Abstract
Objectives: Executive network deficits are putative neurocognitive endophenotypes for obsessive-compulsive disorder (OCD). Yet, unlike alterations in fronto-striatal and limbic connectivity, connectivity in the fronto-parietal (FPN) and cingulo-opercular (CON) networks involved in cognitive control has received little attention.
Methods: The coherence of FPN, CON and fronto-limbic networks was investigated in 39 unmedicated OCD patients, 16 of their unaffected siblings and 36 healthy controls using resting-state functional-connectivity MRI and a seed-based analysis approach.
Results: FPN and CON connectivity was similar for patients and controls. Siblings showed higher connectivity than patients within the CON, and between the CON and FPN compared to patients and controls (trend level). In OCD patients, but not in siblings, fronto-limbic hyperconnectivity was present compared to controls. In contrast to our expectations, no group differences in resting-state connectivity of the cognitive control networks were observed between OCD patients and controls.
Conclusions: The increased within- and between-network connectivity in siblings, but not in patients, could indicate a mechanism of increased cognitive control that may act as a protective mechanism. None of the observed network alterations can be considered an endophenotype for OCD since differences were present in either patients or siblings, but not in both groups.
Introduction
Neurobiological theories of obsessive-compulsive disorder (OCD) have traditionally focussed on disturbances in the cortico-striatal-thalamo-cortical connections, specifically in the orbitofronto-striatal circuit (Saxena et al. Citation1998; Remijnse et al. Citation2006). Recent insights show, however, that this model may be too limited as it does not sufficiently explain structural and functional alterations in other brain areas, such as the cerebellum, parietal and prefrontal cortices (Menzies et al. Citation2008; de Wit et al. Citation2014; Eng et al. Citation2015; Bandelow et al. Citation2016). Several alternative models for OCD have been proposed, including dysfunction of fronto-limbic circuits (involved in emotional processing), ventral and dorsal cognitive control circuits, and sensorimotor circuits involved in habitual behaviour (Milad and Rauch Citation2012; Milad et al. Citation2013; van den Heuvel et al. Citation2015).
Disturbances in the large-scale networks for cognitive control have been hypothesised to play a role in a range of psychiatric symptoms that are also characterised by executive dysfunction (Menon Citation2011; Cole et al. Citation2014). Conversely, compensatory activity in control systems may have a beneficial effect on symptoms and functioning or even protect against developing psychiatric symptoms (Cole et al. Citation2014). Top-down control over goal-directed behaviour is thought to be exerted through the interaction between two systems; stable maintenance of task-mode by the cingulo-opercular network (CON) and flexible control by the fronto-parietal network (FPN) (Dosenbach et al. Citation2007). Strength of within-network connectivity is associated with cognitive performance (Seeley et al. Citation2007; Sheffield et al. Citation2015). There is evidence for the involvement of the FPN and CON in OCD. For example, structural changes have been reported in the dorsal anterior cingulum (dACC) and anterior insula of OCD (de Wit et al. Citation2014). Functional differences between OCD patients and healthy controls during executive tasks were reported in two recent meta-analyses. First, OCD patients, compared to controls, showed increased activation of the FPN and decreased activation in the striatum, ACC and OFC (Del Casale et al. Citation2016). The second meta-analysis also reported decreased activation in striatum and OFC in OCD patients, but in contrast reported increased ACC and decreased FPN activation (Eng et al. Citation2015). The latter study investigated activation patterns for separate executive functions and included fewer studies per task, which could explain some of the differences. In two task-based studies OCD patients and their unaffected relatives showed compensatory brain activation in cognitive control regions, coupled with task-related interference of limbic areas (de Wit et al. Citation2012; de Vries et al. Citation2014; van Velzen et al. Citation2015).
Resting-state functional-connectivity magnetic resonance imaging (rs-fcMRI) has gained popularity in the study of the function of cerebral networks at rest. Several reports have shown higher connectivity in the fronto-limbic (orbitofronto-striatal) circuit in OCD patients (Harrison et al. Citation2009, Citation2013; Sakai et al. Citation2011; Beucke et al. Citation2013) and in their unaffected relatives (Hou et al. Citation2014) relative to healthy subjects, with the exception of one study demonstrating decreased fronto-limbic connectivity in unmedicated OCD patients (Posner et al. Citation2014). Few studies have examined resting-state connectivity in fronto-parietal and cingulo-opercular control networks in OCD. Increased connectivity between the FPN and the default mode network (DMN) was reported in adult OCD patients (Stern et al. Citation2012) and (trend level) decreased connectivity within the CON network was reported in paediatric OCD (Fitzgerald et al. Citation2010).
A case–control design does not allow disentangling whether changes in functional connectivity are a result of genetic vulnerability or are a consequence of having (often chronic) OCD. Second, altered connectivity may be associated with disease (severity) or alternatively reflect compensatory mechanisms. Therefore, in the current family design, we included a group of unaffected siblings of OCD patients in addition to OCD cases and controls. If unaffected relatives show changes similar to OCD patients, these effects are likely to represent a (genetic) vulnerability to the disease (an endophenotype) (Gottesman and Gould Citation2003). Alternatively, changes present in patients but not in their unaffected relatives may be a consequence of the disease. Changes present in the siblings but not (or to a lesser extent) in OCD patients, could indicate a cognitive compensation mechanism that protects genetically vulnerable individuals against developing OCD symptoms.
The goal of this study was to identify network alterations explaining both disease vulnerability and disease resilience. We hypothesise altered connectivity in the FPN and CON in both patients and their siblings, following previously observed abnormalities in these networks during task-based studies in OCD. Second, we hypothesise increased connectivity in fronto-limbic networks in both OCD patients and siblings, as was shown during cognitive processing.
Materials and methods
Participants
Forty-five OCD patients, 19 of their unaffected siblings and 40 healthy controls matched on age, gender, handedness and educational level were included in this study. Results from two task-based studies in the same study group were reported previously (de Wit et al. Citation2012; de Vries et al. Citation2014; van Velzen et al. Citation2015). Patients were recruited through outpatient services contributing to the Netherlands OCD Association study (Schuurmans et al. Citation2012), through Altrecht Academic Anxiety Centre, and through advertisements. Advertisements were used to recruit healthy control subjects. The Structural Clinical Interview for DSM-IV (First et al. Citation1999) was used to screen for axis I diagnoses in all subjects. OCD symptom characteristics and severity were assessed with the Obsessive-Compulsive Inventory-Revised (OCI-R) (Foa et al. Citation2002) and the Yale-Brown Obsessive-Compulsive Symptom Severity scale (Y-BOCS) (Goodman et al. Citation1989), respectively. Severity of depressive and anxiety symptoms was assessed with the Montgomery–Åsberg Depression Rating Scale (MADRS) and the Beck Anxiety Inventory (BAI) (Beck et al. Citation1988). Handedness was determined with the Edinburgh Handedness Inventory (Oldfield Citation1971). Subjects between 18 and 65 years old with a primary diagnosis of OCD without predominant hoarding were included. Psychiatric co-morbidity (including tic disorders) was not an exclusion criterion. Exclusion criteria were psychotic symptoms, a major somatic illness, history of a major neurological illness and MRI contra-indications. All patients were off psychotropic medication for at least 4 weeks. Siblings did not have a lifetime diagnosis of OCD. Healthy controls had no current axis I diagnosis and no (family) history of OCD. The local medical ethical committee (VU university medical centre, Amsterdam) approved the study protocol and all subjects gave written informed consent.
Seven OCD patients, three siblings and four controls were excluded from data analyses due to pathology on the structural scan or excessive head movement (>2 mm/degree absolute translation or rotation over the whole run or >1 mm/degree inter-volume motion). This resulted in a final sample for data analysis of 39 OCD patients, 16 unaffected siblings and 36 controls. Comparisons were made between 39 OCD patients and 36 participants in the control group. To further investigate genetic vulnerability and resilience in connectivity patterns a gender-, age- and handedness-matched three-group comparison was done, including 16 OCD patients, 16 unaffected siblings and 16 healthy controls.
Data acquisition
Scanning was performed on a GE Signa HDxt 3.0-Tesla MRI-scanner (General Electric, Milwaukee, WI, USA). A total of 200 functional images (6-min run) was acquired with an eight-channel circularised head coil using a T2*-weighted single-shot gradient-echo planar imaging sequence (repetition time = 1800 ms; echo time = 35 ms; 64 × 64 matrix; field of view = 21.1 cm; flip angle = 80°) with 34 ascending slices per volume (3.3 × 3.3 mm in-plane resolution; slice thickness = 3.0 mm; inter-slice gap = 0.3 mm). Structural scanning included a sagittal three-dimensional gradient-echo T1-weighted sequence (256 × 256 matrix; voxel size = 1 × 0.977 × 0.977 mm; 172 sections). Participants were instructed to keep their eyes closed and not fall asleep. Padding around the head ensured minimal head movement and earplugs attenuated background noise.
Data processing
The first five scan volumes were discarded to ensure steady-state magnetisation equilibrium. Preprocessing of functional images included realignment, slice time correction, BET brain extraction, reslicing with a 3 × 3 × 3-mm resolution, high-pass filtering (0.01 Hz) and warping to the Montreal Neurological Institute (MNI) standard space. To increase the signal to noise ratio, images were smoothed with a 6-mm full-width-at-half-maximum Gaussian kernel. To remove motion and physiologic signals (heart rate and respiration), the preprocessed images were de-noised using Fix (beta version) (FSL, analysis group, FMRIB, Oxford). For this approach a single-subject independent component analysis (ICA) was run. Second, based on training in the standard dataset (resulting in a 95–99% classification accuracy of noise components), Fix identified those components which were noise related. Third, the noise components were regressed out of the data (Salimi-Khorshidi et al. Citation2014).
Functional-connectivity analyses
Functional-connectivity analyses were carried out with the Statistical Parametric Mapping (SPM) 8 package (Wellcome Institute of Cognitive Neurology, London). Seed regions were chosen from a meta-analysis by Dosenbach et al. (Citation2007) to represent three networks; FPN, CON and fronto-limbic network. The FPN was represented by a combination of four seeds; left and right dorsolateral prefrontal cortex (dlPFC; x = ± 43, y = 22, z = 34) and left and right intraparietal sulcus (IPS; left x = –31, y = –59, z = 42 and right x = 30, y = –61, z = 39). The CON was represented by a combination of three seeds; left and right anterior insula (aIns; left x = –35, y = 14, z = 5 and right x = 36, y = 16, z = 4) and dorsal ACC/dorsomedial prefrontal cortex (dmPFC; x = –1, y = 1, z = 46). The fronto-limbic network was seeded from the ventromedial prefrontal cortex (located in the subgenual ACC (sgACC)) with slightly modified coordinates to exclude white matter voxels (vmPFC/sgACC; x = 1, y = 31, z = –10). The fronto-limbic network is partially overlapping with the network studied by Harrison et al. (Citation2009) seeded from the ventral striatum.
Seeds were created as 6-mm radius spheres around the previously mentioned peak coordinates with the Marsbar toolbox (Brett et al. Citation2002). Afterwards, the four FPN seeds were combined to one seed map representing the whole network and the same was done for the three CON seeds. The seed regions are visualised in .
Figure 1. Seed regions are depicted on the left side. The fronto-parietal network consisted of four seed regions, the cingulo-opercular network of three regions, and the the fronto-parietal network of on seed region (subgenual ACC). On the right side the connectivity from each network seed with the rest of the brain is displayed for each group separately.
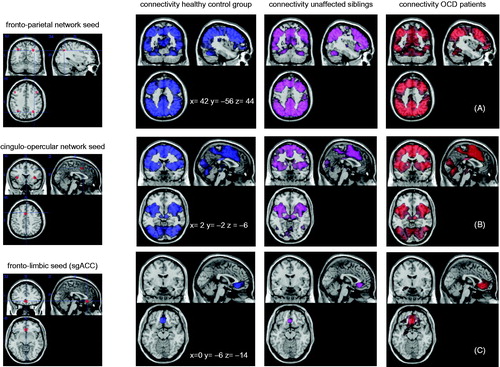
Functional-connectivity maps
The REST toolbox (Song et al. Citation2011) was used to extract the time course of all seeds. To assess the strength of the correlation within the networks, the mean time course of the seeds within each network were correlated to each other (six correlations for the four FPN seeds, three correlations for the three CON seeds). Since the fronto-limbic network consisted of only one seed, within-network connectivity could not be calculated. These correlations were Fisher Z transformed and averaged for each network, as a measure of the strength of the within-network connectivity. Group differences were analysed in SPSS with t-tests (39 OCD and 36 controls) and ANOVA (16 siblings, 16 OCD, 16 controls) followed-up with post-hoc testing. For each network the correlation between OCD patients’ Y-BOCS scores and the strength of within-network connectivity was tested.
Second, the time course of the vmPFC/sgACC seed and the mean time courses of the combined seed maps (one for the FPN and one for the CON) were correlated to all voxels in the brain. Resulting functional-connectivity maps for each network seed were Fisher Z transformed and brought to second level, using one sample t-tests (to visualise the networks for each group separately), two sample t-tests (to compare the 39 OCD patients and 36 healthy controls), and ANOVAs (for the three-group comparisons including 16 OCD patients, 16 unaffected siblings and 16 matched controls). For each group, maps of (whole brain) network connectivity were thresholded at P < 0.05 with family-wise error (FWE) correction. Significance for between-group comparisons was set at P < 0.05 FWE corrected for multiple comparisons at the cluster level with an initial threshold of P < 0.005 uncorrected at the voxel level. For the OCD group, regression analyses were performed with Y-BOCS scores, for each network separately.
Results
Sample characteristics of OCD patients and comparison subjects and of the subgroups matched to the 16 siblings are displayed in and , respectively. Siblings did not have a current axis I diagnoses, except for one sibling with claustrophobia, which did not interfere with MRI scanning.
Table 1. Demographic and clinical characteristics of OCD patients and healthy controls.
Table 2. The matched group of OCD patients, unaffected siblings and healthy controls.
Within-group network connectivity
The connectivity patterns of the FPN and CON revealed widespread connectivity throughout large parts of the cortical mantle and subcortical structures. The fronto-limbic network revealed local connectivity with the vmPFC, similar across the three groups. In the OCD group only, the connectivity pattern from the sgACC seed to the vmPFC spread into neighbouring areas such as the basal ganglia. Seed regions and connectivity patterns for each group are visualised in , thresholded at P < 0.05 with FWE correction. Supplementary Figure 1 (available online) shows the connectivity of the sgACC seed at P < 0.001 uncorrected.
Within-network connectivity
No differences were found for the strength of the within-network connectivity between OCD patients and controls for the two networks, i.e. FPN and CON (see ). The siblings, compared to the OCD patients, showed increased connectivity within the CON (see ). After exclusion of one sibling with claustrophobia, the results did not change (data not shown).
Table 3. Averaged (z transformed) correlation coefficients between the time courses of the seed maps of each network as a measure of within-network connectivity: t-test between average (z transformed) correlations for OCD patients and controls.
Table 4. Averaged (z transformed) correlation coefficients between the time courses of the seed maps of each network as a measure of within-network connectivity: ANOVA with average (z transformed) correlations for OCD patients, siblings and controls.
Network to whole-brain connectivity
Group differences for whole-brain connectivity patterns for the combined FPN and CON seed maps and fronto-limbic network are shown in and ( summarises the results of the OCD patients versus controls comparison, gives the results for the matched three-group comparison). A comparison between 39 OCD patients, 16 siblings and 36 controls, not matched for gender, is reported in the supplements.
Table 5. Effect of group for network to whole-brain connectivity: two-group comparison (39 OCD patients and 36 healthy controls).
Table 6. Effect of group for network to whole-brain connectivity: three-group comparison (16 OCD patients, 16 unaffected siblings and 16 healthy controls).
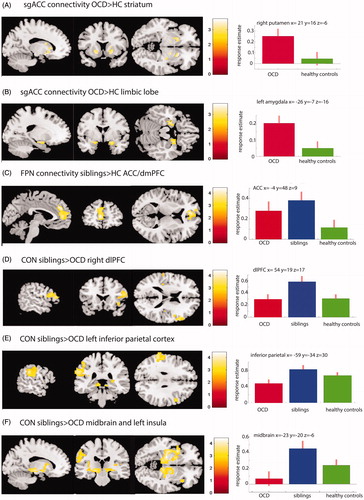
OCD patients did not differ from controls in connectivity patterns for the FPN or CON. When compared to controls, the sibling group showed increased connectivity between the FPN and the rostral ACC (rACC) extending into the dmPFC. Compared to OCD patients, the siblings showed increased connectivity between the CON and three clusters: first, an area in the midbrain including the substantia nigra, the nucleus subthalamicus, extending into the globus pallidus, putamen, and insula; second, left inferior parietal cortex; third, right dlPFC/anterior PFC. Enhanced connectivity between CON and the right dlPFC was also present in the siblings versus healthy controls comparison, but at an uncorrected significance level (ke= 81, Z = 3.89, P < 0.001 uncorrected, see , panel D). In OCD compared with controls, the fronto-limbic network showed increased connectivity with bilateral hippocampus, amygdala, anterior cingulate cortex, ventral striatum (including both putamen and caudate nucleus) extending into the inferior frontal cortex; see . There were no differences between siblings and OCD patients or controls for the fronto-limbic network. Adding age as a covariate to each network did not change any of the results, nor did exclusion of the sibling with claustrophobia.
Figure 2. Group differences in network to whole-brain connectivity. (A and B) Areas with increased connectivity with the sgACC in OCD (n = 39) compared with healthy controls (n = 36). In (A) results are masked for the striatum and in (B) for the limbic lobe for illustration purpose and displayed at P < 0.005 uncorrected. (C) Increased connectivity between the FPN and the dACC/dmPFC in siblings vs healthy controls. (D, E, F) Increased connectivity in siblings vs OCD patients between the CON and the right dlPFC (D), the left inferior parietal cortex (E), and the midbrain areas, extending into the insular cortex (F). Plots on the right side show the response estimates for each group at the given coordinates.
Correlation with Y-BOCS
In OCD patients FPN, CON and sgACC seed to whole-brain connectivity patterns did not correlate with Y-BOCS. In OCD patients, within-network strength in the FPN correlated negatively with Y-BOCS severity (Spearman’s rho = −0.46, P = 0.003) and not with severity of depressive symptoms (MADRS scores). Strength of the connectivity within the CON did not correlate with Y-BOCS.
Discussion
This study used seed-based analysis to investigate patterns of connectivity at rest in OCD patients and their unaffected siblings in two cognitive control networks and a fronto-limbic emotion-related network. We hypothesised the existence of altered connectivity in the FPN and/or CON in patients and we expected similar changes in these networks in unaffected first-degree relatives. In addition we expected increased fronto-limbic connectivity in OCD and their unaffected relatives. First, in the FPN no differences between OCD patients and controls were found, but siblings, compared to controls, showed increased connectivity between the FPN and the rACC/dmPFC. In OCD patients the within-FPN connectivity correlated negatively with disease severity. Second, no differences between patients and controls were observed in the CON. Siblings, compared with OCD patients, showed increased connectivity between the CON and fronto-parietal regions, and increased within-network connectivity (between the three CON seed regions). Third, in line with our hypothesis and previous studies (Harrison et al. Citation2009; Sakai et al. Citation2011), OCD patients, compared with controls, showed increased connectivity in the fronto-limbic network, between the sgACC seed and limbic and striatal regions. In contrast to our hypothesis no differences between siblings and controls or siblings and patients were observed in the fronto-limbic network suggesting that these network abnormalities do not constitute an endophenotype.
Fronto-parietal and cingulo-opercular networks
Contrary to our hypothesis, OCD patients compared with controls did not show differences in the within-network connectivity in FPN and CON or in connectivity between these networks and other brain areas. The negative correlation between disease severity and within-FPN connectivity suggests a role for this network in controlling OC symptoms. Our results are in contrast to some previous studies that did report alterations in cognitive control network connectivity in OCD. Gottlich et al. (Citation2014) found overall stronger within executive network connectivity in OCD that correlated with Y-BOCS severity. In contrast, another study reported reduced connectivity within the prefrontal cortex in 27 OCD patients (half of them medicated), with reduced prefrontal connectivity associated with increased OCD severity (Anticevic et al. Citation2014), which is in line with the negative correlation between FPN and Y-BOCS. Although both studies show alterations in executive network connectivity, their results point in opposite directions. Moreover, different analytical approaches (graph theory and global connectivity) were used in these studies hampering a direct comparison to the current study. Studies that seeded from the striatum reported decreased dorsal striatal to FPN connectivity in OCD patients (Harrison et al. Citation2009; Vaghi et al. Citation2016). Medicated patients were included in both of these studies, but it is also possible that subcortical seeds are more sensitive to detect such differences. Two seed-based studies reported increased connectivity between cognitive control networks and the DMN. Stern et al. (Citation2012) reported increased connectivity in 30 OCD patients (13 medicated) between anterior insula seeds and the DMN and sensori-motor network. Beucke et al. (Citation2014) found increased connectivity between a parietal seed and the DMN network in 46 OCD patients (23 medicated). Inconsistencies between the current study and previous work may be attributed to patient characteristics (such as medication status or co-morbidity). A post-hoc analysis to assess if co-morbidity influenced our results, showed no differences in connectivity in the cognitive control networks between OCD patients without co-morbidity and the control group (see Supplemental material). As our sample was sufficiently large and consisted of medication-free patients, we propose that if altered connectivity of cognitive control networks is present in OCD, it is a subtle effect at most and it may depend on additional factors (e.g. it could be present in only a subset of OCD patients or related to medication status). Alternatively, cognitive control network dysfunction in OCD could be a dynamic feature, appearing in transition phases from rest to task performance rather than purely during resting state (Cocchi et al. Citation2012).
Increased connectivity of cognitive control networks was observed in siblings; compared to the control group between the FPN and rACC/dmPFC, and compared to patients within the CON and between CON and FPN areas. When increased connectivity is interpreted as a manner to maintain normal function, it may indicate inefficiency of the cognitive control networks in siblings. We speculate that increased connectivity between cognitive control networks represents enhanced cognitive control in siblings that is lacking in OCD patients. This explanation is in line with previous reports on the same study population: increased executive network activation was present in siblings and to a lesser extent in OCD patients during a response inhibition (de Wit et al. Citation2012) and working-memory task (de Vries et al. Citation2014) and in both studies increased network activation was related to sustained cognitive performance, suggesting compensatory neural recruitment. In both tasks, altered limbic-frontal connectivity was present in patients, possibly related to the enhanced recruitment of cognitive control networks (de Vries et al. Citation2014; van Velzen et al. Citation2015). Alternatively, in patients, limbic interference may hamper a sufficient increase in compensatory recruitment, resulting in a failure to perform cognitive tasks (de Wit et al. Citation2012; de Vries et al. Citation2014; van Velzen et al. Citation2015). Preliminary results of an emotion regulation paradigm in the same sample of siblings demonstrated a task-related increase of connectivity within the CON during emotion regulation (Thorsen et al. Citation2015). Sadaghiani and D’Esposito (Citation2015) demonstrated that increased coherence of the CON was associated with increase in tonic alertness, a concept that involves suppressing distractions and thereby keeping cognitive facilities available. This suggests that increased connectivity within this network may be a beneficial mechanism, explaining better cognitive performance in the siblings than in patients (de Wit et al. Citation2012; de Vries et al. Citation2014). A speculative interpretation would be that increased tonic alertness could be a protective mechanism against developing OC symptoms. This concurs with decreased connectivity in the CON reported (at trend level) in paediatric OCD (Fitzgerald et al. Citation2010). The rostral part of the ACC and dmPFC that was hyper-connected to the FPN in siblings, is an area related to appraisal of emotional stimuli and implicit emotional control (Ochsner and Gross Citation2005; Kalisch and Gerlicher Citation2014; Servaas et al. Citation2014). During effortful reappraisal its activity is thought to be regulated by lateral prefrontal cortex (Kalisch et al. Citation2005; Ochsner and Gross Citation2005). We speculate that increased connectivity between this region and the FPN indicates that siblings exert increased effortful cognitive control over emotion. shows that connectivity strength between FPN and rACC/dmpfc in OCD patients was between that of the siblings and the control group (but not significantly different from either group), which may indicate that patients also exert increased (but insufficient) cognitive control over this area. In line with these findings, OCD patients with higher symptom severity showed impaired downregulation of the rACC during emotion regulation (de Wit et al. Citation2015). Concluding, increased CON connectivity in unaffected siblings of OCD patients is present at rest, during cognitive processing and emotion regulation and we conjecture that it acts as a mechanism to maintain normal cognitive and emotional function, which may also be the case for increased FPN to rACC/dmpfc connectivity in siblings.
Fronto-limbic network
Increased connectivity from the sgACC seed with other limbic structures in OCD replicates previous findings of increased connectivity between ventral striatum and limbic regions in samples including medicated (Harrison et al. Citation2009, Citation2013; Anticevic et al. Citation2014) and unmedicated OCD patients (Sakai et al. Citation2011). Severe and treatment-resistant OCD patients that were selected for either neurosurgical intervention with capsulotomy or deep-brain stimulation in the ventral striatum had increased metabolism in the sgACC before the intervention. After either procedure, the severity of OCD and depressive symptoms decreased as well as the metabolism in the sgACC, the striatum and the CON (Suetens et al. Citation2014). Finally, a recent voxel-based morphometry study showed a relationship between grey matter density in the vmPFC and amygdala in over 300 OCD patients (Subira et al. Citation2015).
In contrast to previous studies (Harrison et al. Citation2009; Anticevic et al. Citation2014), we found no correlation between fronto-limbic connectivity and OC disease severity. Hou et al. (Citation2014) suggested that increased connectivity between striatum and vmPFC could be an endophenotype for OCD, as both unmedicated patients and unaffected relatives showed similar differences compared with a control group. The current study does not concur with these findings, as the pattern of increased limbic connectivity was not present in unaffected siblings. The studies may not be directly comparable, because even though a similar number of patients and siblings were included, a different analysis approach was used and different seed regions were chosen. We cannot rule out that the sibling group was underpowered to detect a small effect, but also at a lower significance level (P < 0.001 uncorrected), no differences between siblings and healthy controls were observed.
The sgACC and other parts of the vmPFC play a role in the regulation of emotions, by implicit inhibitory control over the amygdala (Etkin et al. Citation2011). Increased connectivity between the sgACC/vmPFC and the striatum and amygdala may therefore imply an increased implicit effort to regulate emotions at rest. Increased connectivity between sgACC and ventral striatum followed sad mood induction in 11 OCD patients, but not in a control group (Fontenelle et al. Citation2012). Alterations in resting-state connectivity in areas indicated in emotion regulation are probably not specific to OCD and are also reported in other affective disorders, such as other anxiety disorders and depression (Kaiser et al. Citation2015). During an emotion regulation task (performed with the same OCD patients and controls) patients recruited more dmPFC (CON) relative to the dlPFC (FPN) during emotion regulation and displayed altered connectivity between the dmPFC and amygdala (de Wit et al. Citation2015). These data suggest that deficiencies in emotion regulation in OCD are mediated by interplay between CON, FPN and limbic networks and point towards a relative dysfunction of the dlPFC.
Methodological considerations
Strengths of this study are the inclusion of a large group of unmedicated OCD patients and the inclusion of a group of unaffected relatives, although the sibling group was relatively small. Differences between OCD patients and healthy controls in the fronto-limbic network were not significant after correction for multiple comparisons in the three-way analysis with the siblings. The subgroup of OCD patients used in the age- and gender-matched three-group comparison, included more males (in order to provide a good gender matching to the siblings) and had non-significantly lower scores on affective symptoms. Although exploratory analyses did not show a relation between fronto-limbic connectivity and gender, anxiety or depression, this may partly explain the differences between the complete sample and the matched three-way comparison. At a lower statistical threshold (of P < 0.001 uncorrected), however, the OCD patients also showed increased connectivity from the sgACC to the putamen (see Supplementary Table 3), suggesting that failure to replicate this finding using a corrected threshold is due to decreased power. The relatively small number of siblings that was included in the study is an important limitation and results should be replicated in larger samples.
Within-network connectivity could not be assessed for the fronto-limbic network, since this consisted of only one seed. This limits the interpretation of the results from the fronto-limbic network. Amygdala seeds were not used because partial dropout of the signal in the amygdala occurred in some subjects. A limitation of resting-state studies in general is that it is unknown what subjects are actually doing during ‘rest’. Possibly OCD patients are not capable to ‘mind wander’ to the same extent as unaffected subjects, as they may need to suppress anxiety and OC symptoms while being in the scanner. Inconsistencies between rs-fcMRI studies may relate to different emotions that patients experience while in the scanner, but also from various approaches for analysing these data (for example graph theoretical analysis, seed-based analysis or ICA) and different ways to reduce the influence of noise. Rs-fcMRI is susceptible to movement and physiological signals and there is increasing awareness for the need to vigorously correct for this noise. While new methods are developed to do this, it is difficult to compare the results from studies that used a different approach. For example, controversy has arisen around a common way to correct for noise; global mean signal regression, which may introduce artificial correlations and distort group differences (Murphy et al. Citation2009; Saad et al. Citation2012). Although a new method for noise correction was used, the partial replication of the fronto-limbic findings described by other groups lends credibility to our approach. To resolve the inconsistencies between resting-state studies, a meta-analytical approach can be used (as was recently done for rs-fcMRI studies of depression; Kaiser et al. Citation2015). To overcome differences caused by methodological confounds, the data processing steps of independent resting-state studies need to be harmonised before pooling the results. This effort is currently undertaken within the ENIGMA (Enhancing NeuroImaging and Genetics through MetaAnalyses) consortium (Thompson et al. Citation2015).
Conclusions
Contrary to our hypothesis, resting-state connectivity of cognitive control networks did not differ between unmedicated OCD patients and a healthy comparison group, implying that it is not a good candidate endophenotype for OCD. Second, unaffected siblings of OCD patients showed increased connectivity within and between cognitive control networks, which may indicate enhanced recruitment of cognitive resources that could be beneficial for cognitive performance and mental health. Finally, increased fronto-limbic connectivity was only present in OCD patients, but not in siblings, which suggests that it is likely not an endophenotype for OCD.
Statement of interest
None to declare.
Supplemental information
Download MS Word (209 KB)Acknowledgements
The authors would like to thank D. Bos, MSc, C. Vriend, PhD, S. J. C. Verfaille, MSc, E.M. Veltman, MD, V. van der Borden, MD and M. Eikelenboom, MSc, for their help with data collection and data management.
Additional information
Funding
References
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, et al. 2014. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 75:595–605.
- Bandelow B, Baldwin D, Abelli M, Altamura C, Dell’Osso B, Domschke K, Fineberg NA, Grunblatt E, Jarema M, Maron E, et al. 2016. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part I: Neuroimaging and genetics. World J Biol Psychiatry. 17:321–365.
- Beck AT, Epstein N, Brown G, Steer RA. 1988. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 56:893–897.
- Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. 2014. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 205:376–382.
- Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kaufmann C, Kathmann N. 2013. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 70:619–629.
- Brett M, Anton J, Valabregue R, Jean-Baptiste P. 2002. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Jun 2–6; Sendai, Japan. Available on CD-ROM in NeuroImage, Vol. 16, No 2.
- Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, Yucel M. 2012. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Hum Brain Mapp. 33:1089–1106.
- Cole MW, Repovs G, Anticevic A. 2014. The frontoparietal control system: a central role in mental health. Neuroscientist. 20:652–664.
- de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, van Balkom AJ, van der Wee NJ, Veltman DJ, van den Heuvel OA. 2014. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 76:878–887.
- de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchon JM, Stein DJ, Fouche JP, Soriano-Mas C, Sato JR, et al. 2014. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 171:340–349.
- de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, van Balkom AJ, Veltman DJ, van den Heuvel OA. 2012. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 169:1100–1108.
- de Wit SJ, van der Werf YD, Mataix-Cols D, Trujillo JP, van Oppen P, Veltman DJ, van den Heuvel OA. 2015. Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychol Med. 45:3059–3073.
- Del Casale A, Rapinesi C, Kotzalidis GD, De Rossi P, Curto M, Janiri D, Criscuolo S, Alessi MC, Ferri VR, De Giorgi R, et al. 2016. Executive functions in obsessive-compulsive disorder: an activation likelihood estimate meta-analysis of fMRI studies. World J Biol Psychiatry. 17:378–393.
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 104:11073–11078.
- Eng GK, Sim K, Chen SH. 2015. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: an integrative review. Neurosci Biobehav Rev. 52:233–257.
- Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 15:85–93.
- First MB, Spitzer RL, Gibbon M, Williams JB. 1999. Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P (version 2.0) edn). New York: Biometrics Research, New York State Psychiatric Institute.
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth KC, Maynor MR, Welsh RC, Hanna GL, Taylor SF. 2010. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry. 68:1039–1047.
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. 2002. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess. 14:485–496.
- Fontenelle LF, Harrison BJ, Pujol J, Davey CG, Fornito A, Bora E, Pantelis C, Yucel M. 2012. Brain functional connectivity during induced sadness in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 37:231–240.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. 1989. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 46:1006–1011.
- Gottesman II, Gould TD. 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 160:636–645.
- Gottlich M, Kramer UM, Kordon A, Hohagen F, Zurowski B. 2014. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. 35:5617–5632.
- Harrison BJ, Pujol J, Cardoner N, Deus J, Alonso P, Lopez-Sola M, Contreras-Rodriguez O, Real E, Segalas C, Blanco-Hinojo L, et al. 2013. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry. 73:321–328.
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, et al. 2009. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 66:1189–1200.
- Hou JM, Zhao M, Zhang W, Song LH, Wu WJ, Wang J, Zhou DQ, Xie B, He M, Guo JW, et al. 2014. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci. 39:304–311.
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. 2015. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 72:603–611.
- Kalisch R, Gerlicher AM. 2014. Making a mountain out of a molehill: on the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci Biobehav Rev. 42:1–8.
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, Allen P, Dolan RJ. 2005. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 17:874–883.
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506.
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. 2008. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 32:525–549.
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S. 2013. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 70:608–618.
- Milad MR, Rauch SL. 2012. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 16:43–51.
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 44:893–905.
- Ochsner KN, Gross JJ. 2005. The cognitive control of emotion. Trends Cogn Sci (Regul. Ed.). 9:242–249.
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113.
- Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB. 2014. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 35:2852–2860.
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. 2006. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 63:1225–1236.
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. 2012. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2:25–32.
- Sadaghiani S, D’Esposito M. 2015. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb Cortex. 25:2763–2773.
- Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishimura T, Fukui K. 2011. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry. 26:463–469.
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 90:449–468.
- Saxena S, Brody AL, Schwartz JM, Baxter LR. 1998. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry. (Suppl 35):26–37.
- Schuurmans J, van Balkom AJ, van Megen HJ, Smit JH, Eikelenboom M, Cath DC, Kaarsemaker M, Oosterbaan D, Hendriks GJ, Schruers KR, et al. 2012. The Netherlands Obsessive Compulsive Disorder Association (NOCDA) study: design and rationale of a longitudinal naturalistic study of the course of OCD and clinical characteristics of the sample at baseline. Int J Methods Psychiatr Res. 21:273–285.
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356.
- Servaas MN, Riese H, Ormel J, Aleman A. 2014. The neural correlates of worry in association with individual differences in neuroticism. Hum Brain Mapp. 35:4303–4315.
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, III, Daniel RJ, Silverstein SM, Godwin D, Barch DM. 2015. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 73:82–93.
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. 2011. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 6:e25031.
- Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. 2012. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 7:e36356.
- Subira M, Cano M, de Wit SJ, Alonso P, Cardoner N, Hoexter MQ, Kwon JS, Nakamae T, Lochner C, Sato JR, et al. 2015. Structural covariance of neostriatal and limbic regions in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 41:150012.
- Suetens K, Nuttin B, Gabriels L, Van Laere K. 2014. Differences in metabolic network modulation between capsulotomy and deep-brain stimulation for refractory obsessive-compulsive disorder. J Nucl Med. 55:951–959.
- Thompson PM, Andreassen OA, Arias-Vasquez A, Bearden CE, Boedhoe PD, Brouwer RM, Buitelaar J, Cannon DM, Cavalleri GL, Cohen RA, et al. 2015. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 145:389–408.
- Thorsen AL, de Wit SJ, de Vries FE, Cath DC, Veltman DJ, van der Werf YD, Hansen B, Kvale G, van den Heuvel OA. 2015. The endophenotype of emotional regulation in obsessive-compulsive disorder (OCD). Proceedings of the Meeting of the International College of Obsessive-Compulsive Spectrum disorders.
- Vaghi MM, Vertes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, Sule A, Zaman R, Voon V, Kundu P, et al. 2016. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. 81:708–717.
- van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, Denys D, Goudriaan AE. 2015. Brain circuitry in compulsivity. Eur Neuropsychopharmacol. 26:810–827.
- van Velzen LS, de Wit SJ, Curcic-Blake B, Cath DC, de Vries FE, Veltman DJ, van der Werf YD, van den Heuvel OA. 2015. Altered inhibition-related frontolimbic connectivity in obsessive-compulsive disorder. Hum Brain Mapp. 36:4064–4075.
