Abstract
Objectives
To investigate the relationship between patient age and the selection and dosage of antipsychotic drugs (APDs) for treatment of schizophrenia. We describe age effects for multiple individual APDs, thus allowing comparisons between drugs.
Methods
Prescription data of 32,062 inpatients with schizophrenia from 2000 to 2017 were obtained from the Drug Safety Program in Psychiatry (AMSP) database. APD selection and dosage were related to patient age with sex as an influencing variable. Moreover, a systematic search of current guideline recommendations on APD treatment in patients with schizophrenia aged ≥65 years was performed.
Results
Eighty percentof elderly patients (≥65 years) received a second-generation APD, most commonly risperidone. The dosage of APDs increased with age until about age 40 years, then decreased slowly at first and more steeply beyond age 55 years. The influence of age as well as sex on dosage partly differed between the individual drugs. Only one of eight schizophrenia guidelines systematically addressed specific aspects of pharmacotherapy in older adults.
Conclusions
In clinical routine, age has a significant impact on selection and dosing of APDs. Information on optimising pharmacotherapy in older adults with schizophrenia from clinical trials is needed. Guidelines should be improved regarding APD therapy specifically for older adults.
Introduction
The use of antipsychotic drugs (APDs) in the treatment of schizophrenia is essential in all age groups and efficacy in older age is often comparable to younger age (Tampi et al. Citation2019). However, dosing and tolerability of psychotropic drugs (PDs) have primarily been studied in younger patients. As a result, only limited data on age-specific dosing leaves clinicians with insufficient guidance on dose adjustment as patients age (Suzuki et al. Citation2011; Tampi et al. Citation2019). This issue is of critical importance, especially in light of concerns about increased morbidity and mortality associated with APDs (U.S. Food and Drug Administration – Center for Drug Evaluation and Research Citation2005; Wang et al. Citation2005; Sacchetti et al. Citation2010; Schneider et al. Citation2020). Pharmacokinetics and pharmacodynamics of APDs are less predictable because older people 1) are a heterogeneous group of individuals ranging from healthy to multimorbid, 2) may be more sensitive to pharmacodynamic effects, 3) often suffer from more than one morbidity resulting in polypharmacy and an increased risk of drug-drug interactions, 4) are under-represented in clinical trials, and 5) older people’s bodies absorb and eliminate drugs differently from younger people’s (Uchida et al. Citation2009). Pharmacokinetic changes in older adults include a reduced renal and hepatic clearance and a higher volume of distribution of lipophilic APDs (hence prolongation of their elimination half-life) whereas pharmacodynamic changes involve increased sensitivity to APDs (Uchida et al. Citation2009). The minimum required and effective dose of APDs is expected to change with increasing age due to altered kinetics, receptor reserve, and possibly the natural course of schizophrenia (Uchida et al. Citation2009; Citation2009; Uchida and Mamo Citation2009).
Older adults are particularly vulnerable to adverse effects caused by APDs, including delirium, extrapyramidal symptoms, arrhythmias, and postural hypotension. Multimorbidity, polypharmacy, and decreased physiological reserve contribute to greater susceptibility to adverse drug reactions (ADRs). For example, pre-existing cardiovascular disease may aggravate cardiac risks of APDs due to QT prolongation or anticholinergic effects. Drug-drug interactions may impair metabolism of APDs resulting in enhanced toxicity. In vivo brain imaging studies have consistently demonstrated an age-related decrease in striatal D2-receptor binding sites by 5–10% per decade (Wang et al. Citation1998; Ishibashi et al. Citation2009), potentially resulting in a higher risk for both clinical and adverse effects at a given level of APD occupancy.
Drug regulatory agencies have launched initiatives to increase the quantity and quality of information on drug use in older adults (Expert Working Group of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Citation1994; European Medicines Agency Citation2011; U.S. Department of Health and Human Services – Food and Drug Administration Citation2020). These include ensuring that dossiers contain sufficient data to evaluate the safety and efficacy of drugs in older adults. Further, product information should accurately reflect any findings or missing data in the geriatric population to support informed prescribing. Because most APDs were approved before the adoption of this strategy, information in the summary of product characteristics (SmPCs) about the use of APDs in older adults and especially about dose adjustments is sparse.
The aim of this study was to investigate age effects on prescription of APDs in a large population of inpatients with schizophrenia. In addition to previous studies primarily assessing dosing practice (Uchida et al. Citation2008; Greil et al. Citation2013), we also considered whether certain APDs are preferred and others avoided as patients age. Furthermore, recommendations for geriatric APD dose adjustments were identified in guidelines and SmPCs and compared to real-life dosing practices.
Methods
Data source
The ongoing AMSP ("Arzneimittelsicherheit in der Psychiatrie"/Drug Safety Program in Psychiatry) drug surveillance program assesses drug prescriptions and severe ADRs in psychiatric inpatients from German-speaking countries in routine clinical treatment. AMSP’s methodology has been described in detail elsewhere (Grohmann et al. Citation2004). In brief, pharmaco-epidemiological data are collected on two index dates per year, including primary psychiatric diagnoses, prescribed drugs, dosage, age, and sex of patients under surveillance. Data on secondary and tertiary psychiatric diagnoses have been documented since 2007. The Ethics Committees of the University of Munich and the Hannover Medical School have approved evaluations based on the AMSP database (Nr. 8100_BO_S_2018). This study adheres to the Declaration of Helsinki and its later amendments. The AMSP program does not interfere with the ongoing clinical treatment of the patients under surveillance.
Study population and design
Data from 60 university, community, and state psychiatric hospitals and departments from Germany (72.9%), Switzerland (18.0%), and Austria (8.6%) participating in AMSP from 2000 to 2017 was analysed. All patients with current primary diagnosis of schizophrenia, i.e. with ICD-10 (International Classification of Diseases, Tenth Revision) codes F20, were included. Age, sex, psychiatric and somatic comorbidity (corresponding ICD-10 codes), and prescriptions (drug name, daily dose, or for long-acting injectable antipsychotic drugs [LAIs], dose, and dosing interval) were collected from the AMSP database.
Classification of PDs
PDs were classified as follows: APDs, antidepressant drugs, tranquilising and hypnotic drugs (benzodiazepines, benzodiazepine-like drugs and others), lithium salts, anticonvulsant drugs, and antiparkinsonian drugs. APDs were further divided into first-generation antipsychotic drugs (FGAs) and second-generation antipsychotic drugs (SGAs). Both groups included oral and LAI formulations. FGAs were subclassified as high-potency and low-potency FGAs.
Data analysis
Dose equivalents were used to combine dose data from oral and LAI formulations of a drug or to estimate the patient’s total APD dose. Dose equivalents based on defined daily dose (DDD) published by the Collaborative Centre for Drug Statistics Methodology of the World Health Organisation were calculated because 1) unlike chlorpromazine equivalents, DDDs are available for most drugs, including all APDs, 2) DDDs are an internationally recognised measure, 3) with particular relevance to our project, DDDs of APDs are based on the treatment of psychosis rather than other indications for which lower doses are often used, and 4) DDDs are also reported for LAIs (Danivas and Venkatasubramanian Citation2013; Leucht et al. Citation2016). In patients treated with ≥1 APD, total APD dose exposure was calculated by adding the DDD of each APD.
We compared dosages among seven age groups (in years; ≤24, 25–34, 35–44, 45–54, 55–64, 65–74, and ≥75). Only APDs prescribed to ≥25 patients per age group were included in the analysis. In the following, age groups ≥ 65 years are referred to as older adults.
Because dose data did not fit a normal distribution, we applied log-transformation to remove or reduce skewness. Means of log-transformed data were compared by analysis of variance (ANOVA) with the factor age group, followed by Bonferroni-adjusted significance tests for pairwise comparisons between age groups. The same method was used for APDs with ≥10 male and female users per age group using the factors sex and age group. A previous AMSP publication revealed that the average dosage of the most commonly prescribed APDs decreased significantly from 2000 to 2015 (Toto et al. Citation2019). We therefore included the survey year as a covariate in ANOVA. Means and 95% confidence intervals (CI) were back-transformed for the purpose of graphical representation of data. Pearson Chi-square tests were conducted to compare prescription rates between age groups.
Jonckheere-Terpstra tests were used to determine if there was a statistically significant trend of lower daily doses of APDs over the period 2000–2017. The significance level was set at p < 0.05. Statistical analyses were performed using R version 4.0.2. and IBM SPSS Statistics version 27.
SmPC and guideline search
SmPCs were obtained from https://www.pharmnet-bund.de and https://www.swissmedicinfo.ch/. SmPCs of APDs commonly prescribed in our population were included. We performed a database search for schizophrenia treatment guidelines via PubMed, guidelinecentral.com, and https://www.awmf.org. Only currently valid guidelines were included. Full text documents were screened for recommendations on pharmacological treatment of older adults with schizophrenia regarding drug selection and dosage.
Results
Study population
During the study period 2000–2017, 32,062 patients with a primary diagnosis of schizophrenia were treated in the participating hospitals. The mean age of patients was 41.6 ± 14.4 years (range 9–102 years). Patients between 25 and 34 years of age formed the largest group (25.1%), and only 5.2% and 2.2% of patients were in the age groups of 65–74 years and ≥75 years, respectively. Overall, more men than women were hospitalised with a diagnosis of schizophrenia (57.4% vs. 42.6%). However, the proportion of women increased with age and predominated in the older age groups ().
Table 1. Demographic and clinical characteristics of the study population.
Diagnosed in 83.9% of patients, paranoid schizophrenia (F20.0) was the most common disorder. The frequency of other subtypes ranged from 0.3% to 4.0% (); 27.7% of patients had ≥1 psychiatric secondary diagnosis of which mental and behavioural disorders due to substance use were most common (17.1% of patients).
Selection of APDs
Most patients used ≥1 APD (96.0%), predominantly SGAs (81.0%). Besides APDs, benzodiazepine (35.0%), antidepressant (16.5%), antiparkinsonian (15.6%, mostly biperiden), and anticonvulsant drugs (14.0%, mostly valproate) were commonly used (Table S1).
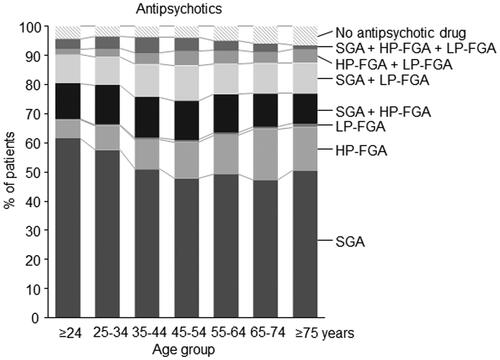
shows prescription rates of APD classes by age group. Treatment with SGAs without additional administration of FGAs was most common overall (52.7%) and slightly more common in younger age groups. In contrast, prescriptions of high-potency FGAs as single-class treatment (overall 10.5% of patients) increased with age from 5.3% in patients ≤24 years to 17.4% and 14.9% in patients 65-74 and ≥75 years, respectively (p < 0.001). The use of low-potency FGA as single-class treatment was rare (0.60%). Co-treatment of APDs from multiple classes was most common in the middle-aged groups. Overall, 36.0% of patients were concomitantly treated with APDs from multiple classes.
Figure 1. Prescribing frequencies of the three main classes of antipsychotic drugs by age group. Shown is the percentage of all patients with a primary diagnosis of schizophrenia treated with at least one antipsychotic drug from either a single class of antipsychotic drugs or as simultaneous prescription from two or from three different classes of antipsychotic drugs. HP-FGA: high potency first generation antipsychotic drugs; LP-FGA: low potency first generation antipsychotic drugs; SGA: second generation antipsychotic drugs.
shows prescription rates of APDs by age. Overall, clozapine was the most used SGA (26.8%). Clozapine prescriptions initially increased with age but decreased in elderly patients (p < 0.001). Olanzapine and quetiapine prescription rates decreased slightly in elderly patients, while the use of risperidone increased slightly (p < 0.05 each). Aripiprazole and amisulpride were rarely used in older patients.
Figure 2. Prescription frequencies of antipsychotic drugs by drug class and age group. FGA: first generation antipsychotic drugs; SGA: second generation antipsychotic drugs. Note that the overall prescription rate within an age group may exceed 100% due to the simultaneous prescription of different compounds.
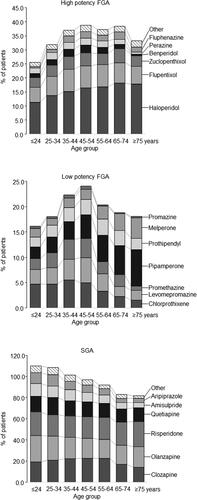
Haloperidol was the most used FGA overall (15.0%) with prescription increasing with age from 11.5% to 18.1% and 17.8% in the two older age groups, respectively (p < 0.001). Prescriptions of benperidol and perazine significantly decreased. Among low-potency FGAs, pipamperone prescriptions markedly increased with age from 2.2% to 5.7% and 7.2% in the two older age groups, respectively (p < 0.001). The use of melperone also increased with age (p < 0.001), whereas chlorprothixene and levomepromazine prescriptions significantly decreased.
Dosage of APDs
Median daily doses of amisulpride, aripiprazole, clozapine, haloperidol, melperone, pipamperone, quetiapine, risperidone, and zuclopenthixol (p < 0.001 each) – but not flupentixol (p = 0.364) and olanzapine (p = 0.431) – showed a significant decrease from 2000 to 2017 (Supplemental Figure S1).
and show dosage according to age group, expressed as mean DDD with 95% CI, for SGAs and FGAs, respectively. There was a statistically significant difference between groups for all APDs except amisulpride. Post-hoc comparisons of age groups showed a decrease in mean dose in the older compared to the younger age groups. The dose of drugs such as amisulpride, aripiprazole, flupentixol, and pipamperone was not or only slightly (<25%) reduced in patients ≥65 years compared to patients <65 years. Dosage of clozapine and haloperidol was reduced by more than 50% in patients ≥75 years ().
Figure 3. Dosage by age group of second-generation antipsychotic drugs (amisulpride, aripiprazole, clozapine, olanzapine, risperidone, and quetiapine) commonly prescribed in the study population. There was a statistically significant difference between groups as determined by ANOVA for all antipsychotic drugs except amisulpride. * Significant difference (p < 0.05) against each of the age groups ≤64 years and # significant difference (p < 0.05) against the age groups 25-44 years as revealed by Bonferroni-adjusted posthoc tests for pairwise comparisons. DDD: defined daily dose; LAI: long-acting injectable.
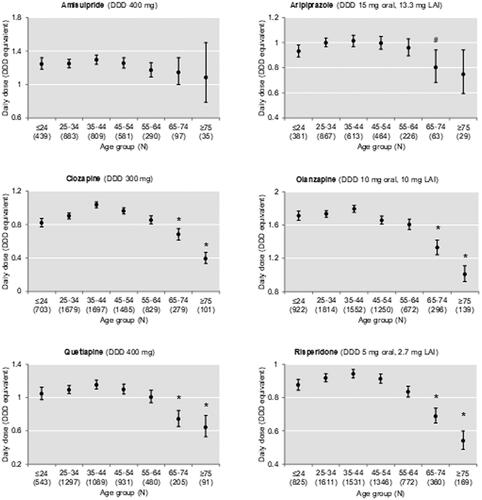
Figure 4. Dosage according to age group of first-generation antipsychotic drugs (flupentixol, haloperidol, melperone, pipamperone, and zuclopenthixol) commonly used in the study population. Data are shown as mean prescribed dose with 95% CI. There was a statistically significant difference between age groups as determined by ANOVA for all antipsychotic drugs. *Significant difference (p < 0.05) against each of the age groups ≤64 years; # significant difference (p < 0.05) against the age group 35–44 years; § significant difference (p < 0.05) against age groups 25–34 years, 35–44 years, and 45–54 years; and significant difference (p < 0.05) against age groups 25–34 years, 35–44 years, 45–54 years, and 55–64 years, as revealed by Bonferroni-adjusted post-hoc tests for pairwise comparisons. DDD: defined daily dose; LAI: long-acting injectable drugs.
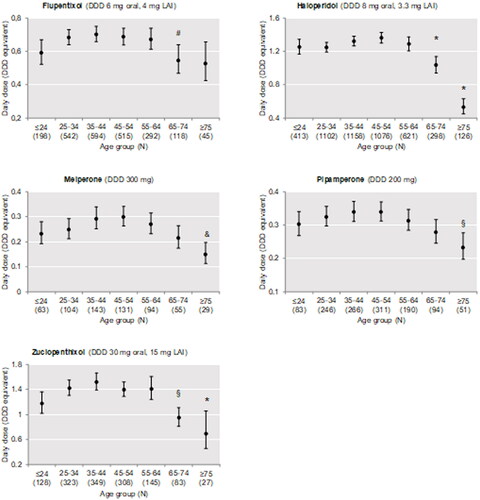
Table 2. Dosage of antipsychotic drugs in elderly patients compared with patients younger than 65 years.
Additionally, sex affected dosage of haloperidol (p < 0.001), clozapine (p < 0.001), olanzapine (p = 0.004), quetiapine (p = 0.042), and risperidone (p = 0.004) (Supplemental Figure S2).
Most patients were treated with ≥1 APD. DDDs were used to compare APD dose exposure among age groups (Supplemental Figure S3). Dose exposure increased with age until around age 40 (peak DDD equivalent 1.90, 95% CI 1.87-1.97), then decreased slowly at first and more steeply beyond 55 years of age (minimum DDD equivalent 0.81, 95% CI 0.75-0.87 in patients aged ≥75 years).
Information in SmPCs and guidelines
summarises the statements identified in the SmPCs regarding the use and dosage of APDs in older adults. Specific dosage recommendations in milligrams for older adults with schizophrenia were given for clozapine, olanzapine, risperidone, haloperidol, pipamperone, and zuclopenthixol. Recommendations for the remaining APDs were less specific (i.e. to use lower starting doses and to titrate up more cautiously). The mean doses of risperidone and zuclopenthixol prescribed in the AMSP population exceeded the maximum doses for older adults as specified in the SmPCs, whereas the doses of haloperidol and pipamperone were below the respective recommendations (Table S2).
Table 3. Oral dosages of antipsychotic drugs recommended for older adults in German and Swiss drug labels.
We identified eight treatment guidelines of schizophrenia (Scottish Intercollegiate Guidelines Network Citation2013; National Institute for Health and Care Excellence (NICE)) Citation2014; Galletly et al. Citation2016; Grover et al. Citation2017; Remington et al. Citation2017; Deutsche Gesellschaft für Psychiatrie und Psychotherapie Citation2019; Barnes et al. Citation2020; Guideline Writing Group Citation2021). summarises statements identified in the guidelines specifically addressing the management of older patients, particularly treatment with APDs in patients aged ≥65 years.
Table 4. Synopsis of current schizophrenia guidelines and their statements on pharmacotherapy in older adults with schizophrenia.
Discussion
The objective of the study was to investigate the relationship between patient age and the selection and dosage of APDs used in the treatment of schizophrenia by examining age-dependent prescriptions of APDs in 32,062 inpatients with schizophrenia. This study complements a previous report describing changes in APD prescription practice in the AMSP study population from 2000 to 2015 (Toto et al. Citation2019).
We observed changes in the spectrum of APDs used depending on the age of the patients. For example, clozapine, olanzapine and quetiapine prescription rates decreased in older adults, while use of risperidone increased. This observation raises the question whether the described age-related preference or disfavouring of certain APDs are evidence-based. There are only a few comparative studies of APDs in the treatment of geriatric schizophrenia (Marriott et al. Citation2006) and evidence-based guideline recommendations for specific drugs are lacking. Experts recommend favouring SGAs over FGAs and classify risperidone as first-line treatment of geriatric schizophrenia (Alexopoulos et al. Citation2004), as was observed in this study. This choice may be further reinforced by the current evidence-based recommendation of risperidone for the treatment of behavioural and psychological symptoms of dementia in geriatric patients (Livingston et al. Citation2017). Generally, the choice of first-line APD for the treatment of schizophrenia should be based on the likely benefits and potential ADRs of the drug in question (National Institute for Health and Care Excellence (NICE)) Citation2014). An advantage of risperidone in geriatric patients is its lower potential for anticholinergic ADRs, especially compared to clozapine, quetiapine, and olanzapine (Guideline Writing Group Citation2021). Overall, we observed a lower utilisation of APDs with strong anticholinergic properties such as clozapine, olanzapine, and quetiapine in older patients.
Dose levels of APDs used in the treatment of schizophrenia in younger adults (<65 years) fit well with dose levels as reported in clinical trials. For example, the age-dependent mean DDDs observed in our study were 0.84–0.94 (corresponding to 4.2–4.7 mg/day) for risperidone and 1.60-1.79 (corresponding to 16.0–17.9 mg/day) for olanzapine. The reported mean modal doses for risperidone and olanzapine were 4.8–7.2 mg/day and 12.4–17.2 mg/day, respectively (Tran et al. Citation1997; Conley and Mahmoud Citation2001).
We found an inverted U-shaped relationship of dosage and age. A very similar relationship between age and dose was previously observed in a Japanese cohort of 1418 inpatients and outpatients with schizophrenia (Uchida et al. Citation2008). In one of the first cross-sectional studies to address the issue of APD dose requirements in older adults with schizophrenia, researchers observed an inverse relationship between age and APD dose in a group of 64 outpatients in San Diego (Jeste et al. Citation1993). This inverse age-dose correlation was also observed in another cross-sectional prescription survey of 86 outpatients with schizophrenia ≥45 years treated with LAIs in Pittsburgh (Mamo et al. Citation2002; Uchida and Mamo Citation2009).
Due to their small sample size, these previous studies had to pool APDs from different classes based on chlorpromazine equivalents for the analyses. Because of the large number of patients in our study, we were able to examine individual APDs separately and highlight drug-specific differences in the relationship between patient age and dosage. While dose of certain drugs, such as amisulpride, aripiprazole, flupentixol, and pipamperone was not or only slightly reduced (<25%) in older patients compared to the usual adult dose, doses of clozapine and haloperidol were reduced by more than 50% in patients ≥75 years. The sex difference in dose requirements for APDs is generally more pronounced in younger patients and decreases with older age.
Evidence-based selection and dosage of APDs in older adults with schizophrenia
To our knowledge, there are no published dose-finding studies, i.e. systematic determination of the optimal therapeutic dose range of APDs in older adults with schizophrenia. The lack of evidence is reflected in the relevant SmPCs, some of which refer to missing study data and generally recommend individualised dose titration. Current schizophrenia guidelines published by leading international providers, such as the National Institute for Health and Care Excellence or the American Psychiatric Association, do not address drug treatment in elderly or geriatric patients at all (National Institute for Health and Care Excellence (NICE)) Citation2014; Guideline Writing Group Citation2021).
Recommendations exist for the use of drugs including APDs in older age, such as The American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication (PIM) Use in Older Adults or the German-Austrian PRISCUS project (Holt et al. Citation2010; By the American Geriatrics Society Beers Criteria Update Expert Citation2019). The Beers criteria generally classify APDs as appropriate for the treatment of schizophrenia in older adults, without recommending specific agents as particularly effective or tolerable in this age group (By the American Geriatrics Society Beers Criteria Update Expert Citation2019). The only exception is the recommendation that patients with Parkinson’s disease as a comorbid condition should be treated preferentially with clozapine, quetiapine, or pimavanserin, which are less likely to aggravate Parkinson’s disease (By the American Geriatrics Society Beers Criteria Update Expert Citation2019). The PRISCUS list recommends the use of SGAs because of their more favourable risk-benefit profile compared with FGAs (Holt et al. Citation2010). Such PIM lists raise awareness of fundamental issues in prescribing APDs for older adults. For example, both olanzapine and clozapine are mentioned as potentially inappropriate medication (PIM) by the PRISCUS list and the Beer’s Criteria due to their anticholinergic properties suggesting that – although the use of both of these APDs may be indicated due to the underlying mental illness – the potential anticholinergic ADRs are taken into consideration when selecting an APD for older adults.
However, PIM lists are poorly suited to assist the clinician in selecting the appropriate drug and dose in specific patient cases because they do not sufficiently differentiate between the two major indications for prescribing APDs, i.e. schizophrenia versus behavioural disorders in dementia (Holt et al. Citation2010). The PRISCUS list provides dosing recommendations for two APDs (i.e. olanzapine, haloperidol) (Holt et al. Citation2010), both of which were used at lower doses in older adults in this study. Further dosing recommendations are mostly lacking (By the American Geriatrics Society Beers Criteria Update Expert Citation2019) (Holt et al. Citation2010).
Potential contributors to the increased sensitivity to APDs in older patients
Substantial dose reductions are consistently observed in elderly patients regardless of geographic region, time period, or patient care setting (i.e. outpatient vs. inpatient) (Jeste et al. Citation1993; Mamo et al. Citation2002; Uchida et al. Citation2008). This suggests that physician awareness and experience as well as current and local standards of care, economic constraints, and patient care setting are less significant factors determining the prescribed dose level as a function of age. This leads to the question of whether age-related changes in pharmacokinetics or pharmacodynamics, as observed for many drugs, also apply to APDs and are responsible for APDs being dosed more cautiously/lower with age.
Concerning pharmacokinetics, most APDs undergo extensive metabolism and are excreted as metabolites in the urine (e.g. clozapine, olanzapine, quetiapine, risperidone, haloperidol, pipamperone, melperone) or in the faeces (e.g. aripiprazole, flupentixol, zuclopenthixol). Amisulpride is weakly metabolised and is predominantly eliminated unaltered in the urine. Several studies have shown a significant reduction in the clearance of many drugs metabolised by phase-1 pathways in the liver of older adults. The main factor is probably represented by the age-related changes in liver size and hepatic blood flow as the activity of drug metabolising enzymes is preserved. Reduction in renal function in older patients may affect the clearance of renally eliminated APDs such as amisulpride. Accordingly, many studies have shown that age has a significant effect on serum concentrations of various APDs such as clozapine, olanzapine, risperidone, and quetiapine (Castberg et al. Citation2017), while a few studies have failed to confirm these results (Bigos et al. Citation2008; Uchida et al. Citation2009). In particular, studies that adopted a population pharmacokinetic modelling approach using data from dose-finding and pivotal trials were often unable to show a significant effect of age (Albitar et al. Citation2020) due to the exclusion of older patients from participation in these trials .
Penetration of the blood-brain barrier (BBB), a complex, heterogeneous, and dynamic tissue, is a prerequisite for CNS action of APDs. Ageing can disrupt BBB integrity, resulting in a decline in overall BBB function and even leakage of normally BBB impermeable molecules (Verheggen et al. Citation2020). Among other factors, BBB dysfunction may contribute to increased sensitivity to the effects of APDs in the elderly, although direct evidence is lacking (Zeevi et al. Citation2010; Erickson and Banks Citation2019).
Older patients are particularly vulnerable to ADRs caused by APDs, perhaps in part due to an age-related decrease in striatal D2 receptor binding sites (Wang et al. Citation1998; Ishibashi et al. Citation2009). However, this explanatory model is one-dimensional because APDs not only affect the D2 receptor-dependent pathways in different brain regions, but also have high affinities for other receptor systems, depending on the drug. An increased risk of ADRs may lead physicians to prescribe APDs to older patients at a lower therapeutic dose than to younger patients and may partially explain our study’s observation.
Age-related changes in APD dosing, as observed in our study, might also be influenced by illness severity and symptomatology. Jeste et al. observed a paradox clinical profile in the life course of schizophrenia: i.e. ageing in schizophrenia is associated with poorer physical health but improved psychosocial functioning, including lower burden of psychotic symptoms, reduction of psychotic relapse requiring hospitalisation, and better self-management (Jeste et al. Citation2011). The different state of health in older in comparison to younger individuals (Cohen et al. Citation2015) likely influences APD prescription. Older patients with schizophrenia include both individuals with a chronic course after an early onset and those with a late and very late onset of this disorder and, in this respect, are a heterogeneous group (Howard et al. Citation2000). Women are usually older at onset and suffer from more severe paranoid symptoms than men with onset of schizophrenia in later life (Abel et al. Citation2010). The male:female ratio of our inpatient population was approximately 3:1 for very young patients and steadily decreased to a ratio of 1:3 for elderly patients. Women often receive lower doses of APDs than men, as shown in our study, and the reasons for this are probably multiple. Differences in body size and fat percentage as well as better response to ADPs have been discussed. Oestrogen effects on the expression of psychopathological symptoms have been demonstrated, leading to the recommendation that modification or dose adjustment of APDs should be considered after menopause (Deutsche Gesellschaft für Psychiatrie und Psychotherapie Citation2019).
Strengths and limitations of the study
In comparison to previous studies, our study provides more detailed information on age-related APD use among patients with schizophrenia. The multicentre design and large representative sample allow us to examine age-related changes in the selection and dosage of individual APDs. This study’s naturalistic design reflects real-life clinical practice. Further, due to the inpatient setting, AMSP assesses actual drug utilisation rates versus prescription rates, as is often the case in studies with an outpatient setting.
However, some limitations must also be considered. Detailed information about the disorder, beyond ICD-10 coding, such as age at onset, course of illness, outcome, or occurrence of ADRs is not available. Moreover, only inpatients were included. Due to the acute nature of the disease in hospitalised patients, medication is not necessarily comparable to the outpatient setting regarding choice of substances, number of concomitantly used APDs, and dosage. We do not know if the patient was already on stable antipsychotic therapy at the time of the cut-off date survey, in the dose titration phase, or if the drug therapy had just been switched. Temporal changes in prescription practices were observed and corrected using a statistical approach.
Conclusion
Age significantly impacts APD selection and dosing in older adults. The extent of dose reduction in elderly patients differs between individual drugs. We observed an additional dose reduction of some APDs in patients ≥75 years than in patients aged 65–74 years. More clinical trials on the use of APDs in older patients with schizophrenia are needed, and guidelines should better address treatment of patients ≥65 years. We recognise that the number of clinical trials on pharmacotherapy of schizophrenia in older adults is limited, but this does not justify the complete omission of this information from many guidelines.
Supplemental Table S2
Download MS Word (12.6 KB)Supplemental Table S1
Download MS Word (13.4 KB)Supplemental Figure S3
Download MS Word (105.3 KB)Supplemental Figure S2
Download MS Word (118.5 KB)Supplemental Figure S1
Download MS Word (4.5 MB)Acknowledgments
We gratefully acknowledge the support of the participating hospitals.
Disclosure statement
SB, RG, and ST are project managers of the AMSP program. ST is a member of the advisory board for Otsuka and Janssen-Cilag and has received speaker’s honoraria from Janssen-Cilag, Lundbeck/Otsuka, Recordati Pharma GmbH, and Servier. JS took part in an educational event sponsored by Otsuka/Lundbeck. All other authors state they have no conflicts of interest to declare.
The research presented in this manuscript did not receive any specific grants or funding. The AMSP drug safety project is facilitated by non-profit associations in Germany, Austria, and Switzerland. The AMSP project has been supported with unrestricted educational and research grants since 1993 by the following companies:
German companies: Abbott GmbH & Co. KG, AstraZeneca GmbH, Aventis Pharma Deutschland GmbH GE–O/R/N, Bayer Vital GmbH, Boehringer Mannheim GmbH, Bristol-Myers-Squibb, Ciba Geigy GmbH, Desitin Arzneimittel GmbH, Duphar Pharma GmbH & Co. KG, Eisai GmbH, Esparma GmbH Arzneimittel, GlaxoSmithKline Pharma GmbH & Co. KG, Hoffmann-La Roche AG Medical Affairs, Janssen-Cilag GmbH, Janssen Research Foundation, Knoll Deutschland GmbH, Lilly Deutschland GmbH Niederlassung Bad Homburg, Lundbeck GmbH & Co. KG, Novartis Pharma GmbH, Nordmark Arzneimittel GmbH, Organon GmbH, Otsuka-Pharma Frankfurt, Pfizer GmbH, Pharmacia & Upjohn GmbH, Promonta Lundbeck Arzneimittel, Recordati Pharma GmbH, Rhone-Poulenc Rohrer, Sanofi-Synthelabo GmbH, Sanofi-Aventis Deutschland, Schering AG, SmithKlineBeecham Pharma GmbH, Solvay Arzneimittel GmbH, Synthelabo Arzneimittel GmbH, Dr. Wilmar Schwabe GmbH & Co., Thiemann Arzneimittel GmbH, Troponwerke GmbH & Co. KG, Upjohn GmbH, Wander Pharma GmbH, and Wyeth-Pharma GmbH.
Austrian companies: Astra Zeneca Österreich GmbH, Boehringer Ingelheim Austria, Bristol-Myers Squibb GmbH, CSC Pharmaceuticals GmbH, Eli Lilly GmbH, Germania Pharma GmbH, GlaxoSmithKline Pharma GmbH, Janssen-Cilag Pharma GmbH, Lundbeck GmbH, Novartis Pharma GmbH, Pfizer Med Inform, and Wyeth Lederle Pharma GmbH.
Swiss companies: AHP (Schweiz) AG, AstraZeneca AG, Bristol-Myers Squibb AG, Desitin Pharma GmbH, Eli Lilly (Suisse) S.A., Essex Chemie AG, GlaxoSmithKline AG, Janssen-Cilag AG, Lundbeck (Suisse) AG, Organon AG, Pfizer AG, Pharmacia, Sanofi-Aventis (Suisse) S.A., Sanofi-Synthelabo SA, Servier SA, SmithKlineBeecham AG, Solvay Pharma AG, Wyeth AHP (Suisse) AG, and Wyeth Pharmaceuticals AG.
Additional information
Funding
References
- Abel KM, Drake R, Goldstein JM. 2010. Sex differences in schizophrenia. Int Rev Psychiatry. 22(5):417–428.
- Albitar O, Harun SN, Zainal H, Ibrahim B, Sheikh Ghadzi SM. 2020. Population pharmacokinetics of clozapine: a systematic review. Biomed Res Int. 2020:9872936.
- Alexopoulos GS, Streim J, Carpenter D, Docherty JP. 2004. Using antipsychotic agents in older patients. J Clin Psychiatry. 65(Suppl 2):5–99. discussion 100-102; quiz 103-104.
- Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, Gregory CJ, Haddad PM, Howes OD, Jones I, et al. 2020. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 34(1):3–78.
- Bigos KL, Pollock BG, Coley KC, Miller DD, Marder SR, Aravagiri M, Kirshner MA, Schneider LS, Bies RR. 2008. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 48(2):157–165.
- By the American Geriatrics Society Beers Criteria Update Expert P. 2019. American Geriatrics Society 2019 updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 67(4):674–694.
- Castberg I, Westin AA, Skogvoll E, Spigset O. 2017. Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatr Scand. 136(5):455–464.
- Cohen CI, Meesters PD, Zhao J. 2015. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2(4):340–350.
- Conley RR, Mahmoud R. 2001. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry. 158(5):765–774.
- Danivas V, Venkatasubramanian G. 2013. Current perspectives on chlorpromazine equivalents: Comparing apples and oranges!. Indian J Psychiatry. 55(2):207–208.
- Deutsche Gesellschaft für Psychiatrie und Psychotherapie PuNeV. 2019. S3-Leitlinie Schizophrenie. [accessed 2021 Mar 30]. https://www.awmf.org/uploads/tx_szleitlinien/038-009l_S3_Schizophrenie_2019-03.pdf.
- Erickson MA, Banks WA. 2019. Age-associated changes in the immune system and blood(-)brain barrier functions. Int J Mol Sci. 20(7):1632.
- European Medicines Agency. 2011. EMA geriatric medicines strategy (EMA/CHMP/137793/2011). EMEA; [accessed 2021 Jan 28]. https://www.ema.europa.eu/en/documents/other/geriatric-medicines-strategy_en.pdf.
- Expert Working Group of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 1994. Guideline for industry, studies in support of special populations: geriatrics. [accessed 2021 Oct 19] https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-7-studies-support-special-populations-geriatrics-step-5_en.pdf.
- Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, Kulkarni J, McGorry P, Nielssen O, Tran N. 2016. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust NZ J Psychiatry. 50(5):410–472.
- Greil W, Haberle A, Schuhmann T, Grohmann R, Baumann P. 2013. Age and adverse drug reactions from psychopharmacological treatment: data from the AMSP drug surveillance programme in Switzerland. Swiss Med Wkly. 143:w13772.
- Grohmann R, Engel RR, Ruther E, Hippius H. 2004. The AMSP drug safety program: methods and global results. Pharmacopsychiatry. 37(Suppl 1):S4–S11.
- Grover S, Chakrabarti S, Kulhara P, Avasthi A. 2017. Clinical Practice Guidelines for Management of Schizophrenia. Indian J Psychiatry. 59(Suppl 1):S19–S33.
- Guideline Writing Group. 2021. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. Washington, DC: American Psychiatric Association.
- Holt S, Schmiedl S, Thurmann PA. 2010. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 107(31–32):543–551.
- Howard R, Rabins PV, Seeman MV, Jeste DV. 2000. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 157(2):172–178.
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, Ishiwata K. 2009. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse. 63(4):282–290.
- Jeste DV, Lacro JP, Gilbert PL, Kline J, Kline N. 1993. Treatment of late-life schizophrenia with neuroleptics. Schizophr Bull. 19(4):817–830.
- Jeste DV, Wolkowitz OM, Palmer BW. 2011. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 37(3):451–455.
- Leucht S, Samara M, Heres S, Davis JM. 2016. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 42 Suppl 1(Suppl 1):S90–S94.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, et al. 2017. Dementia prevention, intervention, and care. Lancet. 390(10113):2673–2734.
- Mamo DC, Sweet RA, Chengappa KN, Reddy RR, Jeste DV. 2002. The effect of age on the pharmacological management of ambulatory patients treated with depot neuroleptic medications for schizophrenia and related psychotic disorders. Int J Geriatr Psychiatry. 17(11):1012–1017.
- Marriott RG, Neil W, Waddingham S. 2006. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006:CD005580.
- National Institute for Health and Care Excellence (NICE) 2014. Psychosis and schizophrenia in adults: prevention and management. [accessed 30 Mar 2021]. www.nice.org.uk/guidance/cg178.
- Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. 2017. Guidelines for the Pharmacotherapy of Schizophrenia in Adults. Can J Psychiatry. 62(9):604–616.
- Sacchetti E, Turrina C, Valsecchi P. 2010. Cerebrovascular accidents in elderly people treated with antipsychotic drugs: a systematic review. Drug Saf. 33(4):273–288.
- Schneider M, Pauwels P, Toto S, Bleich S, Grohmann R, Heinze M, Greiner T. 2020. Severe weight gain as an adverse drug reaction of psychotropics: Data from the AMSP project between 2001 and 2016. Eur Neuropsychopharmacol. 36:60–71.
- Scottish Intercollegiate Guidelines Network. 2013. SIGN 131 • Management of schizophrenia [accessed 30 Mar 2021]. https://www.sign.ac.uk/assets/sign131.pdf.
- Suzuki T, Remington G, Uchida H, Rajji TK, Graff-Guerrero A, Mamo DC. 2011. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 28(12):961–980.
- Tampi RR, Young J, Hoq R, Resnick K, Tampi DJ. 2019. Psychotic disorders in late life: a narrative review. Ther Adv Psychopharmacol. 9:2045125319882798.
- Toto S, Grohmann R, Bleich S, Frieling H, Maier HB, Greil W, Cordes J, Schmidt-Kraepelin C, Kasper S, Stubner S, et al. 2019. Psychopharmacological treatment of schizophrenia over time in 30 908 inpatients: data from the AMSP study. Int J Neuropsychopharmacol. 22(9):560–573.
- Tran PV, Hamilton SH, Kuntz AJ, Potvin JH, Andersen SW, Beasley C, Jr., Tollefson GD. 1997. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 17(5):407–418.
- U.S. Department of Health and Human Services - Food and Drug Administration. 2020. Geriatric information in human prescription drug and biological product labeling, guidance for industry. [accessed 15 Apr 2021]. https://www.fda.gov/media/142162/download.
- U.S. Food and Drug Administration – Center for Drug Evaluation and Research. 2005. FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. [accessed 15 Apr 2005]. www.fda.gov/cder/drug/advisory/antipsychotics.htm.
- Uchida H, Mamo DC, Mulsant BH, Pollock BG, Kapur S. 2009. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J Clin Psychiatry. 70(3):397–405.
- Uchida H, Mamo DC. 2009. Dosing of antipsychotics in schizophrenia across the life-spectrum. Prog Neuropsychopharmacol Biol Psychiatry. 33(6):917–920.
- Uchida H, Pollock BG, Bies RR, Mamo DC. 2009. Predicting age-specific dosing of antipsychotics. Clin Pharmacol Ther. 86(4):360–362.
- Uchida H, Suzuki T, Mamo DC, Mulsant BH, Tanabe A, Inagaki A, Watanabe K, Yagi G, Tomita M. 2008. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 16(7):584–593.
- Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild E, Palm WM, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. 2020. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience. 42(4):1183–1193.
- Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA. 2005. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 353(22):2335–2341.
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, et al. 1998. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 30(1):56–61.
- Zeevi N, Pachter J, McCullough LD, Wolfson L, Kuchel GA. 2010. The blood-brain barrier: geriatric relevance of a critical brain-body interface. J Am Geriatr Soc. 58(9):1749–1757.
