients with bipolar depression. These findings appear consistent with tolerability results from randomised clinical trials comparing lurasidone with quetiapine (Loebel et al. Citation2013; Loebel et al. Citation2014b) and with olanzapine (Meltzer et al. Citation2011) in patients with schizophrenia.
In recent bipolar depression treatment guidelines, quetiapine monotherapy or lurasidone as monotherapy and adjunctive with lithium/valproate were recommended as first-line treatment for patients with bipolar depression (University of Florida Citation2015). The results of this NMA support these recommendations and provide insight regarding the differential clinical profile of these agents.
Limitations
Provided that similarity and consistency assumptions hold, network meta-analyses can yield useful information about the relative efficacy and tolerability of different treatments that have not been directly compared to one another (Jansen & Naci Citation2013). If the evidence base informing the analysis is found to be heterogeneous, however, this may modify observed treatment effects. Meta-regression, which can potentially adjust for such effect modifiers, was not run due to the absence of a sufficiently large number of trials per comparison that is required to render meta-regression feasible at the aggregate level.
Because the included studies used different imputation methods for the handling of missing data, continuous efficacy measures may not be comparable in some cases. Comparability could not be determined based on the information reported in the included studies, which generally only reported outcomes using one imputation method. We used MMRMs, as opposed to last observation carried forward, for continuous outcomes wherever available.
The NMA used data derived from studies of 6 or 8 weeks duration (depending on the trial). Although it is not known whether trial duration is a treatment effect modifier, it is possible that these differences may have biased the analysis results. It is also uncertain whether the NMA findings apply to bipolar II depression, as most of the studies did not include this patient population.
The frequency of EPS was not consistently reported across studies. Studies reporting frequency of EPS used inconsistent definitions and in some cases included akathisia (McElroy et al. Citation2010; Suppes et al. Citation2010; Young et al. Citation2010), whereas the study by Loebel et al. (Citation2014a) reported akathisia as a distinct adverse event. Some studies did not indicate how EPS was defined (Calabrese et al. Citation2005; Thase et al. Citation2006; Samalin et al. Citation2011). Standardised EPS definitions across clinical trials would facilitate determination of more accurate estimates of the frequency of this adverse event. Only two publications reported data for akathisia separately from EPS (Thase et al. Citation2008; Loebel et al. Citation2014a), and therefore this adverse event was not included as an adverse event in the current NMA.
While our study expanded upon the tolerability outcomes of other meta-analyses, the current study did not include all important metabolic parameters. Commonly included in the warnings and precautions sections of the product labelling of atypical antipsychotics are hyperglycaemia, diabetes mellitus and dyslipidemia, and these outcomes warrant investigation in future evidence synthesis.
This analysis was limited to atypical antipsychotics used as monotherapy for the treatment of bipolar depression. Lurasidone is approved for both monotherapy and adjunctive treatment with lithium or valproate for patients with bipolar depression in the US and Canada. The extent to which other atypical antipsychotics can be used as adjunctive treatment for patients with bipolar depression warrants future studies. The ability to generalise these results to usual clinical care, where combination therapy is common, also requires further study.
The original studies were not powered to detect statistically significant differences between antipsychotics for indirect comparisons using NMA. Therefore, the absence of statistically significant differences does not indicate that differences between treatments do not exist, but that differences cannot be detected based on the current evidence base and the modelling methods and assumptions used.
Conclusions
Results from this indirect NMA suggest that lurasidone is more efficacious than aripiprazole and ziprasidone, and has comparable efficacy to quetiapine and olanzapine monotherapies for the management of bipolar depression. Lurasidone was also found to have statistically significantly lower rates of weight gain compared to quetiapine and olanzapine, and a lower likelihood of somnolence compared to quetiapine and ziprasidone. Overall, this NMA suggests that lurasidone is an efficacious treatment option offering a favourable tolerability profile for patients with bipolar depression.
Statement of interest
The study was sponsored by Sunovion Pharmaceuticals Inc. Michael Ostacher is a consultant who did not receive funding from Sunovion Pharmaceuticals Inc for the write-up of this manuscript. Max Schlueter is an employee of QuintilesIMS and received funding from Sunovion Pharmaceuticals Inc. Dionysios Ntais was an employee of QuintilesIMS at the time of this manuscript?s development. Daisy Ng-Mak and Antony Loebel are employees of Sunovion Pharmaceuticals Inc. Pankaj Patel was an employee of Sunovion Pharmaceuticals Inc. at the time of this manuscript?s development.
Acknowledgements
The study was sponsored by Sunovion Pharmaceuticals Inc. Natasha Lenton, an employee of Sunovion Pharmaceuticals Canada Inc., provided input and guidance on the preparation and implementation of the analysis. Krithika Rajagopalan, an employee of Sunovion Pharmaceuticals Inc., reviewed and provided input on the content of the draft manuscript. Ashley Pitcher and Tim Reason, employees of QuintilesIMS, provided input and guidance on the design and implementation of the NMA, and contributed to the development of the manuscript.
References
- Calabrese JR, Keck PE Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH, Cutler AJ, McCoy R, Wilson E, Mullen J. 2005. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 162:1351–1360.
- Cruz N, Sanchez-Moreno J, Torres F, Goikolea JM, Valenti M, Vieta E. 2010. Efficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: a meta-analysis. Int J Neuropsychopharmacol. 13:5–14.
- Dilsaver Steven C, Chen YW, Swann AC, Shoaib AM, Tsai-Dilsaver Y, Krajewski KJ. 1997. Suicidality, panic disorder and psychosis in bipolar depression, depressive-mania and pure-mania. Psychiatry Res. 73:47–56.
- Franklin R, Zorowitz S, Corse AK, Widge AS, Deckersbach T. 2015. Lurasidone for the treatment of bipolar depression: an evidence-based review. Neuropsychiatr Dis Treat. 11:2143.
- Frye MA, Prieto ML, Bobo WV, Kung S, Veldic M, Alarcon RD, Moore KM, Choi DS, Biernacka JM, Tye SJ. 2014. Current landscape, unmet needs, and future directions for treatment of bipolar depression. J Affect Disord. 169(Suppl 1):S17–S23.
- Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barnes T, Cipriani A, Coghill DR, Fazel S, Geddes JR, Grunze H, et al. 2016. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 30:495–553.
- Higgins, JPT, Green S, 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane collaboration. Available from: www.cochrane-handbook.org.
- Hirschfeld RMA. 2004. Bipolar depression: the real challenge. Eur Neuropsychopharmacol. 14:S83–S88.
- Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC. 2011. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value in Health. 14:429–437.
- Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N. 2011. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value in Health. 14:417–428.
- Jansen JP, Naci H. 2013. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 11:159.
- Judd Lewis L, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, Coryell W, Maser JD, Keller MB. 2005. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 62:1322–1330.
- Kemp DE. 2014. Managing the side effects associated with commonly used treatments for bipolar depression. J Affect Disord. 169(Suppl 1):S34–S44.
- Latuda Prescribing Information, 2012. 2013. Available from: http://www.latuda.com/LatudaPrescribingInformation.pdf
- Li HF, Gu NF, Zhang H, Wang YG, Tan QR, Yang PD, Ning YP, Zhang HG, Lu Z, Xu XF, et al. 2014. The efficacy and safety of quetiapine extended release (xr) as mono-therapy in the treatment of Chinese patients with bipolar I or II depression. Conference abstract presented at the 16th Annual Conference of the International Society for Bipolar Disorders, March 18?21, 2014, Seoul, Korea. Bipolar Disord. 16(Suppl. 1):62–70.
- Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, Pikalov A, Potkin SG. 2013. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial'. Schizophr Res. 145:101–109.
- Loebel A, Cucchiaro J, Silva R, Kroger H, Hsu J, Sarma K, Sachs G. 2014a. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 171:160–168.
- Loebel AD, Siu CO, Cucchiaro JB, Pikalov AA, Harvey PD. 2014b. Daytime sleepiness associated with lurasidone and quetiapine XR: results from a randomized double-blind, placebo-controlled trial in patients with schizophrenia. CNS Spectr. 19:197–205.
- Lombardo I, Sachs G, Kolluri S, Kremer C, Yang R. 2012. Two 6-week, randomized, double-blind, placebo-controlled studies of ziprasidone in outpatients with bipolar I depression: did baseline characteristics impact trial outcome? J Clin Psychopharmacol. 32:470–478.
- Lunn DJ, A, Thomas N, Best D. 2000. Spiegelhalter, 'Winbugs: a Bayesian modelling framework: concepts, structure, and extensibility. Statist Comput. 10:325–337.
- McElroy SL, Weisler R, Chang H, Olausson W, Paulsson B, Brecher B, Agambaram M, Merideth V, Nordenhem CA, Young AH. 2010. A double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II). J Clin Psychiatry. 71:163–174.
- Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, Kalali AH, Schweizer E, Pikalov A, Loebel A. 2011. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 168:957–967.
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, et al. 2011. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 68:241–251.
- Miller S, Dell'Osso B, Ketter TA. 2014. The prevalence and burden of bipolar depression. J Affect Disord. 169(Suppl 1):S3–S11.
- Murphy FC, Sahakian BJ. 2001. Neuropsychology of bipolar disorder. Br J Psychiatry. 178:s120–s127.
- NICE. 2014. Bipolar disorder: the NICE guideline on the assessment and management of bipolar disorder in adults, children and young people. National Clinical Guideline Number 185. Available from: https://www.nice.org.uk/guidance/cg185
- Samalin L, Garnier M, Llorca PM. 2011. Clinical potential of lurasidone in the management of schizophrenia. Ther Clin Risk Manag. 7:239–250.
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. 2007. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Informat Decision Making. 7:1.
- Seroquel XR Prescribing Information, 2009. 2013. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/ucm089126.pdf
- Simon GE, Ludman EJ, Untzer J, Operskalski BH, Bauer MS. 2008. Severity of mood symptoms and work productivity in people treated for bipolar disorder. Bipolar Disord. 10:718–725.
- Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. 2002. Bayesian measures of model complexity and fit. J Royal Statis Soc Ser B. 64:583–639.
- Suppes T, Datto Minkwitz C, Nordenhem M, Walker AC, Darko D. 2010. Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression. J Affect Disord. 121:106–115.
- Taylor DM, Cornelius V, Smith L, Young AH. 2014. Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis. Acta Psychiatr Scand. 130:452–469.
- Thase ME, Jonas A, Khan A, Bowden CL, Wu X, McQuade RD, Carson WH, Marcus RN, Owen R. 2008. Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol. 28:13–20.
- Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, Calabrese JR. 2006. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol. 26:600–609.
- Tohen M, McDonnell DP, Case M, Kanba S, Ha K, Fang YR, Katagiri H, Gomez JC. 2012. Randomised, double-blind, placebo-controlled study of olanzapine in patients with bipolar I depression. Br J Psychiatry. 201:376–382.
- Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, Mitchell PB, Centorrino F, Risser R, Baker RW, et al 2003. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 60:1079–1088.
- University of Florida. 2015. The University of South Florida, Florida Medicaid Drug Therapy Management Program. 2015 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. Available from: http://www.medicaidmentalhealth.org/_assets/file/Guidelines/Web_2015-Psychotherapeutic Medication Guidelines for Adults_Final_Approved1.pdf
- Vieta E, Locklear J, Günther O, Ekman M, Miltenburger C, Chatterton ML, Åström M, Paulsson B. 2010. Treatment options for bipolar depression: a systematic review of randomized, controlled trials. J Clin Psychopharmacol. 30:579–590.
- Wang M, Tong JH, Huang DS, Zhu G, Liang GM, Du H. 2014. Efficacy of olanzapine monotherapy for treatment of bipolar I depression: a randomized, double-blind, placebo controlled study. Psychopharmacology (Berl). 231:2811–2818.
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. 2009. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol. 169:1158–1165.
- Welton NJ, Sutton AJ, Cooper NJ, Abrams KR, Ades AE. 2012. Evidence Synthesis for Decision Making in Healthcare. 1st ed. Wiley.
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. 2015. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 10:e0116820.
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O'Donovan C, MacQueen G, McIntyre RS, Sharma L, et al. 2013. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 15:1–44.
- Young AH, McElroy S, Bauer L, Philips M, Chang N, Olausson W, Paulsson BB, Brecher M. 2010. A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I). J Clin Psychiatry. 71:150–162.
Appendix
Convergence of the NMA was assessed by visual inspection of density, history and autocorrelation plots. If autocorrelation was observed, chains were thinned until autocorrelation was no longer present Welton et al. Citation2012. In all cases a burn-in of at least 50,000 simulations was discarded. All results were presented based on a further sample of at least 50,000 simulations or until convergence was achieved. Monte Carlo error, which reflects both the number of simulations and the degree of autocorrelation, was examined to ensure that it was no more than 5% of the posterior standard deviation of the treatment effect measures of interest Welton et al. Citation2009.
Data on missing standard errors associated with the change in continuous outcomes from baseline were imputed using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 Higgins & Green Citation2011.
Baseline and intervention effect parameters were given flat uninformative normal (0, 1000) priors. The between-study standard deviation was given a flat uniform distribution with an appropriately large range.
Appendix Table 1. Base-case results for the efficacy outcomes of change from baseline in MADRS score, CGI-BP-S score, response and remission.
Appendix Table 2. Base-case results for the tolerability outcomes of weight change, somnolence, extrapyramidal symptoms and overall discontinuation.
Appendix Table 3. Sensitivity analysis results for the efficacy outcomes of change from baseline in MADRS score, CGI-BP-S score, response and remission.
Appendix Table 4. Sensitivity analysis results for the tolerability outcomes of weight change from baseline, somnolence, extrapyramidal symptoms and overall discontinuation.
Appendix Table 5. Sensitivity analysis results for the efficacy and safety outcomes (excluding Wang et al. (Citation2014) from the base-case analysis).
Appendix Table 7. Sensitivity analysis results for the tolerability outcomes (excluding Wang et al. (Citation2014) from the base-case analysis).