Abstract
Objectives
The present study introduces the assessment of depression and depressive symptoms in the German National Cohort (NAKO), a population-based mega cohort. Distribution of core measures, and associations with sociodemographic factors are examined.
Methods
The current analysis includes data from the first 101,667 participants (NAKO data freeze 100,000). Depression and depressive symptoms were assessed using a modified version of the depression section of the Mini-International Neuropsychiatric Interview (MINI), self-reported physician’s diagnosis of depression, and the depression scale of the Patient Health Questionnaire (PHQ-9).
Results
A lifetime physician’s diagnosis of depression was reported by 15.0% of participants. Of those, 47.6% reported having received treatment for depression within the last 12 months. Of the subset of 26,342 participants undergoing the full depression section of the modified MINI, 15.9% were classified by the MINI with a lifetime depressive episode. Based on the PHQ-9, 5.8% of the participants were classified as currently having a major or other depression by the diagnostic algorithm, and 7.8% according to the dimensional assessment (score ≥ 10). Increased frequency of depression measures and higher depression scores were observed in women and participants with lower education level or a family history of depression.
Conclusions
The observed distributions of all depression measures and their associations with sociodemographic variables are consistent with the literature on depression. The NAKO represents a valuable epidemiologic resource to investigate depression, and the range of measures for lifetime and current depression allows users to select the most suitable instrument for their specific research question.
Introduction
Depression is a leading cause of disability worldwide (James et al. Citation2018). It often co-occurs with somatic and other mental disorders, especially anxiety disorders (Steffen et al. Citation2020). The burden of disease attributed to depression has markedly increased in the last 30 years (James et al. Citation2018). In Germany, depression is responsible for the highest number of days of sick leave, and numbers continue to rise (Baumeister et al. Citation2015; Schneider et al. Citation2019). The lifetime and 12-month prevalence of depression in population-based studies have been reported to vary between 12% and 17% (Jacobi et al. Citation2004; Busch et al. Citation2013), and 6% to 10%, respectively (Jacobi et al. Citation2004; Busch et al. Citation2013; Bretschneider et al. Citation2018). Major risk factors for depression include genetic predisposition, socioeconomic factors, stress, and being female (Krishnan and Nestler Citation2010). The aetiology of depression is still poorly understood and, so far, no established objective biological marker is available to assist the diagnosis of depression (Kennis et al. Citation2020). According to the two main diagnostic systems, i.e. the International Classification of Diseases (ICD), and the Diagnostic and Statistical Manual of Mental Disorders (DSM), which is often used for research settings (Tyrer Citation2014), the cardinal symptoms of depression are a persistent feeling of sadness and/or loss of interest in almost all activities (World Health Organization Citation1993; American Psychiatric Association Citation1994). Further symptoms include disturbances of sleep and appetite, as well as psychomotor activation or retardation. In addition, fatigue or a lack of energy, a decrease in activity, feelings of worthlessness or guilt, poor concentration, and suicidality are common symptoms (World Health Organization Citation1993; American Psychiatric Association Citation1994). The profile, intensity, and duration of these symptoms, the impact on occupational and social functioning, the longitudinal course with frequent recurrent episodes, and co-morbidities can vary widely across individuals (Fried and Nesse Citation2015). A diagnosis of a major depressive disorder (MDD), according to the DSM-IV, requires the presence of at least five of the nine listed depressive symptoms, including at least one of the two cardinal symptoms (A1 or A2) (Criterion A) (see ) (American Psychiatric Association Citation1994). Additionally, clinically significant distress or impairment must be present (Criterion C), and a diagnosis of bipolar disorder, substance abuse or general medical condition, or bereavement as a cause must be excluded (Criteria B, D, E) (see ) (American Psychiatric Association Citation1994).
Table 1. Description of depression symptoms and diagnosis criteria as per the DSM-IV.
The heterogeneity of the depressive symptomatology represents a challenge for researchers and clinicians. Hence, depending on the research question, many different aspects need to be considered, such as severity and impairment, age at onset, symptom patterns, co-morbidities, lifetime (i.e. ever vs. never) or current occurrence of depression, and clinical treatment (Krishnan and Nestler Citation2010; Cai et al. Citation2020; Buch and Liston Citation2021). Diagnosis of a depressive disorder must be based on an expert interview, but validated self-report instruments to assess depressive symptoms and depression severity are available for large-scale epidemiological studies. These self-report instruments can be leveraged to investigate the mechanisms of depression in non-clinical cohorts. The value of this approach is supported by recent large-scale genome-wide association studies, which indicate a large genetic overlap of the biology involved in the vulnerability for depression and continuous measures of depressive symptoms in the general population (Wray et al. Citation2018) as well as in patients (Jermy Hagenaars, et al. Citation2020). Furthermore, specific definitions, subtypes and aspects of depression may differ in their underlying neurobiology (Milaneschi et al. Citation2017; Cai et al. Citation2020; Hagenaars et al. Citation2020), stressing the need for comprehensive phenotypic assessment. The data gathered from the German National Cohort (NAKO) (Schipf et al. Citation2020) comprises the largest population-based source of expert- and self-rated depression and depression symptoms in Germany. The study of depression in large-scale cohorts requires a balance between comprehensive assessment and efficiency with regard to the number of participants. This was addressed in the NAKO by combining self-reported data using validated questionnaires, and the MINI interview carried out by a trained study assistant – with the depression screening part administered to the entire sample, and the complete depression section administered to a subset of the sample (Level2; see below).
The aim of the present analysis is to give an overview of 1) the instruments used in the NAKO to assess lifetime and current depression as well as depressive symptoms, 2) the distribution of core variables of the depression measures, 3) the association of the depression measures with sociodemographic factors such as sex, age, family history of depression, and study centre, and 4) the association between the depression measures and measures of stress and anxiety.
Methods
The NAKO (German National Cohort Consortium Citation2014) is a population-based cohort study aiming to investigate the causes of common chronic diseases and their preclinical stages, examining 205,000 randomly selected participants (German National Cohort Consortium Citation2014). Baseline examination took place between 2014 and 2019 and comprised physical examinations, standardised interviews and questionnaires, and the collection of biomaterial (German National Cohort Consortium Citation2014). All study centres’ local ethics committees had given approval and the study was conducted in accordance with the Declaration of Helsinki. All participants had provided written consent for study participation. At baseline, data were acquired in the 18 study centres at two levels; all participants underwent Level-1 assessment (L1; ∼3–4 hours) and a subset of ∼20% of the participants was randomly selected to undergo the more comprehensive and more detailed Level-2 assessment (L2; ∼5 hours). The current analysis was based on the baseline assessment of the first 101,667 participants (NAKO data freeze 100,000; application NAKO-399). A detailed description of the NAKO has been published elsewhere (German National Cohort Consortium Citation2014). A current overview of the assessment of neuropsychiatric functions and conditions is presented in the editorial article of this series (Berger et al. Citation2022), along with detailed analyses of specific neuropsychiatric measures, i.e. anxiety and panic symptoms (Erhardt et al. Citation2022), childhood maltreatment (Klinger-König et al. Citation2022) and cognition (Kleineidam et al. Citation2022; Schmiedek et al. Citation2022).
Instruments
The assessment of a self-reported physician’s diagnosis and the MDD modules of the Mini-International Neuropsychiatric Interview (MINI, German version 5.0.0) (Lecrubier et al. Citation1999) were conducted by a trained study assistant as part of the face-to-face interview. Other depression measures were assessed via self-report questionnaires answered by the participants on a touchscreen device.
MINI interview
The MINI is a standardised, structured psychiatric interview for the diagnosis of different psychiatric disorders (Sheehan Citation1998; Lecrubier et al. Citation1999). It is shorter than other structured interviews (e.g. CIDI, SCID (Robins et al. Citation1988; First and Gibbon Citation2004)) and easy to administer. It is divided into different modules including 17 Axis I disorders, a suicidality module, and one Axis II disorder. It is compatible with the two major international classification systems: the DSM-IV (American Psychiatric Association Citation1994), and the International Classification of Diseases, 10th Revision (ICD-10) (World Health Organization Citation1993) from the WHO, which is mostly used in the clinical context. Each diagnostic module starts with screening questions, which correspond to the main criteria of the disorder. In the NAKO, the MINI v. 5.0.0 (Lecrubier et al. Citation1999) module ‘Episode of Major Depression’ was adapted for use by the NAKO neuropsychiatric expert group. While the original version focusses on a current episode of depression, i.e. symptoms during the last two weeks, the MINI adapted for the NAKO assesses the occurrence and number of lifetime episodes, with specific symptoms recorded for the last episode. Thus, in the NAKO, an initial question asking about lifetime occurrence of depression was added (‘Have you had periods of two weeks or more during your life when you felt depressed or disinterested?’). Only participants responding ‘Yes’ to this filter question continued with further questions from the depression module, answering a question to assess when such period had last occurred, followed by the two original screening questions of the MINI, assessing the cardinal symptoms of a depressive disorder (Criteria A1 and A2). If the MINI Screen was positive (at least one of Criteria A1 and A2 was answered positively), it could then be followed by the questions assessing Symptoms A3–A9.
While all participants received the screening questions, L2 participants received the remaining set of the questions from the depression module in case the MINI Screen was positive. Some questions in the remainder of the depression module were also slightly modified. While the original MINI assesses Symptom 3 of Criterion A (changes in appetite of weight) with one question (coded as ‘yes’ if one of the four characteristics [appetite/weight gain/loss] applies), in the NAKO, change in appetite and weight are assessed with two separate questions. Furthermore, the answer categories for appetite, weight, and sleep were split such that individuals can further specify individual symptoms and direction of the change (e.g. increased or decreased). In addition, participants were asked whether they experienced impairment in social or occupational functioning during the depressive episode (i.e. Criterion C, a mandatory criterion for the diagnosis of a major depressive disorder in ICD-10 and DSM-IV), how many episodes have occurred throughout the lifetime, the age of onset (i.e. of the first episode), and the duration of the longest depressive episode.
Participants were assigned a positive lifetime MINI Screen or NAKO MINI Classification (see below) according to the manual. Following DSM-IV, one of the two cardinal symptoms (A1&A2), and a total of at least five symptoms from A1–A9 must be positive to fulfil the diagnosis 'major depressive episode'. For this, the Criterion A items for symptom 3 (appetite; weight change), and symptom 4 (psychomotor retardation; restlessness), and items indicating the direction or manifestation of a symptom in more detail (appetite, weight, sleep) were recoded into a binary variable indicating the presence or absence of each symptom. In case of missing values on any of the Symptoms A1–A9, MINI Screen and Criterion A were only assigned when the data was conclusive. A ‘NAKO MINI Classification’ was defined as the affirmation of depressive symptomatology as described above (Criterion A) and impairment (Criterion C). DSM-IV Criteria B, D and E were not assessed in the MINI interview, and could therefore not be included in the NAKO MINI Classification. The time since onset of the last episode was calculated as the difference in months between the current age and time-point of the last episode. When only the year, but not the month was reported, time since onset of the last episode was calculated using 1 July of the respective year. Age at onset was set to missing if the age was ten years or lower. Time since onset of the last episode and age at onset are presented for the L2 participants assigned a NAKO MINI Classification ().
Table 2. Descriptive statistics of demographic and depression measures in the NAKO.
Self-reported physician’s diagnosis of depression
In the face-to-face interview, participants were asked to indicate whether they had ever been diagnosed with depression by a physician. If participants answered ‘yes’, the time of the first diagnosis (either age or calendar year) was assessed. Participants were also asked if they had been treated for depression during the previous 12 months by a physician or a psychologist. Age at onset was calculated using age/year of diagnosis and the age/year at/of the NAKO baseline examination.
Family history of depression
As part of the family history question set, participants were asked whether their parents had suffered from ‘depression’ (not further specified) and to indicate the age at onset of the affected parent, if applicable.
Patient health questionnaire PHQ-9
PHQ-9 is the depression module of the Patient Health Questionnaire (Kroenke et al. Citation2001) and a nationally and internationally established self-report screening instrument (Kroenke et al. Citation2010; Manea et al. Citation2015). It was developed for screening of depression and contains nine items corresponding to the symptoms comprising Criterion A in DSM-IV (Kroenke et al. Citation2001) (see ). The items relate to the presence of the symptoms in the last two weeks and can be answered on a scale of 0 (‘not at all’) to 3 (‘almost every day’). In addition, a tenth item is used to assess the impairment caused by the symptoms (Not at all (0); Some (1); Relatively strong (2); Very strong impairment (3)). This item is to be answered if at least one of the nine symptom items was answered with ‘several days (1)’ or higher. However, this item is usually not included in the evaluation. The first nine items can be summarised into a score (range 0–27) representing depressive symptom severity, with suggested grading of 0–4 minimal, 5–9 mild, 10–14 moderate, 15–19 moderately severe, and 20–27 severe (Kroenke et al. Citation2001). A cut-off score of ≥ 10 (moderate to severe symptoms) is commonly used to indicate the presence of a current depressive episode (Löwe et al. Citation2004, Citation2017) and was selected in the present analyses for comparability with other studies. Alternatively, a categorical PHQ diagnosis of a Depressive Syndrome can be made based on the following diagnostic algorithm: Each symptom is counted as present if the item is answered with at least ‘more than half the days’ (answer choice 2), with the exception of suicidal tendency (item 9), which is counted as present if the answer is at least ‘several days’ (answer choice 1). Analogous to the A Criterion of the DSM-IV for the diagnosis of an episode of major depression, at least one of Symptoms A1 or A2 (item 1 or item 2) must be present. If this is the case, participants with a symptom count (PHQ Items 1–9) of five or more are assigned the diagnosis ‘Major Depressive Syndrome’ and those with two to four ‘Other Depressive Syndrome’. PHQ-9 sum scores and categorical variables indicating current symptom severity category, current depressive episode using the cut-off (PHQ-9 ≥ 10), and current ‘Major ‘or ‘Other Depressive Syndrome’ using the diagnostic algorithm were calculated for all participants without missing values on the respective items following the manual.
Stress and anxiety symptoms – PHQ-Stress and GAD-7
To assess perceived stress and anxiety, the stress module of the PHQ (PHQ-Stress) (Löwe et al. Citation2004) and the General Anxiety Disorder-7 (GAD-7) (Spitzer et al. Citation2006) were used (for a detailed description see Erhardt et al. Citation2022). Sum scores were calculated for PHQ-Stress (10 items; range 0–20) and GAD-7 (7 items; range 0–21) for all participants without missing values on the respective items.
Education
Education was categorised following the International Standard Classification of Education 97 (ISCED97) (UNESCO United Nations Educational and Scientific and Cultural Organization Citation2003) as described for the NAKO by Dragano et al. (Citation2020). Education was coded as lower (ISCED97 level 1/2), intermediate (ISCED97 level 3/4) and higher (ISCED97 level 5/6). As the classification of job education has not been finalised at the time of data analyses, participants who were not yet classified were excluded from the respective analyses.
Statistical analyses
Analyses were conducted using the R open-source environment for statistical computation and graphics (v3.5.1). Frequencies and mean scores and standard deviations are reported. Associations between continuous scores were assessed using Pearson correlations. To assess the association of depression measures with the family history of depression, the number of affected parents (0, 1, 2) was calculated for all participants with data available from both parents. Odds ratios (OR) and mean estimates and 95% confidence intervals were calculated with logistic/linear regression models, using the participants reporting no family history of depression for both parents as reference group, while adjusting for age, sex and education level. Graphics were created using ggplot2 (Wickham Citation2011), and curves depicting smoothed estimates of mean scores and frequencies of depression measures against age, were estimated using the gam() function as implemented in the ggplot2 package (see ; method = ‘gam’).
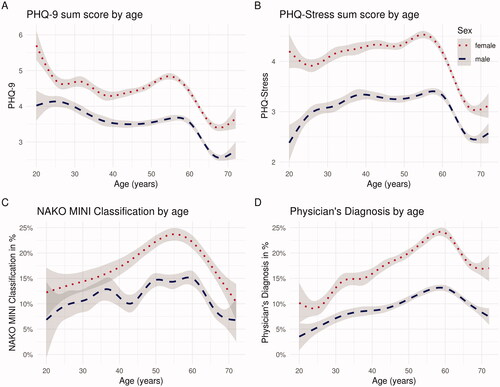
Figure 1. Smoothed estimates of mean of Current (A) PHQ-9 and (B) PHQ-Stress Ratings and Frequency of Lifetime (C) NAKO MINI Classification and (D) Physician’s Diagnosis of Depression by Sex and Age. Note. The grey areas represent the 95% confidence interval. MINI = Mini-International Neuropsychiatric Interview. NAKO = German National Cohort. PHQ-9 = Depression Scale of the Patient Health Questionnaire (PHQ-9). Based on (NAKO data freeze 100,000; application NAKO-399).
Results
Sample characteristics
Participants aged 73 years and older (n = 6) at the time point of examination were excluded as they deviated more than three years from the targeted age range, leaving 101,667 participants for the analyses. Those had a mean age of 52.0 years (SD = 12.4 years) and 54,463 (53.6%) were women. At least one valid value for MINI-Screen, physician’s diagnosis or PHQ-9 score was available for 101,599 participants (99.9%). An overview of the sample characteristics and descriptive statistics is shown in . Frequencies are given in reference to the subjects with valid data records for the respective measure. The number of subjects with available data for one measure is given in , and the number of subjects with available data for the combination of different depression measures is reported in Supplementary Table S5.
Self-reported physician’s diagnosis of depression
A lifetime diagnosis of depression made by a physician was reported by 15.0% of the participants (women: 19.0%; men: 10.4%), and 47.6% of these (women = 48.8%; men = 45.2%) had received treatment for depression within the last 12 months, corresponding to 7.1% of the total sample (women = 9.3%; men = 4.7%).
MINI interview
In the entire sample, 27.4% of the participants (women: 31.7%; men: 22.5%) were positive in the MINI Screen. Of the participants who underwent the in-depth L2 examination, 19.1% (women: 23.4%; men: 14.5%) fulfilled Criterion A (see also ) and 15.9% (women: 19.4%; men: 12.2%) fulfilled the NAKO MINI Classification (Criteria A and C). On average, the age at onset of participants with a lifetime NAKO MINI Classification was 36.4 years (SD = 13.6), and 6.67 years (SD = 8.0) had passed since the last episode. Of the participants with a MINI lifetime diagnosis, 26.4% reported that less than a year had passed since the last episode.
PHQ-9 and PHQ-Stress assessment
Overall, the participants had a mean PHQ-9 score of 3.9 (SD = 3.7) (women: M = 4.4, SD = 3.9; men: M = 3.4, SD = 3.5). A score of ten or higher was observed in 7.8% of the participants (women: 9.4%; men: 6.1%). Using the PHQ diagnostic algorithm, 2.9% of the participants (women: 3.4%; men; 2.4%) were assigned ‘Major Depressive Syndrome’, and an additional 2.9% of the participants (women: 3.0%; men: 2.7%) were assigned ‘Other Depressive Syndrome’. Percentiles and frequencies of the categorical severity levels are reported by sex and age groups in Supplementary Table S1 and S2.The sample showed a mean PHQ-Stress score of 3.6 (SD = 3.1) with a clear sex-specific difference (women: M = 4.1, SD = 3.3; men: M = 3.1, SD = 2.9).
Association of depression and depressive symptoms with sex, age, education, and study center
Differences with regard to sex for all depression measures are shown in . In general, women reported higher frequencies of all categorical measures of depression as well as a higher current depression score as measured by the PHQ-9. This sex difference was observed across all age groups (see ; Supplementary Table S3).
With regard to age, the proportion of respondents with both a lifetime physician’s diagnosis of a depressive disorder or a positive NAKO MINI Classification was highest for the age group of 50–59, while the current PHQ-9 score was highest among participants aged 20–29 (; ).
Table 3. Descriptive statistics of depression measures by Age Group in the NAKO.
Overall, lower rates of depression measures and lower depression score were observed in participants with higher education levels compared to those with lower levels (; stratified by sex in Supplementary Table S4).
Table 4. Descriptive statistics of depression measures by ISCED-97 education level in the NAKO.
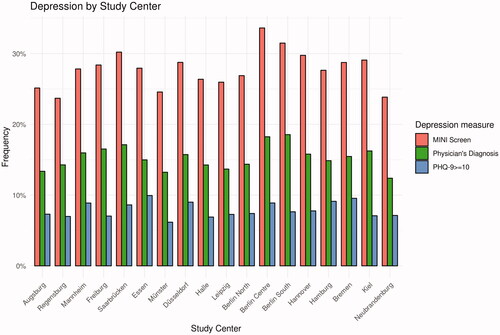
There was some variation in the observed frequencies of the assessed depression measures between the study centres. The frequency of a positive MINI Screen ranged from 23.7% (Regensburg) to 33.6% (Berlin Centre), physician’s diagnosis of depression from 12.4% (Neubrandenburg) to 18.6% (Berlin South), and depression by PHQ-9 Cut-off ≥ 10 from 6.2% (Münster) to 9.9% (Essen) ().
Figure 2. Frequency of physician’s diagnosis of depression (lifetime), positive MINI screen (lifetime), and PHQ-9 ≥ 10 (4 weeks) by Study Centre. Note. MINI = Mini-International Neuropsychiatric Interview. NAKO = German National Cohort. PHQ-9 = Depression Scale of the Patient Health Questionnaire (PHQ-9). Based on (NAKO data freeze 100,000; application NAKO-399).
Association of depression and depressive symptoms with family history
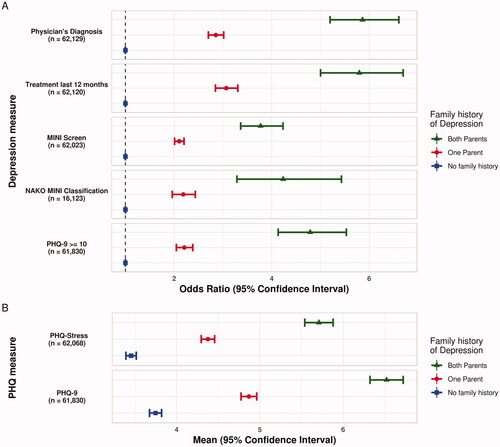
A relatively high number of missing values was observed for the self-report module on family history. This was either due to participants not answering this section at all (family history father = 15.9%, family history mother = 14.5%) or due to answer options ‘do not know or do not want to provide the information’ (family history father = 13.1%, family history mother = 8.7%). The reported frequencies of a positive family history of depression ranged from 16.5% (age group 60–72) to 19.7% (age group 30–39; ). Both higher frequencies and higher means for measures of depression were observed in participants with a positive family history, and this was most pronounced in those who reported that both parents had a history of depression. depicts the OR for the included depression measures according to the family history of depression. In addition, shows the estimated mean scores from PHQ-9 and PHQ-Stress in dependence on family history (both analyses adjusting for age, sex and education level).
Figure 3. (A) Odds Ratio and 95% confidence interval for measures of depression by reported family history of depression. (B) Distribution of PHQ-9 and PHQ-stress mean scores and 95% confidence interval by Reported Family History of Depression in the NAKO. Note. Participants reporting no history of depression in their parents are presented as the reference group (blue). Estimates are adjusted for age, sex and education level (lower/intermediate/higher). MINI = Mini-International Neuropsychiatric Interview. NAKO = German National Cohort. PHQ-9 = Depression Scale of the Patient Health Questionnaire (PHQ-9). n = subjects with complete data included in the respective analysis. Based on (NAKO data freeze 100,000; application NAKO-399).
Association between measures of depression, stress and anxiety
Overall, the applied measures of depression were all positively associated with each other. shows the overlap between the different instruments used in the NAKO.
Table 5. Proportion of positive diagnosis or screening and mean of depression measures (columns) for participants negative or positive for the different categorical depression measures (rows) in the NAKO.
Participants who reported a physician’s diagnosis of depression had a higher proportion of positive screening or diagnosis in other categorical depression measures. They also had higher sum scores of PHQ-9, PHQ-Stress, and GAD-7. If they additionally reported treatment in the last 12 months by a psychiatrist or a psychologist, mean PHQ-9, PHQ-Stress and GAD-7 scores were further increased. Compared to the total sample, participants who screened positive in the MINI or received a NAKO MINI Classification showed a higher frequency of physician’s diagnosis or treatment in the last 12 months, and their PHQ-9, PHQ-Stress, and GAD-7 sum scores were increased (). PHQ-9, PHQ-Stress, and GAD-7 sum scores were highly correlated (PHQ-9-PHQ-Stress: r = .66; PHQ-9-GAD-7: r = .77; PHQ-Stress-GAD-7: r = .67).
Discussion
In the baseline examination of the NAKO, a range of established and validated instruments has been applied to assess different aspects of depression. It has to be noted that prevalence and mean values of the depression measures presented, have not been weighted, e.g. for sex, age and other characteristics, and can therefore not be interpreted as representative for the German population. However, contextualising the results, we refer to the numbers observed in other population based studies (see below).
Lifetime depression
In the NAKO data freeze 100,000, about every fourth participant was positive on the MINI Screen. This Screen is available for all NAKO participants and assesses the lifetime presence of depressed mood and anhedonia (Symptoms A1 and A2). A presence of at least one of these two cardinal symptoms of depression is a prerequisite for a diagnosis of depression, and therefore shared by all possible manifestations or symptom combinations. Recent studies (based on molecular genetic data) indicate that while a detailed assessment of depression symptoms gives important information on severity or depression subtypes, the cardinal symptoms already reflect a large share of the genetic aetiology of depression (Jermy, Glanville, et al. Citation2020). The frequency of the NAKO MINI Classification of 15.9% among the L2 participants (i.e. those with the most comprehensive assessment) observed in the MINI interview was comparable to other previous studies in the German population, assessing lifetime depression with a clinical interview (Jacobi et al. Citation2004; Martin et al. Citation2017). It has to be noted that the MINI 5.0.0 in its original version does not assess impairment (Criterion C). In the present study, the NAKO MINI Classification was based on DSM-IV Criteria A and C; a higher frequency was observed when only taking Criterion A into account (19.1% vs. 15.9%). For the self-reported physician’s diagnosis, the frequency of lifetime depression in the NAKO was 15.0%, while the hitherto largest German Survey on Mental Health, the DEGS study, comprising close to 8000 individuals reported 11.6% (Busch et al. Citation2013), while on the other hand, in the UK Biobank 21.2% of the subjects report a lifetime depression diagnosis by a health care professional (Davis et al. Citation2020). In the NAKO, about half of the participants with a physician’s diagnosis of depression reported treatment for depression within the last 12 months. This is in line with depression being an often recurring and chronic disorder. In the DEGS study 52% of the participants reporting a lifetime physician’s diagnosis indicated they experienced depression in the last 12 months (Busch et al. Citation2013).
Current depressive symptoms
The frequency of clinically relevant, current depression, defined by a PHQ-9 cut-off score of ≥10, was higher (7.8%) than the one measured with the categorical diagnostic algorithm (major depressive syndrome 2.9%; other depressive syndrome 2.9%). Very similar frequencies were observed in the representative German survey of adults DEGS (Busch et al. Citation2013; Maske et al. Citation2015), and slightly lower numbers are reported for PHQ-9 mean and PHQ-9 cut-off score of > =10 for the UK Biobank (Chaplin et al. Citation2021). Meta-analytical studies investigating different cut-offs for the PHQ-9 in comparison to clinical interviews indicate that the most commonly used cut-off of ≥10 represents a good compromise between sensitivity and specificity, and reported a sensitivity of 70%–88% and a specificity of 84%–89% (Manea et al. Citation2012; Moriarty et al. Citation2015; Levis et al. Citation2019). Meta-analytic studies also report a higher sensitivity for the cut-off of ≥10 compared to the algorithm scoring method (Manea et al. Citation2015; He et al. Citation2020). This cut-off was also applied in the NAKO; however, future studies using NAKO data are free to adapt this threshold according to their research question depending on the application context (Grafe et al. Citation2004).
Association of depression and depressive symptoms with sex, age, education and study center
Considerable differences across age groups and sex as well as educational levels were observed. For all categorical depression measures, both lifetime and current, a substantially higher frequency was observed in women. This is in line with the observation that female sex is a major risk factor for depression (Krishnan and Nestler Citation2010; Bretschneider et al. Citation2018). Similarly, higher scores of current depression, stress, and anxiety were observed in women, which is also in line with other large German studies (Busch et al. Citation2013; Maske et al. Citation2015; Martin et al. Citation2017).
The distribution based on the continuous and categorical assessment in different age groups is similar to the one described in other samples from Germany (Busch et al. Citation2013; Maske et al. Citation2016). Here, it is noteworthy that both lifetime and 12-month frequencies of physician’s diagnosis or NAKO MINI Classification, increase with age to a peak in the group 50–59 years and then decrease for the older participants. In contrast, the highest mean PHQ-9 scores were observed in the youngest participants (20–29 years), with a second peak in the group 50–59 years. Considering the broad range of age at disorder onset, an increase of the lifetime prevalence of depression is to be expected, when data is acquired in a longitudinal way (Streiner et al. Citation2009). However, reports of lower lifetime depression in older participants have been observed previously in cross-sectional population samples (Busch et al. Citation2013; Maske et al. Citation2016) and have been attributed to several factors, including cohort effects, increased mortality in persons with depression (Laursen et al. Citation2016), and selection bias as well as recall or memory errors (Streiner et al. Citation2009; Takayanagi et al. Citation2014). Of interest is also the relatively high PHQ-9 scores, together with a relatively low frequency of treatment within the last 12 months observed in the subjects of 20–29 years, highlighting the importance of assessing participants as early as age 20 as implemented in the NAKO. The wider age range of participants in the NAKO in distinction to other well-characterized mega cohorts, e.g. the UK-Biobank which only included participants ≥40 years old (Sudlow et al. Citation2015) enables the assessment of health in individuals from different age groups across the life span. This is of special importance for mental disorders, which tend to have a low age of onset (Kessler et al. Citation2007), and a long undiagnosed period (Tegethoff et al. Citation2016).
Higher frequency and scores of depression measures were associated with lower education level, which has also been reported previously (Lorant et al. Citation2003; Bretschneider et al. Citation2017; Schlax et al. Citation2019). Substantial differences in the frequencies of the different lifetime and current depression measures were observed between the study centres. These differences are likely related to sociodemographic differences between participants in the several study centres (Erhart and von Stillfried Citation2012); however, further detailed analyses are needed to investigate the underlying contributing factors.
Association of depression and depressive symptoms with family history
Family history is an established risk factor for depression (Angst et al. Citation2003). In line with the higher prevalence of depression in women (Maske et al. Citation2016; Martin et al. Citation2017; Bretschneider et al. Citation2018), a history of depression was reported almost twice as often for mothers as for fathers of the NAKO participants (see ). Depression measures were more frequent in participants with a family history, and this was most pronounced in participants with two affected parents. This has been reported previously (Martin et al. Citation2017) and is in agreement with the notion that a family history of depression can be conceptualised as a marker for a genetic risk for depression, but might also reflect the effect of the familial psychosocial environment (Sander and McCarty Citation2005).
Association between measures of depression, stress and anxiety
As depicted in , all measures of depression and also measures of stress and anxiety were positively associated with each other. However, it is important to note that the different measures of depression used in the NAKO assess different aspects of the condition and therefore full agreement should not be expected. A major difference is that the instruments cover different time periods: while the MINI and the physician’s diagnosis are lifetime measures, the PHQ-9 assesses depressive symptoms during the last two weeks. Many participants reporting a history of depression have recovered from the disorder. This is indicated by the observation that in the participants with a lifetime MINI Classification, the mean time since onset of the last episode was around seven years and by the observation that every second participant with a lifetime physician’s diagnosis did not receive treatment during the last year. Secondly, the measures differ in their operationalisation of depression: for example, the MINI assesses the DSM criteria via direct interview of the participant, while the physician’s diagnosis represents the past assessment of a physician which depends on help-seeking behaviour and detection in a primary care setting (Jacobi et al. Citation2002) in which mental disorders are often underdiagnosed (Sartorius et al. Citation1995; Jacobi et al. Citation2002; Wittchen and Pittrow Citation2002; Trautmann and Beesdo-Baum Citation2017). Additionally, the criteria for depression differ between the measures; in Germany the diagnosis of depression by a physician is carried out in accordance with the ICD-10, which differs in the exact criteria applied and can also include mild episodes only requiring the presence of two to three additional, non-cardinal symptoms (World Health Organization Citation1993; American Psychiatric Association Citation1994). Similarly, the PHQ-9 diagnostic algorithm and other depression measures require the presence of cardinal symptoms (i.e. persistent sadness and loss of interest), while a sum score of > =10 in the PHQ-9 can also be reached by only scoring high on items reflecting Criteria A3–9. Therefore, it is evident that different categorical measures of depression assess different aspects of the disorder. Accordingly, the moderate agreement between clinical interview, self-reported physician’s diagnosis, and PHQ-9 observed in this study is consistent with previously reported data (Maske et al. Citation2015, Citation2016). All depression measures showed a positive association with the related constructs stress and anxiety measured by the PHQ-Stress and the GAD-7 (File Citation1996). More details on those scales in the NAKO study are given in Erhardt et al. (Citation2022).
In future analyses of depression in the NAKO cohort study, researchers need to take the differences between the depression measures into account. The depression measure needs to be selected based on the specific research question, e.g. what time reference and whether a continuous depression score or a categorical measure of depression is of interest. Depending on the time reference, the MINI Screen and the physician’s diagnosis can be selected for lifetime depression, while the PHQ-9 is a measure of current depression. For analyses restricted to the L2 subset of participants, the complete MINI interview is available and should be considered for lifetime assessment. It is possible to include and assess or statistically adjust for the effects of all depression measures, considering the large sample size of the NAKO. Of note, recent studies indicate that combining the information of several measures of depression can further inform about risk, e.g. an increased genetic risk for depression has been observed in participants fulfilling depression criteria based on more than one measure of depression (Martin et al. Citation2017; Glanville et al. Citation2020).
Limitations
All collected information from the NAKO presented here was self-reported by the participants. This is the standard situation for most of the used instruments, such as the MINI or the PHQ-9. However, for some measures, especially family history or physician’s diagnosis, additional sources of information would be favourable. Linking electronic health records of participants to the study data as planned for the NAKO would make it possible to estimate whether a bias might exist in the reporting behaviour of the participants. It has to be noted that the MINI was adapted for the NAKO study to assess some aspects in more detail, and to assess life-time depression symptomatology. It cannot be excluded that this introduces some form of bias to the data. Also, to reduce recall bias, we chose to inquire the symptoms during the last episode, and therefore cannot exclude that symptoms that were not reported might have been present in earlier episodes. Additionally, participants with severe depressive symptoms might have been impaired or less motivated to participate in the study, and therefore might be under-represented in the sample. In general, regarding the response rate of ∼20% (Schipf et al. Citation2020), effects of a selection bias on some measures cannot be excluded. Furthermore, DSM-IV Criteria B,D,E (see ) which need to be evaluated to exclude bipolar disorder, substance abuse or a general medical conditions or bereavement as a cause for depression, were not assessed in the MINI interview, and could not be used as differential diagnostic criteria.
Summary and outlook
Depression is a heterogeneous disorder, and the detailed and comprehensive assessment of current and lifetime depression in the NAKO offers a unique opportunity to analyse its exact frequency and aetiology in a mega cohort. The observed distributions of the different depression measures, their associations with each other, and with the assessed sociodemographic and psychological variables are coherent and in line with those reported in the literature, indicating the robustness of the approach and generalisability of the findings. The availability of valid and reliable data on depression, depressive symptoms, and impairment of functioning in the general population is a valuable prerequisite for health policymakers, therapists, and researchers to improve prevention and therapy. For this, a detailed assessment is needed, as genetic studies indicate that the different aspects of depression such as specific symptoms or age at onset differ in their biological aetiologies (Kendler et al. Citation2005; Martin et al. Citation2017; Hagenaars et al. Citation2020). A broad range of risk factors contribute to the aetiology of depression, and conversely depression represents a risk for other psychiatric and somatic disorders (Maier and Falkai Citation1999). Thus, the collection of data on sociodemographic factors, health behaviour, histories of mental and somatic disorders, in combination with physical and cognitive function measures, magnetic resonance imaging, and biomaterials enables the investigation of those relationships in detail (Schipf et al. Citation2020). In the future, the value of the NAKO data for the analyses of mental disorders will be further increased by the inclusion of longitudinal data (including an assessment during the COVID-19 pandemic; Peters et al. Citation2020), electronic health records, and results of genotyping and other omics data.
Statement of interest
HJ.Grabe has received travel grants and speakers honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag. J. Deckert is the co-recipient of a grant of the Bavarian State Government to BioVariance and an investigator in a European grant to P1Vital. Wolfgang Hoffmann received lecture fees and/or travel support from Roche, AMGEN, Pfizer, and a royalty from Janssen-Cilag.
Supplementary Material
Download MS Word (53.6 KB)Acknowledgement
We thank all study participants, all staff at the NAKO study centres, the data management and integration centre, and the NAKO head office who enabled the conduction of the study and made the collection of all data possible.
Additional information
Funding
References
- American Psychiatric Association A. 1994. Diagnostic and statistical manual of mental disorders (DSM-IV).
- Angst J, Gamma A, Endrass J. 2003. Risk factors for the bipolar and depression spectra. Acta Psychiatr Scand Suppl. 108(418):15–19.
- Baumeister SE, Schomerus G, Schmidt C-O, Möckel F, van den Berg N, Hoffmann W, Völzke H, Grabe HJ. 2015. Change in depressive symptoms and mental health-related quality of life in northeast Germany between 1997-2001 and 2008-2012. Int J Public Health. 60(1):33–39.
- Berger K, Rietschel M, Rujescu D. 2022. Editorial – The value of ‘mega cohorts’ for psychiatric research. World J Biol Psychiatry. [ahead of print]. doi:10.1080/15622975.2021.2011405
- Bretschneider J, Janitza S, Jacobi F, Thom J, Hapke U, Kurth T, Maske UE. 2018. Time trends in depression prevalence and health-related correlates: results from population-based surveys in Germany 1997-1999 vs. 2009-2012. BMC Psychiatry. 18(1):394.
- Bretschneider J, Kuhnert R, Hapke U. 2017. Depressive symptoms among adults in Germany. Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung.
- Buch AM, Liston C. 2021. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology. 46(1):156–175.
- Busch M, Maske U, Ryl L, Schlack R, Hapke U. 2013. Prevalence of depressive symptoms and diagnosed depression among adults in Germany.
- Cai N, Choi KW, Fried EI. 2020. Reviewing the genetics of heterogeneity in depression: operationalizations, manifestations and etiologies. Hum Mol Genet. 29(R1):R10–R18.
- Cai N, Revez JA, Adams MJ, Andlauer TFM, Breen G, Byrne EM, Clarke TK, Forstner AJ, Grabe HJ, Hamilton SP, et al. 2020. Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet. 52(4):437–447.
- Chaplin AB, Jones PB, Khandaker GM. 2021. Sexual and physical abuse and depressive symptoms in the UK Biobank. BMC Psychiatry. 21(1):1–10.
- Davis KAS, Coleman JRI, Adams M, Allen N, Breen G, Cullen B, Dickens C, Fox E, Graham N, Holliday J, et al. 2020. Mental health in UK Biobank – development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 6(2):e18.
- Dragano N, Reuter M, Greiser KH, Becher H, Zeeb H, Mikolajczyk R, Kluttig A, Leitzmann M, Fischer B, Jockel KH, et al. 2020. [Socio-demographic and employment-related factors in the German National Cohort (GNC; NAKO Gesundheitsstudie)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 63(3):267–278.
- Erhardt A, Gelbrich G, Klinger-König J, Streit F, Kleineidam L, Riedel-Heller S, NAKO Investigators, Schmiedek F, Wagner M, Grabe H, et al. 2022. Generalized anxiety and panic symptoms in the German National Cohort (NAKO). World J Biol Psychiatry. [ahead of print]. doi: 10.1080/15622975.2021.2011409
- Erhart M, von Stillfried D. 2012. Analyse regionaler Unterschiede in der Prävalenz und Versorgung depressiver Störungen auf Basis vertragsärztlicher Abrechnungsdaten–Teil 1 Prävalenz. Berlin: Zentralinstitut für die kassenärztliche Versorgung in Deutschland.
- File SE. 1996. Recent developments in anxiety, stress, and depression. Pharmacol Biochem Behav. 54(1):3–12.
- First MB, Gibbon M. 2004. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). Comprehen Handbook Psychol Assessment. 2:134–143.
- Fried EI, Nesse RM. 2015. Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR*D study. J Affect Disord. 172:96–102.
- German National Cohort Consortium. 2014. The German National Cohort: aims, study design and organization. Eur J Epidemiol. 29:371–382.
- Glanville KP, Coleman JRI, Howard DM, Pain O, Hanscombe KB, Jermy B, Arathimos R, Hübel C, Breen G, O’Reilly PF, et al. 2020. Multiple measures of depression to enhance validity of Major Depressive Disorder in the UK Biobank. medRxiv.2020.2009.2018.20196451.
- Grafe K, Zipfel S, Herzog W, Lowe B. 2004. Screening for psychiatric disorders with the Patient Health Questionnaire (PHQ). Results from the German validation study. Diagnostica. 50(4):171–181.
- Hagenaars SP, Coleman JRI, Choi SW, Gaspar H, Adams MJ, Howard DM, Hodgson K, Traylor M, Air TM, Andlauer TFM, et al. 2020. Genetic comorbidity between major depression and cardio‐metabolic traits, stratified by age at onset of major depression. Am J Med Genet B Neuropsychiatr Genet. 183(6):309–330.
- He C, Levis B, Riehm KE, Saadat N, Levis AW, Azar M, Rice DB, Krishnan A, Wu Y, Sun Y, et al. 2020. The accuracy of the patient health questionnaire-9 algorithm for screening to detect major depression: an individual participant data meta-analysis. Psychother Psychosom. 89(1):25–37.
- Jacobi F, Hofler M, Meister W, Wittchen HU. 2002. [Prevalence, detection and prescribing behavior in depressive syndromes. A German federal family physician study]. Nervenarzt. 73(7):651–658.
- Jacobi F, Wittchen HU, Holting C, Hofler M, Pfister H, Muller N, Lieb R. 2004. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS). Psychol Med. 34(4):597–611.
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 392(10159):1789–1858.
- Jermy BS, Glanville KP, Coleman JRI, Lewis CM, Vassos E. 2020. What’s in a diagnosis? A genetic decomposition of major depression. medRxiv.2020.2012.2015.20247015.
- Jermy BS, Hagenaars SP, Glanville KP, Coleman JRI, Howard DM, Breen G, Vassos E, Lewis CM. 2020. Using major depression polygenic risk scores to explore the depressive symptom continuum. bioRxiv.2020.2002.2025.962704.
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. 2005. Age at onset and familial risk for major depression in a Swedish national twin sample. Psychol Med. 35(11):1573–1579.
- Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. 2020. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 25(2):321–338.
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. 2007. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 20(4):359–364.
- Kleineidam L, Stark M, Riedel-Heller S, Pabst A, Schmiedek F, Streit F, Rietschel M, Klinger-König J, Grabe HJ, Erhardt A, et al. 2022. The assessment of cognitive function in the German National Cohort (NAKO) – Associations of demographics and psychiatric symptoms with cognitive test performance. World J Biol Psychiatry. [ahead of print]. doi: 10.1080/15622975.2021.2011408
- Klinger-König J, Streit F, Erhardt A, Kleineidam L, Schmiedek F, NAKO Investigators, Wagner M, Deckert J, Rietschel M, Berger K, et al. 2022. The Assessment of childhood maltreatment and its associations with affective symptoms in adulthood: results of the german national cohort (NAKO). World J Biol Psychiatry. [ahead of print]. doi: 10.1080/15622975.2021.2011406
- Krishnan V, Nestler EJ. 2010. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 167(11):1305–1320.
- Kroenke K, Spitzer RL, Williams JB. 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 16(9):606–613.
- Kroenke K, Spitzer RL, Williams JB, Lowe B. 2010. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 32(4):345–359.
- Laursen TM, Musliner KL, Benros ME, Vestergaard M, Munk-Olsen T. 2016. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 193:203–207.
- Lecrubier Y, Weiller E, Herugeta T, Amorim P, Bonora L, Lépine J. 1999. Mini International Neuropsychiatric Interview German Version 5.0. 0. München: Psychiatrischen Universitätsklinik München.
- Levis B, Benedetti A, Thombs BD. 2019. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 365:l1476.
- Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. 2003. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 157(2):98–112.
- Löwe B, Spitzer RL, Gräfe K, Kroenke K, Quenter A, Zipfel S, Buchholz C, Witte S, Herzog W. 2004. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. J Affect Disord. 78(2):131–140.
- Löwe B, Spitzer RL, Zipfel S, Herzog W. 2017. Autorisierte deutsche Version des “Prime MD Patient Health Questionnaire (PHQ)”.
- Maier W, Falkai P. 1999. The epidemiology of comorbidity between depression, anxiety disorders and somatic diseases. Int Clin Psychopharmacol. 14:S1–S6.
- Manea L, Gilbody S, McMillan D. 2012. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 184(3):E191–196.
- Manea L, Gilbody S, McMillan D. 2015. A diagnostic meta-analysis of the Patient Health Questionnaire-9 (PHQ-9) algorithm scoring method as a screen for depression. Gen Hosp Psychiatry. 37(1):67–75.
- Martin J, Streit F, Treutlein J, Lang M, Frank J, Forstner AJ, Degenhardt F, Witt SH, Schulze TG, Cichon S, et al. 2017. Expert and self-assessment of lifetime symptoms and diagnosis of major depressive disorder in large-scale genetic studies in the general population: comparison of a clinical interview and a self-administered checklist. Psychiatr Genet. 27(5):187–196.
- Maske UE, Busch MA, Jacobi F, Beesdo-Baum K, Seiffert I, Wittchen HU, Riedel-Heller S, Hapke U. 2015. Current major depressive syndrome measured with the Patient Health Questionnaire-9 (PHQ-9) and the Composite International Diagnostic Interview (CIDI): results from a cross-sectional population-based study of adults in Germany. BMC Psychiatry. 15(1):77.
- Maske UE, Buttery AK, Beesdo-Baum K, Riedel-Heller S, Hapke U, Busch MA. 2016. Prevalence and correlates of DSM-IV-TR major depressive disorder, self-reported diagnosed depression and current depressive symptoms among adults in Germany. J Affect Disord. 190:167–177.
- Milaneschi Y, Lamers F, Peyrot WJ, Baune BT, Breen G, Dehghan A, Forstner AJ, Grabe HJ, Homuth G, Kan C, et al. 2017. Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry. 74(12):1214–1225.
- Moriarty AS, Gilbody S, McMillan D, Manea L. 2015. Screening and case finding for major depressive disorder using the Patient Health Questionnaire (PHQ-9): a meta-analysis. Gen Hosp Psychiatry. 37(6):567–576.
- Peters A, Rospleszcz S, Greiser KH, Dallavalle M, Berger K. 2020. The impact of the COVID-19 pandemic on self-reported health. Dtsch Arztebl Int. 117(50):861–867.
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA. 1988. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 45(12):1069–1077.
- Sander JB, McCarty CA. 2005. Youth depression in the family context: familial risk factors and models of treatment. Clin Child Fam Psychol Rev. 8(3):203–219.
- Sartorius N, Ustun T, Organization WH 1995. Mental illness in general health care: an international study. Chichester: Wiley.
- Schipf S, Schöne G, Schmidt B, Günther K, Stübs G, Greiser KH, Bamberg F, Meinke-Franze C, Becher H, Berger K, et al. 2020. [The baseline assessment of the German National Cohort (NAKO Gesundheitsstudie): participation in the examination modules, quality assurance, and the use of secondary data]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 63(3):254–266.
- Schlax J, Junger C, Beutel ME, Munzel T, Pfeiffer N, Wild P, Blettner M, Kerahrodi JG, Wiltink J, Michal M. 2019. Income and education predict elevated depressive symptoms in the general population: results from the Gutenberg health study. BMC Public Health. 19(1):430.
- Schmiedek F, Kroehne U, Goldhammer F, Prindle JJ, Lindenberger U, Klinger-König J, Grabe HJ, Riedel-Heller SG, Pabst A, Streit F, et al. 2022. General cognitive ability assessment in the German National Cohort (NAKO) – The block-adaptive number series task. World J Biol Psychiatry. [ahead of print]. doi: 10.1080/15622975.2021.2011407
- Schneider F, Erhart M, Hewer W, Loeffler LA, Jacobi F. 2019. Mortality and medical comorbidity in the severely mentally ill: a German Registry Study. Dtsch Arztebl Int. 116(23–24):405–411.
- Sheehan D. 1998. MINI-Mini International neuropsychiatric interview-english version 5.0. 0-DSM-IV. J Clin Psychiatry. 59:34–57.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 166(10):1092–1097.
- Steffen A, Nübel J, Jacobi F, Bätzing J, Holstiege J. 2020. Mental and somatic comorbidity of depression: a comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry. 20(1):15.
- Streiner DL, Patten SB, Anthony JC, Cairney J. 2009. Has ‘lifetime prevalence’ reached the end of its life? An examination of the concept. Int J Methods Psychiatr Res. 18(4):221–228.
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. 2015. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12(3):e1001779.
- Takayanagi Y, Spira AP, Roth KB, Gallo JJ, Eaton WW, Mojtabai R. 2014. Accuracy of reports of lifetime mental and physical disorders: results from the Baltimore Epidemiological Catchment Area study. JAMA Psychiatry. 71(3):273–280.
- Tegethoff M, Stalujanis E, Belardi A, Meinlschmidt G. 2016. Chronology of onset of mental disorders and physical diseases in mental-physical comorbidity – a national representative survey of adolescents. PLoS One. 11(10):e0165196.
- Trautmann S, Beesdo-Baum K. 2017. The treatment of depression in primary care: a cross-sectional epidemiological study. Deutsches Ärzteblatt International. 114(43):721.
- Tyrer P. 2014. A comparison of DSM and ICD classifications of mental disorder. Adv Psychiatr Treat. 20(4):280–285.
- UNESCO United Nations Educational, Scientific and Cultural Organization. 2003. International Standard Classification of Education, ISCED 1997. Advances in Cross-National Comparison: A European Working Book for Demographic and Socio-Economic Variables.195-220.
- Wickham H. 2011. ggplot2. WIREs Comp Stat. 3(2):180–185.
- Wittchen HU, Pittrow D. 2002. Prevalence, recognition and management of depression in primary care in Germany: the Depression 2000 study. Hum Psychopharmacol. 17(S1):S1–S11.
- World Health Organization. 1993. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Vol. 2. Geneva; World Health Organization.
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, et al. 2018. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 50(5):668–681.
