Abstract
Objectives
Second generation antipsychotics (SGAs) induce weight gain and dyslipidemia, albeit with important intervariability. Insulin-like growth factor binding protein (IGFBP)-2 is proposed as a circulating biomarker negatively associated with waist circumference and hypertriglyceridemia. Thus, we tested whether metabolic alterations developed upon the use of SGAs are associated with plasma IGFBP-2 levels.
Methods
A cross-sectional study was performed in 87 men newly diagnosed with schizophrenia and administered for approximately 20 months with olanzapine or risperidone as their first antipsychotic treatment. Plasma IGFBP-2 concentration, anthropometric data, as well as glucose and lipid profiles were determined at the end of the treatments.
Results
IGFBP-2 levels were similar between patients using olanzapine or risperidone and were negatively correlated with waist circumference, insulin sensitivity, and plasma triglycerides (TG). A higher proportion of men with a hypertriglyceridemic (hyperTG) waist phenotype was found in patients with IGFBP-2 levels lower than 220 ng/mL (43% for olanzapine and 13% for risperidone) compared to those with IGFBP-2 above this threshold (10% and 0%, respectively).
Conclusions
IGFBP-2 may have a role in altering metabolic risk in schizophrenic patients using SGAs. Longitudinal studies are required to evaluate whether IGFBP-2 can predict the development of a hyperTG waist phenotype in this population.
Introduction
Schizophrenia (SZ) is often associated with poor nutritional and physical activity habits, making afflicted individuals at risk for the development of metabolic dysfunctions and reduced life expectancy (Vancampfort et al. Citation2015, Citation2017). Moreover, studies in the past decade have demonstrated that treatment of drug-naive SZ patients with second generation antipsychotics (SGAs) often triggers larger appetite and additional weight gain (Jeon and Kim Citation2017). This greatly exacerbates an already reduced cardiorespiratory fitness in this vulnerable population, and further worsens insulin resistance as well as dyslipidemia (Volpato et al. Citation2013; Wei Xin Chong et al. Citation2016). In this regard, olanzapine treatment often appears more detrimental to cardiovascular health than risperidone (Vancampfort et al. Citation2017; Barton et al. Citation2020). However, apart from the likely contributions of genetics, the underlying causes for the large inter-variability in the extent of the metabolic alterations occurring in patients using SGAs are not fully established (Deng Citation2013). Thus, despite the crucial usefulness of SGAs for the clinical management of schizophrenia, additional data is required to better understand their cardiometabolic impact, which may ultimately help curb the high rate of premature mortality caused by cardiovascular disease in these patients (Tiihonen et al. Citation2009).
Recent research on obesity and metabolic abnormalities with non-psychiatric populations support that having low levels of the circulating Insulin-like Growth Factor (IGF) Binding-Protein 2 (IGFBP-2) may play an important role in developing a host of metabolic abnormalities that increase the risk for cardiovascular diseases (Heald et al. Citation2006; Carter et al. Citation2014; Yau et al. Citation2018; Carter et al. Citation2019; Haywood et al. Citation2019). IGFBP-2 is a 36 kDa circulating protein that is mainly produced by hepatocytes, and has been positively associated with insulin sensitivity through IGF-independent actions involving the α5β1 integrins and the receptor tyrosine phosphatase (RPTP)β receptor (Hoeflich and Russo Citation2015). A direct role has been experimentally demonstrated in rodents in which overexpression of IGFBP-2 prevents the development of age- and diet-induced insulin resistance, adipogenesis and body fat accretion (Wheatcroft et al. Citation2007). In line with this, low circulating levels of IGFBP-2 have been consistently observed in overweight humans with dyslipidemia and insulin resistance in an incremental manner (Heald et al. Citation2006; van den Beld et al. Citation2012; Carter et al. Citation2014; Yau et al. Citation2015; Citation2018). Moreover, low IGFBP-2 has recently been proposed as a top biomarker for cardiovascular diseases (Ho et al. Citation2018) and type 2 diabetes (Shah et al. Citation2019) . In 2014, our group reported data from a cross-sectional study in overweight men (Carter et al. Citation2014) in which a circulating IGFBP-2 concentration of ∼220 ng/mL represented a threshold below which individuals met the NCEP ATP III criteria for the clinical diagnosis of the metabolic syndrome (Brown Citation2004), including hypertriglyceridemic (hyperTG) waist phenotype (in men, waist circumference ≥ 90 cm + plasma TG ≥ 2.0 mmol/L), a simple clinical marker of visceral obesity and a robust atherogenic marker (Lemieux et al. Citation2000).
Interestingly, associations between altered levels of IGFBP-2 have been suggested with a number of brain diseases, such as Alzheimer’s disease (Royall et al. Citation2015; Lane et al. Citation2017; Bonham et al. Citation2018) and bipolar disorder (Milanesi et al. Citation2017). In addition, a role for IGFBP-2 has been suggested for the control of anxiety (Schindler et al. Citation2017), onset of depression (Basta-Kaim et al. Citation2014) and for structural plasticity in post-traumatic stress disorder (Burgdorf et al. Citation2017). Thus, there is a possible link between IGFBP-2 and mental health. However, no study has yet directly examined the relationships between IGFBP-2 and SZ. Herein, we hypothesised that having high plasma IGFBP-2 levels is associated with reduced metabolic complications in SZ patients using SGA. To this end, we took advantage of a sample including 42 olanzapine and 45 risperidone users from a previously published cross-sectional study (Almeras et al. Citation2004), and (1) determined the circulating concentrations of IGFBP-2 in these patients; (2) characterised the relationship between IGFBP-2 levels and a variety of well-established risk factors for cardiovascular diseases; and 3) investigated possible differences according to the drug used.
Methods and procedures
Study participants
The present study reports IGFBP-2 data from a cohort previously described in detail (Almeras et al. Citation2004). Details about patients’ recruitment and approval by the ethics review boards have also been published (Almeras et al. Citation2004). Informed written consent was obtained from all participants prior to their inclusion in the study. Samples were taken and deposited in a biobank for future analyses. The biobank is administered by a management framework. This specific study has received ethics approval from the institutional review board of the IUCPQ (#2017-2824, 21433).
Briefly, a total of 87 adult men newly diagnosed with DMS-IV schizophrenia by referring psychiatrists had to be treated for at least six months with either olanzapine or risperidone as their first and only SGA, with no prior exposure to other antipsychotic drugs. The sample was restricted to men, sex being an important determinant of the metabolic parameters and body fat distribution assessed in the present study. Exclusion criteria were: previous exposure to olanzapine, risperidone, clozapine or quetiapine (no other SGA were available in Canada at that time); recent smoking cessation; endocrine diseases; current treatment with medication targeting blood pressure, body weight, or glucose or lipid metabolism. Psychiatric history and psychiatric evaluation were recorded as described (Almeras et al. Citation2004). At the end of the drug treatment, anthropometric data were recorded and blood samples were collected from the antecubital vein after a 12-h overnight fast for biochemical analyses. Thus, the data reported here are a transversal portrait of cardiometabolic variables, including IGFBP-2, that were quantified at one time-point only, i.e. the end of the study.
Anthropometric and blood measurements
Weight, height (Gordon et al. Citation1988), and waist circumferences (van der Kooy et al. Citation1993) were measured using standardised procedures previously described. Body mass index (BMI) was calculated. Plasma glucose was measured enzymatically (Olympus, America Inc., PA) (Richterich and Dauwalder Citation1971), whereas plasma insulin levels were determined by radioimmunoassay (Desbuquois and Aurbach Citation1971). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin concentrations (Matthews et al. Citation1985). Plasma triglycerides (TG) and cholesterol levels were determined in plasma as described (Almeras et al. Citation2004). Plasma apo B and apo A1 concentrations were measured according to standardised procedures (Moorjani et al. Citation1987; Riepponen et al. Citation1987). The LDL peak particle diameter was obtained from non-denaturing 2–16% polyacrylamide gradient gel electrophoresis as also described (Blackburn et al. Citation2004).
IGFBP-2 quantification
Plasma IGFBP-2 levels were measured on frozen samples by ELISA (Mediagnost, Reutlingen, Germany) according to the manufacturer’s instructions. The detection limit was 0.2 ng/mL; the inter-assay coefficient of variability was 7.2%.
Statistical analyses
Data are reported as mean ± SD in Tables and as mean ± SEM in Figures. Differences in IGFBP-2 levels between olanzapine and risperidone groups were evaluated using one-way ANOVA. Relationships between cardiometabolic variables and plasma IGFBP-2 levels are reported using Pearson correlations. To further determine the impact of changes in IGFBP-2, subjects were also divided according to their IGFBP-2 levels, using 220 ng/mL as the cut-off as previously suggested (Carter et al. Citation2014). Differences in cardiometabolic variables between these four groups were assessed by two-way ANOVA using Drugs (D; olanzapine vs. risperidone) and IGFBP-2 (BP2; levels above vs. below 220 ng/mL) as main factors. Categorical variables were compared by chi-squared test. A p-value <0.05 was considered significant. All statistical analyses were performed using the GraphPad Prism statistical system (San Diego, CA, USA).
Results
Plasma samples from 87 men were processed (42 olanzapine and 45 risperidone users). The mean treatment duration with SGA was 20 months (). Age, body weight, waist circumference, fasting glucose, HOMA-IR and illness severity were similar between the two groups, although risperidone treatment was associated with higher functioning (). However, olanzapine-treated patients showed a higher cholesterol/HDL-chol ratio, as well as higher levels of triglycerides and blood pressure compared to risperidone users ().
Table 1. Proportions of schizophrenic patients with a hyperTG waistTable Footnotea according to their anti-psychotic drugs and IGFBP-2 levels.
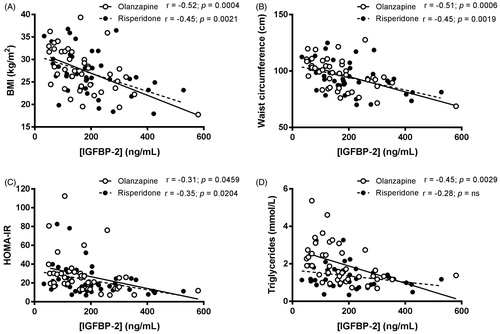
Mean circulating concentrations of IGFBP-2 in the whole cohort were 186 ± 109 ng/mL. Plasma levels of IGFBP-2 were not significantly different between patients treated with olanzapine and risperidone (p = 0.26, ). In the entire cohort, circulating levels of IGFBP-2 were negatively and significantly correlated with BMI (r = −0.49; R2 = 0.2393; p < 0.0001), waist circumference (r = −0.49; R2 = 0.2373; p < 0.0001), triglyceridemia (r = −0.39; R2 = 0.1488; p = 0.0003) and HOMA-IR index (r = −0.34; R2 = 0.1143; p = 0.0016). When drug treatments were specifically compared, these relationships remained similar (), except for triglyceridemia, which was negatively associated with IGFBP-2 levels only in olanzapine, but not in risperidone users ().
Figure 1. Plasma concentrations of IGFBP-2 in schizophrenic patients treated with olanzapine or risperidone for approximately 20 months. Each dot represents one individual. Bars represent mean ± SEM.
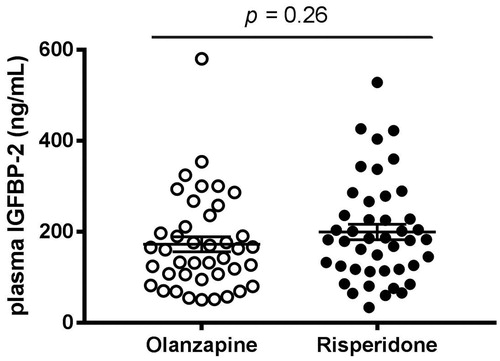
Figure 2. Simple regression analyses between plasma concentrations of IGFBP-2 and BMI (A), waist circumference (B), HOMA-IR index (C) and plasma triglycerides (D) in schizophrenic patients treated with olanzapine (open circles, plain line) or risperidone (black circles, dotted line) for approximately 20 months.
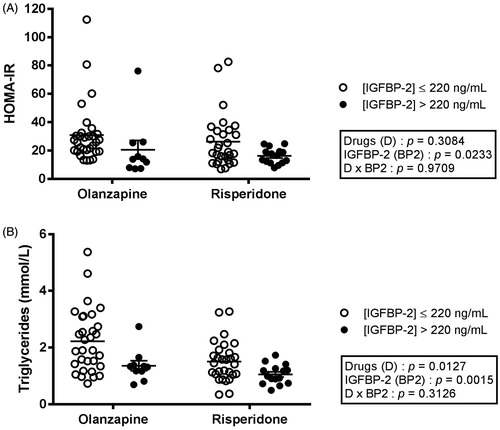
As shown in , only 10 olanzapine-treated and 15 risperidone-treated subjects had IGFBP-2 plasma concentrations above 220 ng/mL, levels suggested as threshold for many determinants of the metabolic syndrome (Carter et al. Citation2014). Regardless of the drug treatment, patients with IGFBP-2 higher than 220 ng/mL were significantly older (p = 0.0187), but were nevertheless characterised with lower body weight (p = 0.0299) than subjects with lower IGFBP-2 (). Interestingly, patients with high IGFBP-2 also had lower BMI (, p = 0.0014), and waist circumference (, p = 0.0032), than individuals with IGFBP-2 levels ≤ 220 ng/mL. Moreover, division of the groups on the basis of IGFBP-2 levels revealed robust differences in LDL particle size (, p = 0.0103), fasting insulin (, p = 0.0151), as well as insulin resistance (HOMA-IR, , p = 0.0233). Both main factors (IGFBP-2 levels and SGA drugs) were significantly linked with triglyceridemia (). In contrast, systolic and diastolic blood pressures did not differ according to IGFBP-2 levels, but rather by the administered drugs ().
Figure 3. BMI (A) and waist circumference (B) in patients treated with olanzapine or risperidone and dichotomised according to their IGFBP-2 levels. Each dot represents one individual. Bars represent mean ± SEM.
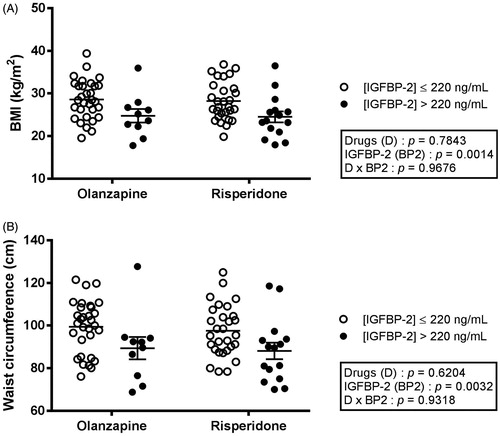
Figure 4. HOMA-IR index (A) and plasma triglycerides (B) in patients treated with olanzapine or risperidone and dichotomised according to their IGFBP-2 levels. Each dot represents one individual. Bars represent mean ± SEM.
To further investigate a possible drug-specific effect, we stratified the olanzapine- and risperidone-treated patients according to their IGFBP-2 levels and examined for each drug-group the association between IGFBP-2 level and the hyperTG waist phenotype (waist circumference ≥ 90 cm and triglyceride levels ≥ 2.0 mmol/L), which constitutes a strong atherogenic marker (Lemieux et al. Citation2000). In patients characterised with IGFBP-2 levels ≤ 220 ng/mL, 44% of olanzapine (14 out of 32) and 13% of risperidone users (4 out of 30) showed the hyperTG waist phenotype (). In patients with IGFBP-2 concentrations above 220 ng/mL, these proportions were significantly (p = 0.0178) much lower, at 10% (1 out of 10) and 0% (0 out of 15), respectively ().
Discussion
A notable and central observation of the present study is the significant number of SZ patients with circulating IGFBP-2 levels lower than 220 ng/mL, a threshold previously shown to discriminate for patients with the characteristics used for the diagnosis of the metabolic syndrome, namely high waist circumference, hypertriglyceridemia, low HDL-cholesterol, elevated fasting glycemia, and high blood pressure (Carter et al. Citation2014). When dichotomised on the basis of this threshold, we found that patients with high IGFBP-2 were less insulin resistant and had lower BMI and abdominal adiposity as shown by a lower waist circumference, independently of the prescribed drug. In this regard, this study further broadens to patients with SZ the known negative correlation between IGFBP-2 and metabolic syndrome. Thus, our findings suggest that IGFBP-2 levels are linked with the extent of the cardiometabolic alterations developed upon the use of SGAs and reveal patients with higher atherogenic risk, as indicated by their hyperTG waist phenotype.
Our data suggest that the differences in cardiometabolic profiles observed between patients could be, at least in part, associated with their IGFBP-2 levels. Consistent with many other studies, plasma IGFBP-2 levels were negatively associated with markers of fat mass and impaired insulin sensitivity, irrespective of the prescribed drug. However, IGFBP-2 and triglyceride levels were not correlated in risperidone users, suggesting a drug-specific effect in olanzapine-treated patients. These findings support the possibility of weight gain-independent effect of specific SGAs on energy metabolism (Raben et al. Citation2017; Kowalchuk et al. Citation2018) and suggest that variables other than accumulation in fat mass contribute to the drug-induced changes in blood lipids. Thus, the potential role of IGFBP-2 for better identification of individuals at risk for dyslipidemia should be tested experimentally in larger cohorts.
On average, olanzapine and risperidone-treated patients had similar circulating IGFBP-2 levels. In the absence of IGFBP-2 data before the onset of the drug administration, we cannot rule out that olanzapine and risperidone have induced a modulation in hepatic IGFBP-2 production (either directly or indirectly). For example, since SGAs have been proposed to modify leptin signalling pathways (Volpato et al. Citation2013; Goncalves et al. Citation2015), perhaps through changes in the adrenergic neuronal circuitry (Volpato et al. Citation2013; Jeon and Kim Citation2017), and that leptin increases IGFBP-2 levels (Hedbacker et al. Citation2010; Levi et al. Citation2012), this possibility is of high interest. Also, because IGFBP-2 is highly expressed in the brain (Hoeflich et al. Citation2014), in which leptin receptors have profound cardiometabolic effects, exploring the potential impact of SGAs on brain IGFBP-2 levels could reveal an unexpected role in energy balance, which could lead to novel strategies. This possibility also remains to be explored.
Limitations
A major limitation of the present study is the absence of IGFBP-2 data before the beginning of SGA treatment. It is thus not possible to know whether administration of SGA modified IGFBP-2 production or clearance, or whether IGFBP-2 can be considered as a predictive biomarker for the progression of cardiometabolic alterations upon SZ treatment. Moreover, given the cross-sectional nature of the study, and the absence of an untreated group, we cannot disentangle the effect of the disease from that of the drug nor the extent to which IGFBP-2 levels reflect causality, since it is also plausible that altered IGFBP-2 levels only reflect parallel changes in other pathways affected by the drugs. All these hypotheses ought to be tested in thorough prospective studies. Finally, the fact that our sample was restricted to male subjects makes impossible to generalise the present results to female patients.
Conclusion
The development of metabolic alterations induced by the treatment of schizophrenia with SGAs is well documented. However, there is a lack of knowledge pertaining to the factors that explain or contribute to the large inter-variability observed in patients administered with these drugs. Here, the present study shows that IGFBP-2 levels are not associated with psychiatric severity of illness or functioning under treatment with SGAs, but that they are linked with important differences in anthropometric and cardiometabolic outcomes between patients treated with olanzapine and risperidone. Thus, we propose that the extent of cardiometabolic alterations developed upon the use of atypical antipsychotics might, at least in part, be influenced by circulating IGFBP-2 levels.
Author contributions
N.A., M.-F. D., J.-P. D. and F.P. conceived and designed research project. M-A.N. performed IGFBP-2 quantification. M-A.N. and C.R. analysed data. S.C., M-A.N. N.A., M-F. D. and F.P. interpreted the experimental results. C.R. and F.P. prepared the figures. N.A., M-F. D and F.P drafted the manuscript. All authors edited and revised the manuscript. All approved the final version of the manuscript.
Supplementary Table 1
Download MS Word (12.9 KB)Acknowledgements
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Almeras N, Despres JP, Villeneuve J, Demers MF, Roy MA, Cadrin C, Mottard JP, Bouchard RH. 2004. Development of an atherogenic metabolic risk factor profile associated with the use of atypical antipsychotics. J Clin Psychiatry. 65(4):557–564.
- Barton BB, Segger F, Fischer K, Obermeier M, Musil R. 2020. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 19:295–314.
- Basta-Kaim A, Szczesny E, Glombik K, Slusarczyk J, Trojan E, Tomaszewski KA, Budziszewska B, Kubera M, Lason W. 2014. Prenatal stress leads to changes in IGF-1 binding proteins network in the hippocampus and frontal cortex of adult male rat. Neuroscience. 274:59–68.
- Blackburn P, Lemieux I, Lamarche B, Bergeron J, Perron P, Tremblay G, Gaudet D, Després J-P. 2004. Effect of type 2 diabetes on various electrophoretic characteristics of low-density lipoprotein particles in women. Diabetologia. 47(12):2114–2117.
- Bonham LW, Geier EG, Steele NZR, Holland D, Miller BL, Dale AM, Desikan RS, Yokoyama JS, Alzheimer’s D, Neuroimaging I. 2018. Insulin-like growth factor binding protein 2 is associated with biomarkers of Alzheimer’s disease pathology and shows differential expression in transgenic mice. Front Neurosci. 12:476.
- Brown DJ. 2004. New guidelines for low-density lipoprotein levels from the National Cholesterol Education Program (NCEP): a 2004 update. Prog Cardiovasc Nurs. 19(4):165.
- Burgdorf J, Colechio EM, Ghoreishi-Haack N, Gross AL, Rex CS, Zhang XL, Stanton PK, Kroes RA, Moskal JR. 2017. IGFBP2 produces rapid-acting and long-lasting effects in rat models of posttraumatic stress disorder via a novel mechanism associated with structural plasticity. Int J Neuropsychopharmacol. 20(6):476–484.
- Carter S, Lemieux I, Li Z, Almeras N, Tremblay A, Bergeron J, Poirier P, Despres JP, Picard F. 2019. Changes in IGFBP-2 levels following a one-year lifestyle modification program are independently related to improvements in plasma apo B and LDL apo B levels. Atherosclerosis. 281:89–97.
- Carter S, Li Z, Lemieux I, Almeras N, Tremblay A, Bergeron J, Poirier P, Deshaies Y, Despres JP, Picard F. 2014. Circulating IGFBP-2 levels are incrementally linked to correlates of the metabolic syndrome and independently associated with VLDL triglycerides. Atherosclerosis. 237(2):645–651.
- Deng C. 2013. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am. 42(3):545–563.
- Desbuquois B, Aurbach GD. 1971. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 33(5):732–738.
- Goncalves P, Araujo JR, Martel F. 2015. Antipsychotics-induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur Neuropsychopharmacol. 25(1):1–16.
- Gordon C, Chumlea W, Roche A. 1988. Stature, recumbent length, and weight. In: Lohman T, Roche A, Martorell R, editors. Anthropometric standardization reference manual. Champaign (IL): Human Kinetics Books; p. 3–8.
- Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. 2019. The insulin like growth factor and binding protein family: novel therapeutic targets in obesity & diabetes. Mol Metab. 19:86–96.
- Heald AH, Kaushal K, Siddals KW, Rudenski AS, Anderson SG, Gibson JM. 2006. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp Clin Endocrinol Diabetes. 114(7):371–376.
- Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. 2010. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 11(1):11–22.
- Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, Hwang SJ, Massaro JM, Larson MG, Levy D. 2018. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc. 7(14):e008108.
- Hoeflich A, Russo VC. 2015. Physiology and pathophysiology of IGFBP-1 and IGFBP-2 - consensus and dissent on metabolic control and malignant potential. Best Pract Res Clin Endocrinol Metab. 29(5):685–700.
- Hoeflich A, Wirthgen E, David R, Classen CF, Spitschak M, Brenmoehl J. 2014. Control of IGFBP-2 expression by steroids and peptide hormones in vertebrates. Front Endocrinol (Lausanne). 5:43.
- Jeon SW, Kim YK. 2017. Unresolved issues for utilization of atypical antipsychotics in schizophrenia: antipsychotic polypharmacy and metabolic syndrome. Int J Mol Sci. 18(10):2174–2190.
- Kowalchuk C, Castellani L, Chintoh A, Remington G, Giacca A, Hahn M. 2018. Antipsychotics and glucose metabolism: how brain and body collide. Am J Physiol Endocrinol Metab. 316:E1–E15.
- Lane EM, Hohman TJ, Jefferson AL; Alzheimer’s Disease Neuroimaging Initiative. 2017. Insulin-like growth factor binding protein-2 interactions with Alzheimer’s disease biomarkers. Brain Imaging Behav. 11(6):1779–1786.
- Lemieux I, Pascot A, Couillard C, Lamarche Bt, Tchernof André, Alméras Natalie, Bergeron Jean, Gaudet Daniel, Tremblay Gérald, Prud’homme Denis, et al. 2000. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 102(2):179–184.
- Levi J, Huynh FK, Denroche HC, Neumann UH, Glavas MM, Covey SD, Kieffer TJ. 2012. Hepatic leptin signalling and subdiaphragmatic vagal efferents are not required for leptin-induced increases of plasma IGF binding protein-2 (IGFBP-2) in ob/ob mice [Research Support, Non-U.S. Gov’t]. Diabetologia. 55(3):752–762.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28(7):412–419.
- Milanesi E, Zanardini R, Rosso G, Maina G, Barbon A, Mora C, Minelli A, Gennarelli M, Bocchio-Chiavetto L. 2017. Insulin-like growth factor binding protein 2 in bipolar disorder: an expression study in peripheral tissues. World J Biol Psychiatry. 19:610–619.
- Moorjani S, Dupont A, Labrie F, Lupien PJ, Brun D, Gagne C, Giguere M, Belanger A. 1987. Increase in plasma high-density lipoprotein concentration following complete androgen blockage in men with prostatic carcinoma. Metab Clin Exp. 36(3):244–250.
- Raben AT, Marshe VS, Chintoh A, Gorbovskaya I, Muller DJ, Hahn MK. 2017. The complex relationship between antipsychotic-induced weight gain and therapeutic benefits: a systematic review and implications for treatment. Front Neurosci. 11:741
- Richterich R, Dauwalder H. 1971. Determination of plasma glucose by hexokinase-glucose-6-phosphate dehydrogenase method. Schweiz Med Wochenschr. 101(17):615–618. ger.
- Riepponen P, Marniemi J, Rautaoja T. 1987. Immunoturbidimetric determination of apolipoproteins A-1 and B in serum. Scand J Clin Lab Invest. 47(7):739–744.
- Royall DR, Bishnoi RJ, Palmer RF. 2015. Serum IGF-BP2 strongly moderates age’s effect on cognition: a MIMIC analysis. Neurobiol Aging. 36(7):2232–2240.
- Schindler N, Mayer J, Saenger S, Gimsa U, Walz C, Brenmoehl J, Ohde D, Wirthgen E, Tuchscherer A, Russo VC, et al. 2017. Phenotype analysis of male transgenic mice overexpressing mutant IGFBP-2 lacking the Cardin-Weintraub sequence motif: Reduced expression of synaptic markers and myelin basic protein in the brain and a lower degree of anxiety-like behaviour. Growth Horm IGF Res. 33:1–8.
- Shah RV, Hwang SJ, Yeri A, Tanriverdi K, Pico AR, Yao C, Murthy V, Ho J, Vitseva O, Demarco D, et al. 2019. Proteins altered by surgical weight loss highlight biomarkers of insulin resistance in the community. Arterioscler Thromb Vasc Biol. 39(1):107–115.
- Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J. 2009. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 374(9690):620–627.
- van den Beld AW, Blum WF, Brugts MP, Janssen JA, Grobbee DE, Lamberts SW. 2012. High IGFBP2 levels are not only associated with a better metabolic risk profile but also with increased mortality in elderly men. Eur J Endocrinol. 167(1):111–117.
- van der Kooy K, Leenen R, Seidell JC, Deurenberg P, Visser M. 1993. Abdominal diameters as indicators of visceral fat: comparison between magnetic resonance imaging and anthropometry. Br J Nutr. 70(1):47–58.
- Vancampfort D, Rosenbaum S, Probst M, Soundy A, Mitchell AJ, De Hert M, Stubbs B. 2015. Promotion of cardiorespiratory fitness in schizophrenia: a clinical overview and meta-analysis. Acta Psychiatr Scand. 132(2):131–143.
- Vancampfort D, Rosenbaum S, Schuch F, Ward PB, Richards J, Mugisha J, Probst M, Stubbs B. 2017. Cardiorespiratory fitness in severe mental illness: a systematic review and meta-analysis. Sports Med. 47(2):343–352.
- Volpato AM, Zugno AI, Quevedo J. 2013. Recent evidence and potential mechanisms underlying weight gain and insulin resistance due to atypical antipsychotics. Braz J Psychiatry. 35(3):295–304.
- Wei Xin Chong J, Hsien-Jie Tan E, Chong CE, Ng Y, Wijesinghe R. 2016. Atypical antipsychotics: a review on the prevalence, monitoring, and management of their metabolic and cardiovascular side effects. Ment Health Clin. 6(4):178–184.
- Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SC, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, et al. 2007. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 56(2):285–294.
- Yau SW, Azar WJ, Sabin MA, Werther GA, Russo VC. 2015. IGFBP-2 - taking the lead in growth, metabolism and cancer. J Cell Commun Signal. 9(2):125–142.
- Yau SW, Harcourt BE, Kao KT, Alexander EJ, Russo VC, Werther GA, Sabin MA. 2018. Serum IGFBP-2 levels are associated with reduced insulin sensitivity in obese children. Clin Obes. 8(3):184–190.
