Abstract
Objectives
Despite the available therapies for treatment-resistant depression (TRD), there are a limited number that are evidence-based and effective in this hard-to-treat population. Esketamine nasal spray, an intranasal N-methyl-d-aspartate (NMDA) glutamate receptor antagonist, is a novel, fast-acting option in this patient population. This manuscript provides expert guidance on the practicalities of using esketamine nasal spray.
Methods
A group of six European experts in major depressive disorder (MDD) and TRD, with clinical experience of treating patients with esketamine nasal spray, first generated practical recommendations, before editing and voting on these to develop consensus statements during an online meeting.
Results
The final consensus statements encompass not only pre-treatment considerations for patients with TRD, but also specific guidelines for clinicians to consider during and post-administration of esketamine nasal spray.
Conclusions
Esketamine nasal spray is a novel, fast-acting agent that provides an additional treatment option for patients with TRD who have previously failed several therapies. The guidance here is based on the authors’ experience and the available literature; however, further real-world use of esketamine nasal spray will add to existing knowledge. The recommendations offer practical guidance to clinicians who are unfamiliar with esketamine nasal spray.
Introduction
MDD is a complex and debilitating disease, affecting over 25.8 million people worldwide in 2017 (Liu et al. Citation2020). Despite the number of antidepressants available for MDD (Bauer et al. Citation2019), its complexity makes it difficult for many patients to reach remission (Trivedi and Daly Citation2008); approximately 30% of patients do not respond to antidepressant therapy (Al-Harbi Citation2012). Treatment options for MDD are limited by their delayed onset of action, early onset of adverse events (Kaur et al. Citation2019) and low response rates (Gaynes et al. Citation2008). Therefore, there is a need for fast-acting, efficacious therapies to treat patients with TRD (Duman et al. Citation2016).
Research into MDD pathophysiology has implicated abnormalities in glutamatergic transmission, with NMDA glutamate receptors identified as a potential pharmacotherapeutic target for MDD and other mood disorders (Mathews et al. Citation2012). Ketamine, a glutamate receptor antagonist, is a non-competitive, high-affinity NMDA receptor antagonist used ‘off-label’ for its rapid antidepressant effects (Kraus et al. Citation2017). However, ketamine as a therapy is limited due to recreational abuse among users who may develop dependency with repeated dosing (Kraus et al. Citation2017). Esketamine, the racemic S-enantiomer of ketamine, has a fourfold higher affinity for the NMDA receptor than the R-enantiomer (Muller et al. Citation2016). Esketamine nasal spray (Spravato®), a glutamate receptor antagonist, selectively blocks NMDA receptors expressed on gamma-aminobutyric acid-ergic inhibitory interneurons, leading to enhanced glutamatergic firing (Kadriu et al. Citation2019). Clinical studies showed esketamine nasal spray exerted rapid antidepressant effects (least square mean change in Montgomery–Åsberg Depression Rating Scale [MADRS] score −3.3 [95% confidence interval: −5.75, −0.85] 24 h after first dose) in patients with TRD (). In 2019, esketamine nasal spray was the first U.S. Food and Drug Administration (FDA)-approved therapy for TRD to target the glutamatergic system (Kaur et al. Citation2019). The European Medicines Agency (EMA) subsequently approved esketamine nasal spray, in combination with a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI), for adults with treatment-resistant MDD, who have not responded to at least two different treatments with antidepressants in the current moderate-to-severe depressive episode (EMA Citation2019). The initial recommended dose for adults <65 years is 56 mg and for adults ≥65 years or of Japanese ancestry is 28 mg (EMA Citation2019). This differs from the FDA indication, which does not specify the antidepressant class or define the number of treatment failures required for TRD and recommends a universal 56 mg starting dose in conjunction with an oral antidepressant (FDA Citation2019b).
Table 1. Summary of Phase 3 studies of esketamine nasal spray in patients with TRD.
In Europe, esketamine nasal spray is recognised as an efficacious treatment for patients with TRD, with a distinct safety profile and a different, intranasal method of administration (EMA Citation2019), compared with existing antidepressants (Carvalho et al. Citation2016). This manuscript aims to provide practical guidance on esketamine nasal spray to aid clinical treatment decision-making for patients with TRD.
Methods
Six European experts in MDD and TRD with clinical experience with esketamine nasal spray developed this manuscript. Collectively, at the time of writing, they had several years’ experience with esketamine nasal spray, in treating over 120 patients.
The authors agreed to generate practical recommendations, in the form of consensus statements, on identifying and treating patients with esketamine nasal spray, based on their experience of treating patients with esketamine nasal spray and published data on TRD. All authors agreed on four themes to cover in the manuscript and draft statements were discussed, reviewed and approved by all authors.
The authors first discussed the practical recommendations at an online consensus meeting and over emails, before editing and voting on these to develop consensus statements (this process was not anonymous). The six voting options were ‘strongly agree’, ‘mostly agree’, ‘somewhat agree’, ‘somewhat disagree’, ‘mostly disagree’ and ‘strongly disagree’. The authors were unanimous in ‘strongly agreeing’ with 5 of 11 statements. For the remaining statements, the authors voted either ‘strongly agree’ or ‘mostly agree’, and for only one statement, one author voted ‘somewhat agree’. A meeting report summarising key discussion points and the amended consensus statements was reviewed and approved by all authors. The authors received support from a medical writing agency (funded by Janssen Pharmaceutica NV), which facilitated the process and drafted the consensus statements and manuscript, under the direction and guidance of all authors. This work was developed in alignment with Good Publications Practice (GPP3; Battisti et al. Citation2015). Janssen Pharmaceutica NV had no direct or indirect involvement in the development of the consensus statements, topics, or manuscript.
Results: Practical guidance for clinicians prescribing esketamine nasal spray
Here, we present consensus statements on pre-, during and post-treatment considerations for treating patients with TRD with esketamine nasal spray. Statements 1–4 provide advice on factors to consider prior to initiating treatment; statements 5–11 provide advice on during and post-administration clinical supervision and guidance.
Understanding the different facets of treatment-resistant depression
(1) TRD is a complex, multifaceted disorder with many contributing neurobiological, genetic, clinical and psychosocial factors
Specific genes have been associated with an increased risk of TRD; however, studies investigating this association are limited in number and have varied findings. The short allele of the serotonin transporter promoter gene polymorphism is hypothesised to alter the serotonergic system, potentially leading to SSRI-treatment resistance (Coplan et al. Citation2014). More specifically, the short allele was shown to reduce transcription of the serotonin transporter gene (Lesch et al. Citation1996), the target for SSRIs. Additionally, several single nucleotide polymorphisms in genes implicated in the serotonergic system have been identified in TRD (Bartova et al. Citation2019), for example: CREB1, BDNF and 5HTR2A (Schosser et al. Citation2012). There are few studies reporting the genetic complexity of TRD and its impact on overall antidepressant efficacy (Fabbri et al. Citation2019); further studies are needed.
Clinical characteristics are also associated with TRD, including comorbidities, characteristics and symptoms of the current depressive episode, and the patient’s disease and treatment history. Psychiatric or somatic comorbidities of MDD can increase the risk of developing TRD (Kornstein and Schneider Citation2001), including anxiety, substance abuse and personality disorders, and neurodegenerative, neurovascular and autoimmune diseases (Bennabi et al. Citation2019). The characteristics and symptomatology of the current depressive episode can exacerbate the persistence and recurrence of TRD. These include the severity and duration of the current depressive episode, early age of onset (Bennabi et al. Citation2019), melancholic features (Jaffe et al. Citation2019) or suicidal risk (Souery et al. Citation2007), the latter of which is thought to be associated with Val66Met and rs10501087 polymorphisms (Schosser et al. Citation2017). The patient’s disease and treatment history may also impact the risk of TRD, for example, childhood adversity (Tunnard et al. Citation2014), number of prior depressive episodes (Bartova et al. Citation2019), traumatic or stressful life events, or previous non-remission or partial remission (Murphy et al. Citation2017). These clinical factors are thought to contribute to TRD, adding to its complexity.
(2) There are a number of different definitions of TRD; however, to inform clinical decisions in this context, the practical definition of TRD is ‘the failure of at least two different treatments with antidepressants of adequate duration and dose in the current moderate-to-severe episode’ (EMA Citation2019), in line with the EMA’s definition of TRD (EMA Citation2013)
There is currently no universally accepted clinical definition for TRD. The most widely used definitions of TRD are based on at least two treatment failures, irrespective of drug class (Bartova et al. Citation2019; Brown et al. Citation2019). From the authors’ experience, this may be because one treatment failure would place the threshold too low. Despite this, a recent systematic review identified 155 different definitions for TRD; however, many had overlapping criteria and/or were a clinician’s opinion rather than a validated definition (Brown et al. Citation2019). Interestingly, the definition of TRD within clinical research is also variable; a systematic review found that only 17% of intervention studies defined TRD based on at least two treatment failures (Gaynes et al. Citation2020). These findings emphasise the variety of TRD definitions in both clinical practice and research.
Variability in the definition of TRD can be explained by many factors. Terminology can be confusing, for example, TRD and treatment-refractory depression; TRD often refers to two antidepressant failures, whereas treatment-refractory depression refers to three or more failures including electroconvulsive therapy (Kasper and Frazer Citation2019). Additionally, ‘pseudo-resistance’ can occur when treatment resistance arises due to misdiagnosis, individual clinician differences (Murphy et al. Citation2017), inadequate dose and duration of antidepressant treatment, or patient non-compliance (Dold et al. Citation2018). There are no international guidelines on the ‘adequate duration and dose’ of antidepressants; a suggested duration is 4 to 6 weeks (Bennabi et al. Citation2019), with a minimum of 2 to 3 weeks at the target dose (Dold and Kasper Citation2017). Poor tolerability may also limit a patient’s ability to complete an adequate trial of antidepressants (Ashton et al. Citation2005).
Furthermore, clinicians should systematically evaluate improvement as early as possible as early improvement may optimise treatment (Oluboka et al. Citation2018) and is associated with treatment response and remission (Kraus et al. Citation2019). To evaluate treatment response, clinicians can consult various staging methods that define TRD alongside their own clinical judgement (). However, further studies are required to demonstrate the reliability and validity of these staging methods in determining treatment response (Gaynes et al. Citation2020). Alternative methods to determine treatment response include digital platforms such as HumanITcare (Jones Citation2019).
Table 2. Examples of staging methods that can be applied to define TRD.
The recommendations for esketamine nasal spray are based on Phase 3 data () where the study definition of TRD was ‘non-response to an adequate trial (dosage, duration and adherence) of at least two antidepressants in the current episode (of which one was observed prospectively)’ (Popova et al. Citation2019). As the study definition of TRD did not require the second antidepressant to be from a different class, a patient who had received two consecutive SSRIs or SNRIs was considered treatment resistant.
Despite the diversity of TRD definitions, European clinicians who decide to treat patients with TRD with esketamine nasal spray should always refer to the definition in the Summary of Product Characteristics (EMA Citation2019), based on the EMA’s definition of TRD (EMA Citation2013).
Considerations prior to initiating treatment with esketamine nasal spray
(3) When deciding whether to initiate treatment with esketamine nasal spray or alternative third-line treatment options in patients with TRD, the prescribing clinician should carefully consider previous treatment history, comorbidities and current concomitant medication, as well as the individual patient’s circumstances and treatment preferences
Figure 1. How esketamine nasal spray might fit into a proposed treatment pathway for patients with MDD. MDD: major depressive disorder; SNRI: serotonin and norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor.
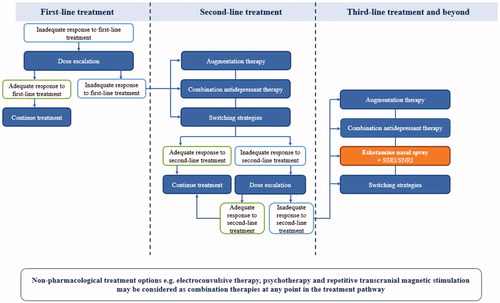
Numerous factors can influence the treatment strategy for TRD, including the patient’s preference and their medical and treatment history. Firstly, patient preference can be influenced by duration and onset of action of treatment, dosing schedule and symptom severity (Gelhorn et al. Citation2011). Given that esketamine nasal spray has a different method of administration via intranasal spray (EMA Citation2019) compared with oral antidepressants, this may also impact a patient’s preference; the authors advise including patients in treatment decision-making. Comorbidities can also determine which treatment for TRD might be suitable. For example, esketamine nasal spray poses a risk to patients with cardiovascular and cerebrovascular conditions due to possible adverse events of elevated blood pressure (EMA Citation2019). If a patient presents with high blood pressure readings (>140/90 mmHg for patients <65 years of age and >150/90 mmHg for patients ≥65 years of age), consider delaying esketamine nasal spray treatment (EMA Citation2019). In summary, clinicians and patients should collaborate in assessing the risks and benefits of each treatment for TRD.
(4) Due to potential acute adverse events, clinical supervision is required for esketamine nasal spray post-administration, which may require additional logistical considerations and resource planning
There are important logistical factors to consider before initiating esketamine nasal spray treatment, summarised in Box 1. Esketamine nasal spray can transiently increase blood pressure; therefore, the EMA advises assessing blood pressure before and after administration. Additionally, appropriate resuscitation equipment and healthcare professionals with training in cardiopulmonary resuscitation should be available when treating patients with clinically significant or unstable respiratory or cardiovascular conditions (EMA Citation2019). Esketamine nasal spray is also associated with other acute adverse events post-administration (EMA Citation2019); therefore, clinical supervision may be required for up to 3 h post-administration (Popova et al. Citation2019). A healthcare professional determines readiness to leave by observing the patient until they appear stable, based on clinical judgement; a supporting checklist is available (EMA Citation2019). In a Phase 3 study of adults (18–64 years) with TRD, 93% of patients treated with esketamine nasal spray were ready to leave at 1.5 h post-dose, with the remaining 7% ready to leave by 3 h (Popova et al. Citation2019). In the authors’ experience, logistical planning becomes easier over time, building on experience from each administration session and reproducibility across sessions. In summary, esketamine nasal spray is associated with specific acute adverse events for which the administrating clinic must be fully equipped, including clinical supervision during and post-administration.
Box 1. Treatment room considerations for esketamine nasal spray treatment.
Provide patients with a comfortable seat that is able to recline to 45 degrees (EMA Citation2019)
In the authors’ experience, a quiet room with minimal background noise and other distractions may help patients to feel calm
In the authors’ experience, during the maintenance phase, cognitive stimuli (e.g. ambient lighting and/or imagery in the room) may be increased, based on clinical judgement
Resuscitation equipment should be available when treating patients with clinically significant or unstable cardiovascular or respiratory risk factors (EMA Citation2019)
In the authors’ experience, it can be helpful for healthcare professionals to remain in proximity to the patient following administration, with easy access to specialised equipment if necessary
The practicalities of esketamine nasal spray administration
Statements 5–11 provide practical guidance on treatment of TRD with esketamine nasal spray in a clinical setting.
(5) Esketamine nasal spray is administered concomitantly with an SSRI/SNRI (EMA Citation2019); evaluate the patient’s treatment response history to decide whether to continue with the current SSRI/SNRI, to begin treatment with a new antidepressant of the same class or to begin treatment with a new antidepressant of a different class (an SSRI/SNRI)
Esketamine nasal spray must be administered concomitantly with an SSRI/SNRI (EMA Citation2019); however, which particular drug depends on clinical judgement. Although it can make intuitive sense to change one treatment variable at a time, in clinical studies, esketamine nasal spray plus a new oral antidepressant showed clinical benefit in comparison with placebo plus a new oral antidepressant, supporting the choice to change two variables at once (Popova et al. Citation2019).
In the case of non-response, or an intolerable adverse-event profile, clinicians should consider switching the current antidepressant (Kudlow et al. Citation2014). In the case of a partial response, clinicians should ensure treatment compliance (QIDS Citation2009), before considering augmentation or combination therapy or medication switch. Please see for further guidance.
Figure 2. An example of the different treatment options available when starting treatment of TRD with esketamine nasal spray. MDD: major depressive disorder; SNRI: serotonin and norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TRD: treatment-resistant depression.
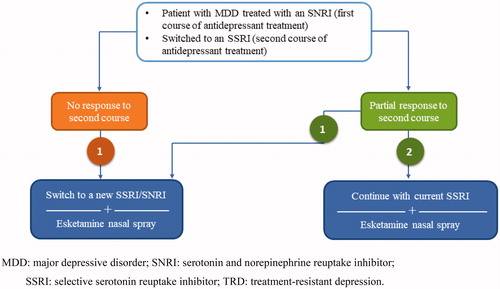
Current evidence from the STAR*D study suggests no difference in response when switching treatment to a new antidepressant of the same class or of a different class (Gaynes et al. Citation2008). For some patients it may be beneficial to choose a new antidepressant of the same class, as lack of tolerability to one SSRI or SNRI does not indicate intolerability to the whole class of antidepressants (Al-Harbi Citation2012). Taken together, these reports suggest that in some cases there may be no benefit to trying a new class compared with staying in the same class as the current antidepressant.
Future studies would be required to show if antidepressants of other classes, e.g. mirtazapine, could be an alternative to administer concomitantly with esketamine nasal spray for TRD. However, esketamine nasal spray is currently only indicated in combination with an SSRI or SNRI (EMA Citation2019).
(6) The dose of esketamine nasal spray is flexible throughout treatment, while the dosing schedule is flexible after the initial maintenance phase. The decision to change the dose or reduce the dosing frequency should be based on clinical evaluation supported by efficacy outcomes
The dosing schedule of esketamine nasal spray is different to previous treatments for TRD and should be regularly assessed. In the 4-week induction phase, esketamine nasal spray dose is flexible, with different twice-weekly doses recommended based on age and race (EMA Citation2019). After the initial 4 weeks, the EMA recommends that patients enter the maintenance phase where esketamine nasal spray can be administered flexibly: once weekly, or every other week (EMA Citation2019). For example, the long-term dosing schedule in the Phase 3 withdrawal study of esketamine nasal spray in adults (18–64 years) with TRD varied; the majority of patients in stable remission were treated every other week (68.9%) for most of the maintenance phase, whereas most of the stable responders were treated more frequently, once a week mostly (54.8%; Daly et al. Citation2019). Sustained or improved efficacy was reported with esketamine nasal spray in 76% of all responders, even at a lower dose frequency, supporting individualisation of treatment frequency (Nijs et al. Citation2020). Additionally, the initial dose from the induction phase can be increased to 84 mg if deemed appropriate (EMA Citation2019). For example, one of the authors’ patients has received esketamine nasal spray, as part of a Phase 3 continuation of care study (ClinicalTrials.gov Citation2020a), since 2017 (3 years prior to the time of writing); they currently receive an 84-mg monthly dose (an investigational dosing regimen) alongside daily venlafaxine. From the authors’ experience, if treatment is tolerable, the dose should be maintained. Clinicians should use their judgement and clinical experience, alongside patient- and clinician-reported outcomes (), to determine response to esketamine nasal spray within a certain timeframe.
Table 3. Summary of some of the outcome measures available to support clinical judgement of efficacy.
Patient education can support compliance and adherence with the esketamine nasal spray dosing regimen; research suggests that patient non-compliance may occur as a result of poor therapy area understanding (Kornstein and Schneider Citation2001). The authors suggest that discussing the definition and causes of TRD, symptom recognition, and the practicalities of esketamine nasal spray treatment with patients could enhance patient adherence to the treatment regimen. The authors also found that reminder calls, SMS or digital calendars encouraged adherence. In case of non-compliance with pre-treatment recommendations – food and liquid avoidance and not using a nasally administered corticosteroid or decongestant – consider delaying or rescheduling administration. From the authors’ experience, patient education measures aid patients’ understanding of, and engagement with, the esketamine nasal spray treatment process.
Clinical considerations for managing treatment-emergent adverse events
(7) Due to the time course of adverse events, patients should be clinically monitored after administration of esketamine nasal spray, particularly for elevated blood pressure, dissociation and sedation (EMA Citation2019)
Generally, in clinical studies, esketamine nasal spray adverse events followed a similar time course; most adverse events were transient, appeared shortly after dosing (during the first 30–40 minutes) and had resolved by 1.5 h after dosing (Popova et al. Citation2019). For example, most cardiovascular adverse events had an onset shortly after dosing and subsided by 1.5 h post-dose (Doherty et al. Citation2020); this time course is consistent with the pharmacokinetic profile of esketamine nasal spray (FDA Citation2019a).
(8) Blood pressure elevation: If a patient’s blood pressure is high or remains elevated for some time, continue to regularly monitor blood pressure and consider prescribing an antihypertensive medication based on your clinical judgement until the values normalise
To mitigate the risk of acute blood pressure elevation, any underlying hypertension (>140/90 mmHg for patients <65 years of age and >150/90 mmHg for patients ≥65 years of age) should be treated and stabilised prior to esketamine nasal spray administration (EMA Citation2019). Additionally, clinicians should check adherence and tolerability to current antihypertensive treatment. In esketamine nasal spray clinical studies, acute blood pressure elevation as an adverse event was more prevalent in patients with a history of hypertension (Doherty et al. Citation2020). This reinforces the importance of managing pre-existing hypertension before initiating esketamine nasal spray treatment.
The treating clinician and patient should be aware that most blood pressure adverse events appear shortly after dosing and are short-lived and self-resolving. In esketamine nasal spray clinical studies, blood pressure increases generally reached a maximum within 40 minutes of dosing and normalised within 1.5–2 h (Doherty et al. Citation2020). Most cases of increased blood pressure were not associated with symptoms, did not result in serious cardiovascular safety sequelae and had no clinically relevant effect on electrocardiogram parameters, and fewer than 2% of patients discontinued due to cardiovascular adverse events (Doherty et al. Citation2020). These data show that acute increases in blood pressure are generally transient and self-resolving.
In clinical studies of esketamine nasal spray, most cases of increased blood pressure were self-resolving without the need for medication (Popova et al. Citation2019; Doherty et al. Citation2020). However, in a short-term, double-blind study, a small proportion of patients without a history of hypertension required an antihypertensive (2.1% in the esketamine arm vs 1.2% in the placebo arm; Doherty et al. Citation2020). Antihypertensives prescribed in the esketamine arm included amlodipine, captopril, losartan, metoprolol and propranolol (Doherty et al. Citation2020). The decision to prescribe an antihypertensive following acute hypertension, and which to prescribe, is based on clinical judgement and the individual patient.
(9) Dissociation: In general, dissociation does not require specific intervention as it is transient and disappears over time; however, in rare cases of severe agitation or anxiety, consider prescribing a benzodiazepine, e.g. lorazepam
Dissociation has a large spectrum of symptoms, in which the severity of symptoms can be subjective; therefore, each patient experience is highly variable and could be positive or negative (Nijenhuis Citation2001). Dissociative symptoms include dissociative amnesia, depersonalisation, derealisation, identity confusion/identity fragmentation, the subjective feeling of being separated from the surrounding environment or one’s body (Nijenhuis Citation2001), and the transient feeling of being disconnected from space and time (EMA Citation2019). While dissociation is experienced differently among patients, it is important to note that dissociation is an adverse event associated with esketamine nasal spray treatment and should not be used therapeutically to gain more insight into the ‘unconscious world’ of the patient.
Patient education is key to managing patient expectations of potential dissociation adverse events (Box 2). A vital aspect of patient education is regular supervision and reassurance from the treating clinician before and during treatment. From the authors’ experience, it can be helpful to prompt patients to think about a positive moment from their life during administration, as this could potentially reduce their risk of experiencing a negative dissociative event. Additionally, it is important to emphasise that in esketamine nasal spray clinical studies, dissociation adverse events were transient and self-limiting, occurring on the day of dosing and in most cases disappearing within 1.5 h post-dose without the need for medical intervention (EMA Citation2019; Popova et al. Citation2019). No medications were utilised specifically for the management of dissociation in clinical trials; however, anxiety or agitation associated with dissociation could be treated with short-acting benzodiazepines; only 10 out of 1,601 patients treated with esketamine nasal spray in the Phase 3 TRD studies received such medication (unpublished data provided by Janssen Pharmaceutica NV). From the authors’ experience, if necessary, medication for symptoms of dissociation can be beneficial. However, in the first instance, the authors found patient education most helpful (Box 2).
Box 2. Discussion topics for clinician-patient conversations around dissociation as an adverse event following esketamine nasal spray administration.
Evidence from the esketamine nasal spray clinical trials
Professional supervision will be provided for the duration of treatment with esketamine nasal spray (EMA Citation2019)
Dissociative adverse events will be monitored by the treating healthcare professional (EMA Citation2019)
Dissociative symptoms vary and can be experienced in different ways; for example, they may be positive or negative (EMA Citation2019)
In general, dissociation occurs shortly after esketamine nasal spray administration (EMA Citation2019; Popova et al. Citation2019)
In general, symptoms of dissociation alleviate relatively quickly on their own (within 1.5 h post-dose; EMA Citation2019; Popova et al. Citation2019)
In general, the severity of dissociative symptoms reduces over time with repeated treatment (EMA Citation2019; Popova et al. Citation2019)
The incidence of severe dissociation in clinical studies was low (<4%; EMA Citation2019)
Of 802 patients enrolled in the long-term safety study, discontinuation due to dissociation occurred in 5 patients (0.6%; Wajs et al. Citation2020)
Authors’ suggestions
It can be helpful to focus on positive and/or mindful thoughts before administration
It can be helpful to focus on positive and/or mindful thoughts before administration
Dissociative adverse events are not a form of therapy and should not be used to better understand the inner mind
(10) Sedation: If a patient experiences severe sedation or becomes unconscious, close monitoring for signs of respiratory depression and change in haemodynamic parameters is recommended
Several factors can help to manage the severity of sedation adverse events following esketamine nasal spray administration. Firstly, patient education helps to manage patient expectations. Clinicians may reassure their patients that cases of sedation are often transient and mild-to-moderate in severity. In clinical studies of esketamine nasal spray, no signs of respiratory distress were observed with sedation adverse events, and sedation was not associated with hypoxaemia (Popova et al. Citation2019). In addition, across all Phase 3 studies (), severe cases of sedation were infrequent (EMA Citation2019). For example, of 802 patients enrolled in the long-term safety study, 5 patients (<1%) experienced severe sedation, as demonstrated by a Modified Observer’s Assessment of Alertness and Sedation (MOAA/S) score of 0 (corresponding to no reaction to painful trapezius squeeze) or 1 (purposeful reflexive withdrawal in response to trapezius squeeze; Wajs et al. Citation2020). Secondly, the authors also found it helpful if the room was properly lit and if a comfortable seat was provided for administration. Thirdly, from the authors’ experience, it was helpful to begin with a low esketamine nasal spray dose and then to only increase the dose as necessary, based on clinical judgement.
In the Phase 3 long-term safety study of esketamine nasal spray, in the cases of sedation (EMA Citation2019), vital signs and oxygen saturation were assessed, as well as any symptoms of respiratory distress (Wajs et al. Citation2020). Based on this, the authors advise regularly assessing vital signs to ensure no respiratory or hypotension problems arise during a potential sedation adverse event. Additionally, from the authors’ experience, it is useful to assess cognitive function and alertness to determine when a patient is no longer experiencing sedation. Based on clinical judgement, consider limiting any concomitant benzodiazepines (Kaur et al. Citation2019) or certain antipsychotics (Miller Citation2004) as these can increase the risk of sedation (Kaur et al. Citation2019).
(11) Consider the frequency and severity of adverse events, dose adjustment, response, patient preference, concomitant medication use and psychiatric history when deciding to continue treatment with esketamine nasal spray
Across clinical studies of esketamine nasal spray, severe adverse events were infrequent (EMA Citation2019); however, regular monitoring is necessary to manage these events if they do occur. Rates of discontinuation due to adverse events in clinical studies were low; 7% of patients discontinued esketamine nasal spray treatment in the 4-week double-blind phase of the Phase 3 study of adults (18–64 years) with TRD (Popova et al. Citation2019), and 3.8% of patients discontinued in the 48-week open-label optimisation/maintenance phase of the long-term safety study (Wajs et al. Citation2020). Moreover, a post hoc analysis of the Phase 3 studies highlighted that, although adverse events such as dissociation were relatively common, they did not necessarily lead to discontinuation (Citrome et al. Citation2020).
The decision on whether to continue treatment with esketamine nasal spray following a severe adverse event is dependent on the type of adverse event and its intensity and duration, concomitant medication, patient preference, general clinical evaluation and presence of elevated blood pressure. For example, one of the authors’ patients who was treated with esketamine nasal spray (56 mg) experienced a hypertensive crisis during their first administration, with their systolic blood pressure increasing to 210 mmHg. Despite being treated with captopril, the patient decided to discontinue esketamine nasal spray treatment (Wajs et al. Citation2020).
Although there is no specific guidance on treatment duration, the EMA recommends continuing treatment with esketamine nasal spray for at least 6 months after depressive symptoms improve. Additionally, clinicians should periodically re-evaluate treatment continuation during the maintenance phase (EMA Citation2013). Data from a withdrawal study (Daly et al. Citation2019) showed that esketamine nasal spray reduced the risk of relapse vs placebo by 70% in patients in stable response (hazard ratio, 0.30; 95% CI, 0.16–0.55). In addition, the median time to relapse was reduced in those patients who were treated with esketamine nasal spray for 20 months vs patients who were treated for only 4 months (635 days vs 88 days, respectively; Daly et al. Citation2019). Further guidance on treatment duration may emerge with additional real-world experience and potential insights from the ongoing long-term safety study (ClinicalTrials.gov Citation2020a). In summary, treatment continuation is a multifactorial and collaborative decision that should be made by the clinician and patient.
Conclusions
Based on the authors’ clinical experience and data available in the literature, this manuscript provides guidance on the practical use of esketamine nasal spray, including treatment initiation and considerations pre-, during and post-administration (summarised in ).
Table 4. Summary of consensus recommendations for esketamine nasal spray.
Esketamine nasal spray is a treatment for TRD, with a novel mechanism of action relative to existing therapies for TRD, which offers an additional option for patients who have already failed several lines of treatment. Current evidence highlights its rapid onset of antidepressant effects (Popova et al. Citation2019), beneficial response rates (Kaur et al. Citation2019), and its generally transient adverse events (Popova et al. Citation2019). Further studies of esketamine nasal spray in patients with TRD are ongoing: a long-term safety study (ClinicalTrials.gov Citation2020a) and a study investigating esketamine nasal spray versus quetiapine extended-release formulation (ClinicalTrials.gov Citation2020b). Esketamine nasal spray is also currently being investigated in patients with MDD who have active suicidal ideation with intent. In this population, esketamine nasal spray showed rapid improvement in depressive symptoms 24 h post-first dose; however, it did not demonstrate a significant improvement in the severity of suicidality compared with placebo (Fu et al. Citation2020; Ionescu et al. Citation2020). Based on these data, Janssen has applied to the EMA for a label extension (JNJ Citation2020).
Here, we provide guidance based on the authors’ clinical experience and the available evidence. Future, real-world use of esketamine nasal spray will add to our collective knowledge about this treatment for patients with TRD.
Statement of interest
S. Kasper – Grants/research support from Janssen, Lundbeck A/S and Schwabe; consultant on advisory boards for Angelini, AOP Orphan Pharmaceuticals AG, Celgene, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sage, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd and Takeda; Speakers’ bureau for Angelini, AOP Orphan Pharmaceuticals AG, Celgene, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sage, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd., Sun Pharmaceutical Industries Ltd and Takeda. W.J. Cubała – Grants, personal fees and non-financial support from Janssen, during the conduct of the study; grants from Acadia, Alkermes, Allergan, Auspex Pharmaceuticals, Biogen, Cortexyme, Ferrier, Forest Laboratories, Gedeon Richter, GW Pharmaceuticals, KCR, Lilly, Lundbeck, Minerva, NIH, NeuroCog, Otsuka, Sanofi and Servier; personal fees from Quintiles; non-financial support from Roche, grants and personal fees from Celon; grants and non-financial support from GW Pharmaceuticals, grants and personal fees from Sanofi, personal fees from Quintiles, non-financial support from Roche, grants and personal fees from Celon. A. Fagiolini – Grants from Angelini, Italfarmaco, Janssen, Lundbeck, Mylan, Otsuka, Pfizer and Angelini; personal fees from Allergan, Apsen, Boehringer Ingelheim, Daiichi Sankyo Brasil Farmacêutica, Doc Generici, FB Health, Italfarmaco, Janssen, Lundbeck, Mylan, Otsuka, Pfizer, Recordati, Sanofi Aventis, Sunovion and Vifor. J.A. Ramos-Quiroga – Speakers’ bureau and/or acted as consultant for Eli Lilly, Janssen-Cilag, Novartis, Shire, Takeda, Bial, Shionogi, Lundbeck, Almirall, Braingaze, Sincrolab, Medice and Rubió in the past 5 years. Travel awards: Janssen-Cilag, Rubió, Shire, Takeda, Shionogi, Bial, Medice and Eli Lilly. Unrestricted educational and research support for the Department of Psychiatry, Hospital Universitari Vall d’Hebron, Barcelona, Catalonia, Spain from Eli Lilly, Lundbeck, Janssen-Cilag, Shire, Ferrer, Oryzon, Roche, Psious and Rubió. D. Souery – Personal fees from Janssen; participated in an advisory board organised by Janssen. A.H. Young – Grants and personal fees from Janssen, LivaNova; grants from COMPASS; personal fees from Sumitomo Dainippon Pharma Co. Ltd, Sunovion, Lundbeck, Allergan, Bionomics; Honorary Consultant for SLaM (NHS UK). Paid lectures and advisory boards for the following companies with drugs used in affective and related disorders: AstraZeneca, Eli Lilly, Lundbeck, Sunovion, Servier, LivaNova, Janssen, Allergan, Bionomics and Sumitomo Dainippon Pharma Co. Ltd. Consultant to Johnson & Johnson. Consultant to LivaNova. Received honoraria for attending advisory boards and presenting talks at meetings organised by LivaNova. Participated in clinical trials sponsored by COMPASS Pathways, Janssen and LivaNova. Grant funding (past and present): NIMH (USA); CIHR (Canada); NARSAD (USA); Stanley Medical Research Institute (USA); MRC (UK); Wellcome Trust (UK); Royal College of Physicians (UK); BMA (UK); UBC-VGH Foundation (Canada); WEDC (Canada); CCS Depression Research Fund (Canada); MSFHR (Canada); NIHR (UK); Janssen (UK). No shareholdings in pharmaceutical companies. Employed by King’s College London.
Author contributions
All authors contributed to the conception and design of the manuscript, drafting and reviewing the manuscript for important intellectual content, and final approval of the manuscript version to be published.
Acknowledgements
The authors thank Janisha Ladva, BSc, of ScientificPathways Ltd, a Nucleus Global company, for providing medical writing support, which was funded by Janssen Pharmaceutica NV in accordance with GPP3 guidelines (http://www.ismpp.org/gpp3). Janssen Pharmaceutica NV was not involved, directly or indirectly, in establishing the content of this manuscript, which was solely driven by the clinical trial findings and the objective experience of the authors. The authors have contributed to this manuscript without receiving any remuneration, reimbursement or any other transfer of value whatsoever.
Additional information
Funding
References
- Al-Harbi KS. 2012. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 6:369–388.
- Ashton AK, Jamerson BD, Weinstein LW, Wagoner C. 2005. Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp. 66:96–106.
- Bartova L, Dold M, Kautzky A, Fabbri C, Spies M, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, et al. 2019. Results of the European group for the study of resistant depression (GSRD) – basis for further research and clinical practice. World J Biol Psychiatry. 20:427–448.
- Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, et al.; International Society for Medical Publication Professionals. 2015. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 163:461–464.,
- Bauer M, Rush AJ, Ricken R, Pilhatsch M, Adli M. 2019. Algorithms for treatment of major depressive disorder: efficacy and cost-effectiveness. Pharmacopsychiatry. 52:117–125.
- Bennabi D, Yrondi A, Charpeaud T, Genty JB, Destouches S, Lancrenon S, Allaili N, Bellivier F, Bougerol T, Camus V, et al. 2019. Clinical guidelines for the management of depression with specific comorbid psychiatric conditions French recommendations from experts (the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental). BMC Psychiatry. 19:50.
- Brown S, Rittenbach K, Cheung S, McKean G, MacMaster FP, Clement F. 2019. Current and common definitions of treatment-resistant depression: findings from a systematic review and qualitative interviews. Can J Psychiatry. 64:380–387.
- Camardese G, Leone B, Serrani R, Walstra C, Di Nicola M, Della Marca G, Bria P, Janiri L. 2015. Augmentation of light therapy in difficult-to-treat depressed patients: an open-label trial in both unipolar and bipolar patients. Neuropsychiatr Dis Treat. 11:2331–2338.
- Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. 2016. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 85:270–288.
- Citrome L, DiBernardo A, Singh J. 2020. Appraising esketamine nasal spray for the management of treatment-resistant depression in adults: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 271:228–238.
- ClinicalTrials.gov. 2020a. Nct02782104: a long-term safety study of esketamine nasal spray in treatment-resistant depression (SUSTAIN-3). [accessed 2020 Apr 07]. https://clinicaltrials.gov/ct2/show/NCT02782104
- ClinicalTrials.gov. 2020b. Nct04338321: a long-term comparison of esketamine nasal spray versus quetiapine extended release, both in combination with a selective serotonin reuptake inhibitor/serotonin-norepinephrine reuptake inhibitor, in participants with treatment resistant major depressive disorder (ESCAPE-TRD). [accessed 2020 May 19]. https://clinicaltrials.gov/ct2/show/NCT04338321
- Conway CR, George MS, Sackeim HA. 2017. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. 74:9–10.
- Coplan JD, Gopinath S, Abdallah CG, Berry BR. 2014. A neurobiological hypothesis of treatment-resistant depression – mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 8:189.
- Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, Lane R, Lim P, Duca AR, Hough D, et al. 2019. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 76:893–903.
- Doherty T, Wajs E, Melkote R, Miller J, Singh JB, Weber MA. 2020. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the clinical development program. CNS Drugs. 34:299–310.
- Dold M, Bartova L, Kasper S. 2020. Treatment response of add-on esketamine nasal spray in resistant major depression in relation to add-on second-generation antipsychotic treatment. Int J Neuropsychopharmacol. 23:440–445.
- Dold M, Bartova L, Mendlewicz J, Souery D, Serretti A, Porcelli S, Zohar J, Montgomery S, Kasper S. 2018. Clinical correlates of augmentation/combination treatment strategies in major depressive disorder. Acta Psychiatr Scand. 137:401–412.
- Dold M, Kasper S. 2017. Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J Psychiatry Clin Pract. 21:13–23.
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. 2016. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 22:238–249.
- EMA. 2013. Guideline on clinical investigation of medicinal products in the treatment of depression. [accessed 2020 May 19]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-depression_en.pdf
- EMA. 2019. Spravato® (esketamine) Summary of Product Characteristics. [accessed 2020 Jan 22]. https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf
- Fabbri C, Corponi F, Souery D, Kasper S, Montgomery S, Zohar J, Rujescu D, Mendlewicz J, Serretti A. 2019. The genetics of treatment-resistant depression: a critical review and future perspectives. Int J Neuropsychopharmacol. 22:93–104.
- FDA. 2019a. Odac briefing document: esketamine nasal spray. [accessed 2020 Mar 22]. https://www.fda.gov/media/121379/download
- FDA. 2019b. Spravato® (esketamine) summary of product characteristics. [accessed 2020 Mar]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, et al. 2019. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 22:616–630.
- Fekadu A, Donocik JG, Cleare AJ. 2018. Standardisation framework for the Maudsley staging method for treatment resistance in depression. BMC Psychiatry. 18:100.
- Feng YP, Parkin D, Devlin NJ. 2014. Assessing the performance of the EQ-VAS in the NHS PROMs programme. Qual Life Res. 23:977–989.
- Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, Hough D, Manji H, Drevets WC, Canuso CM. 2020. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 81:19m13191.
- Gartlehner G, Wagner G, Matyas N, Titscher V, Greimel J, Lux L, Gaynes BN, Viswanathan M, Patel S, Lohr KN. 2017. Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open. 7:e014912.
- Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, Boland E, Weber RP, Randolph C, Bann C, et al. 2020. Defining treatment-resistant depression. Depress Anxiety. 37:134–145.
- Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. 2008. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 75:57–66.
- Gelhorn HL, Secton CC, Classi PM. 2011. Patient preferences for treatment of major depressive disorder and the impact on health outcomes: a systematic review. Prim Care Companion CNS Disord. 13:PCC.11r01161.
- Hahn A, Lanzenberger R, Kasper S. 2019. Making sense of connectivity. Int J Neuropsychopharmacol. 22:194–207.
- Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, Hough D, Drevets WC, Manji H, Canuso CM. 2020. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. pyaa068.
- Jaffe DH, Rive B, Denee TR. 2019. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry. 19:247.
- JNJ. 2020. Janssen seeks expanded use of SPRAVATO® ▼ (esketamine) nasal spray in Europe as a treatment for depressive symptoms in adults with major depressive disorder who have current suicidal ideation with intent. 2020 Jan 15. [accessed 2020 Jul]. https://www.jnj.com/janssen-seeks-expanded-use-of-spravato-esketamine-nasal-spray-in-europe-as-a-treatment-for-depressive-symptoms-in-adults-with-major-depressive-disorder-who-have-current-suicidal-ideation-with-intent
- Jones GG. 2019. Humanitcare: the first real-time symptoms tracking platform for mental illness patients. [accessed 2020 Apr 28]. https://www.compasslist.com/insights/humanitcare-the-first-real-time-symptoms-tracking-platform-for-mental-illness-patients
- Juruena MF, Pariante CM, Papadopoulos AS, Poon L, Lightman S, Cleare AJ. 2009. Prednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistance. Br J Psychiatry. 194:342–349.
- Kadouri A, Corruble E, Falissard B. 2007. The improved Clinical Global Impression Scale (iCGI): development and validation in depression. BMC Psychiatry. 7:7.
- Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA. Jr. 2019. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol. 22:119–135.
- Kasper S, Frazer A. 2019. Editorial for treatment-resistant depression (TRD). Int J Neuropsychopharmacol. 22:83–84.
- Kaur U, Pathak BK, Singh A, Chakrabarti SS. 2019. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci.
- Kautzky A, Baldinger-Melich P, Kranz GS, Vanicek T, Souery D, Montgomery S, Mendlewicz J, Zohar J, Serretti A, Lanzenberger R, et al. 2017. A new prediction model for evaluating treatment-resistant depression. J Clin Psychiatry. 78:215–222.
- Klok MPC, van Eijndhoven PF, Argyelan M, Schene AH, Tendolkar I. 2019. Structural brain characteristics in treatment-resistant depression: review of magnetic resonance imaging studies. BJPsych Open. 5:e76.
- Kornstein SG, Schneider RK. 2001. Clinical features of treatment-resistant depression. J Clin Psychiatry. 62:18–25.
- Kraus C, Kadriu B, Lanzenberger R, Zarate CA, Jr., Kasper S. 2019. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry. 9:127.
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, et al. 2017. Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract. 21:2–12.
- Kudlow PA, McIntyre RS, Lam RW. 2014. Early switching strategies in antidepressant non-responders: current evidence and future research directions. CNS Drugs. 28:601–609.
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 274:1527–1531.
- Li JM, Zhang Y, Su WJ, Liu LL, Gong H, Peng W, Jiang CL. 2018. Cognitive behavioral therapy for treatment-resistant depression: a systematic review and meta-analysis. Psychiatry Res. 268:243–250.
- Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. 2020. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res. 126:134–140.
- Mathews DC, Henter ID, Zarate CA. 2012. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 72:1313–1333.
- McAllister-Williams RH, Arango C, Blier P, Demyttenaere K, Falkai P, Gorwood P, Hopwood M, Javed A, Kasper S, Malhi GS, et al. 2020. The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J Affect Disord. 267:264–282.
- Miller DD. 2004. Atypical antipsychotics: Sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 6:3–7.
- Moret C. 2005. Combination/augmentation strategies for improving the treatment of depression. Neuropsychiatr Dis Treat. 1:301–309.
- Muller J, Pentyala S, Dilger J, Pentyala S. 2016. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 6:185–192.
- Murphy JA, Sarris J, Byrne GJ. 2017. A review of the conceptualisation and risk factors associated with treatment-resistant depression. Depress Res Treat. 2017:4176825.
- Nijenhuis ERS. 2001. Somatoform dissociation. J Trauma Dissociation. 1:7–32.
- Nijs M, Wajs E, Aluisio L, Turkoz I, Daly E, Janik A, Borentain S, Singh JB, DiBernardo A, Wiegand F. 2020. Managing esketamine treatment frequency toward successful outcomes: analysis of phase 3 data. Int J Neuropsychopharmacol. 23:426–433.
- Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, Steffens DC, et al. 2020. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression – TRANSFORM-3. Am J Geriatr Psychiatry. 28:121–141.
- Oluboka OJ, Katzman MA, Habert J, McIntosh D, MacQueen GM, Milev RV, McIntyre RS, Blier P. 2018. Functional recovery in major depressive disorder: providing early optimal treatment for the individual patient. Int J Neuropsychopharmacol. 21:128–144.
- Peng HJ, Zheng HR, Ning YP, Zhang Y, Shan BC, Zhang L, Yang HC, Liu J, Li ZX, Zhou JS, et al. 2013. Abnormalities of cortical-limbic-cerebellar white matter networks may contribute to treatment-resistant depression: a diffusion tensor imaging study. BMC Psychiatry. 13:72.
- Philip NS, Carpenter LL, Tyrka AR, Price LH. 2010. Pharmacologic approaches to treatment resistant depression: a re-examination for the modern era. Expert Opin Pharmacother. 11:709–722.
- Popova V, Daly EJ, Trivedi MH, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, et al. 2019. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 176:428–438.
- Qids D. 2009. Tackling partial response to depression treatment. Prim Care Companion J Clin Psychiatry. 11:155–162.
- Ruhe HG, van Rooijen G, Spijker J, Peeters FP, Schene AH. 2012. Staging methods for treatment resistant depression. a systematic review. J Affect Disord. 137:35–45.
- Rush AJ, Carmody TJ, Reimitz P-E. 2000. The inventory of depressive symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms [Review Article. Int J Methods Psychiatr Res. 9:45–59.
- Sackeim HA, Aaronson ST, Bunker MT, Conway CR, Demitrack MA, George MS, Prudic J, Thase ME, Rush AJ. 2019. The assessment of resistance to antidepressant treatment: rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). J Psychiatr Res. 113:125–136.
- Schosser A, Carlberg L, Calati R, Serretti A, Massat I, Spindelegger C, Linotte S, Mendlewicz J, Souery D, Zohar J, et al. 2017. The impact of BDNF polymorphisms on suicidality in treatment-resistant major depressive disorder: a European multicenter study. Int J Neuropsychopharmacol. 20:782–787.
- Schosser A, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, Kasper S. 2012. European Group for the Study of Resistant Depression (GSRD)-where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol. 22:453–468.
- Snyderman D, Rovner B. 2009. Mental status exam in primary care: a review. Am Fam Physician. 80:809–814.
- Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, Racagni G, Zohar J, Mendlewicz J. 1999. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 9:83–91.
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Serretti A, et al.; Group for the Study of Resistant Depression (GSRD). 2007. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry. 68:1062–1070.
- Strawbridge R, Hodsoll J, Powell TR, Hotopf M, Hatch SL, Breen G, Cleare AJ. 2019. Inflammatory profiles of severe treatment-resistant depression. J Affect Disord. 246:42–51.
- Thase ME, Rush AJ. 1997. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 58:23–29.
- Trivedi MH, Daly EJ. 2008. Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues Clin Neurosci. 10:377–384.
- Tunnard C, Rane LJ, Wooderson SC, Markopoulou K, Poon L, Fekadu A, Juruena M, Cleare AJ. 2014. The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. J Affect Disord. 152-154:122–130.
- Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, George JE, Morrison RL, Sanacora G, Young AH, et al. 2020. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 81:19m12891.
- Wang SM, Han C, Lee SJ, Jun TY, Patkar AA, Masand PS, Pae CU. 2016. Second generation antipsychotics in the treatment of major depressive disorder: an update. Chonnam Med J. 52:159–172.
- Wikberg C, Nejati S, Larsson ME, Petersson EL, Westman J, Ariai N, Kivi M, Eriksson M, Eggertsen R, Hange D, et al. 2015. Comparison between the Montgomery-Asberg Depression Rating Scale-Self and the Beck Depression Inventory II in primary care. Prim Care Companion CNS Disord. 17;10.4088/PCC.14m01758.
- Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, Cao J, Chen GM, Chen NX, Chen W, Cheng C, et al. 2019. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci USA. 116:9078–9083.
