Abstract
Objectives
Because alcohol use disorder (AUD) is often accompanied by mood disorder (MD) and both alcoholism and depression result in activation of the immune system, this study compares serum cytokine levels in the presence of co-morbidity with those in either AUD or MD alone.
Methods
In this naturalistic prospective study the levels of 15 different cytokines were measured in serum samples of patients with MD (n = 43), participants with combined AUD-MD (n = 44) and AUD without MD (n = 42). The levels were compared cross-sectionally among themselves and with those in 50 healthy volunteers.
Results
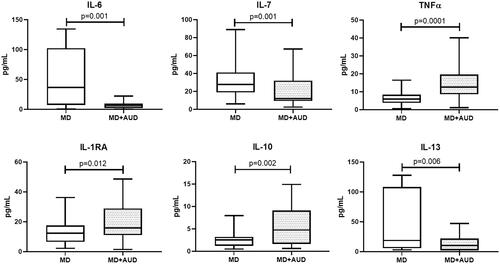
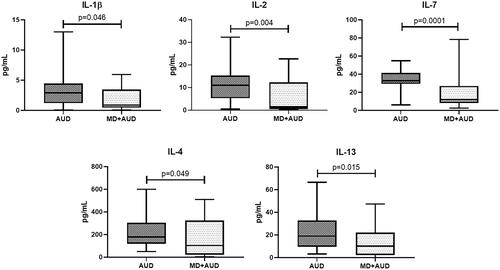
Pro-inflammatory IFN-2α levels were consistently significantly higher and anti-inflammatory IL-1RA significantly lower in all study groups in comparison to healthy volunteers. In the MD only group we found increased IL-6 (p = 0.001), IL-7 (p = 0.001) and IL-13 (p = 0.006) levels, and decreased TNFα (p = 0.0001), IL-1RA (p = 0.012), IL-10 (p = 0.002) compared with group MD + AUD. Patients with AUD only showed elevated levels of IL-1β (p = 0.046), IL-2 (p = 0.004), IL-7 (p = 0.0001), IL-4 (p = 0.049) and IL-13 (p = 0.015) in contrast with MD + AUD group.
Conclusions
Because the interactions of alcohol with peripheral and cerebral immune systems are multifaceted, the pertinent connection to the mechanism how alcohol consumption contributes to the development of mood disorders cannot be properly explored.
Introduction
According to data from the Global Burden of Diseases studies, mental and addictive disorders affected more than 1 billion people globally in 2016. They caused 7% of all global burden of disease as measured in DALYs and 19% of all years lived with disability (Rehm and Shield Citation2019). Higher rates of depression were seen in women, while rates of substance use disorders, including alcohol use disorder (AUD) were higher in men (Rehm and Shield Citation2019). Given the high prevalence of depression and AUD in the general population, co-occurrence of these diseases is expected. A recent systematic review found a 2.4 increased lifetime risk of depression in alcohol use disorders (Saha et al. Citation2022). Conversely, a 2005 systematic review of 35 studies found a median current and lifetime prevalence of alcohol use disorders in depression of 16% (range 5%–67%) and 30% (range 10%–60%), respectively (Sullivan et al. Citation2005) and a recent meta-analysis of 39 studies found a prevalence of 0.208 (95% CI 0.183, 0.235) of individuals with major depressive disorder who also abused alcohol (Hunt et al. Citation2020). Comorbidity of affective and addictive disorders leads to worsening in clinical and dynamic indicators, levels of social adaptation and cognitive functions of patients, and also treatment dropout, suicide attempts, and poorer effect of antidepressant medication (Sullivan et al. Citation2005; Neupane Citation2016; Vasilieva et al. Citation2021). However, the mechanisms by which one disorder affects another remain unclear.
There are a number of common pathogenetic mechanisms of depression (Loonen and Ivanova Citation2016a) and alcoholism, in particular, impaired serotonin metabolism, imbalance in hypothalamus–pituitary–gonadal and hypothalamus–pituitary–adrenocortical axis, and dysfunction of the immune system (Neupane Citation2016; Loonen and Ivanova Citation2016a; Walther et al. Citation2016; Underwood et al. Citation2018). We have previously found a decrease in Brain-Derived Neurotrophic Factor and Neural Cell Adhesion Molecules levels in AUD with and without concomitant mood disorder compared with healthy individuals (Levchuk et al. Citation2020). There is evidence that with excessive and/or prolonged activation of the cytokine network, a deterioration of neural plasticity can be observed (Miller et al. Citation2009) and therefore, in this study, we decided to focus on studying specific functions of the immune system.
After initial criticisms, an involvement of cytokines in the pathophysiology of depression has now become widespread acknowledged (Miller et al. Citation2009; Loonen and Ivanova Citation2016a), however the directionality of this relationship has not yet been elucidated. An increase in the level of a number of pro-inflammatory cytokines is observed against the background of a decrease in anti-inflammatory cytokines in the cerebrospinal fluid, serum and blood plasma of patients with depression (Dowlati et al. Citation2010). Decreased baseline levels of interleukin IL-6 and IL-8 were found in patients who later responded to antidepressant treatment (Lanquillon et al. Citation2000; Liu et al. Citation2020).
Acute and moderate alcohol use is thought to be associated with suppression of inflammatory responses, and chronic heavy alcohol use is thought to increase inflammation (Szabo and Mandrekar Citation2009). Increased C-reactive protein levels have been demonstrated in the presence of nicotine, alcohol, and cannabis use and nicotine dependence in adolescents and young adults (Costello et al. Citation2013). In patients with AUD IL-6 level was positively associated with depression and psychological distress scores, while IL-10 was negatively associated with anxiety score (Martinez et al. Citation2018).
From the above data, it can be inferred that the neuroimmune system plays at least some role in both the development of alcohol dependence and depressive mood disorder and that this results in changes in the levels of inflammation-related immune factors. Exactly what this connection looks like and what the interaction is with the comorbid mood disorders in alcohol use disorder has not yet been sufficiently explored. In an effort to gain some perspective on this, in the current study we compare cytokine levels in patients with AUD with and without mood disorder (MD), and in healthy individuals.
Materials and methods
Design
In a cross-sectional naturalistic study, the serum cytokine levels were compared across three diagnostic groups (patients with “pure” MD, patients with “pure” AUD, patients with comorbidity of AUD as well as MD (AUD-MD)), and healthy controls. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki 1975, revised in Fortaleza, Brazil, 2013), and approved by the Institutional Medical Review Board (Protocol of the local ethics committee at the Research Institute of Mental Health № 101 from 13 June 2017). All participants provided written informed consent.
Participants
Patients with MD (n = 43), participants with AUD-MD (n = 44) and AUD without MD (n = 42), were recruited from the departments of affective and addictive states of Mental Health Research Institute of the Tomsk National Research Medical Centre. Inclusion criteria were: a diagnosis of AUD (F10.2), MD (F31, F32, F33, F34.1) according to ICD-10 (World Health Organization Citation2004), or their comorbidity; ages 18–60 years. We excluded patients with other comorbid mental disorders, for instance schizophrenia, intellectual disability, and alcoholic psychoses, and patients with acute physical diseases. All participants had not taken any psychopharmacological drugs within 6 months prior to admission. The screening for relevant pathology for in/exclusion of subjects, disease development and the severity of the condition was performed through clinical assessment by three trained psychiatrists (O.R., G.S., and N.B.) on the first day of admission. Patients in the state of alcohol withdrawal received benzodiazepine therapy to alleviate withdrawal symptoms. The duration of alcohol withdrawal as estimated by the treating psychiatrists was on average 2–4 days after admission. The control group consisted of 50 healthy volunteers recruited through local advertisements at the MHRI and Siberian State Medical University. Healthy individuals were screened using a self-report questionnaire. The questionnaire screens for both physical and mental pathology, e.g. endocrine, neurological, gynaecological and psychiatric disorders.
Measurements
Phenotype measures
Depressive symptoms were assessed using the 17 item Hamilton Depression Rating Scale (HAMD-17). Anxiety symptoms were measured using the Hamilton Anxiety Scale (HARS). Anhedonia, a core symptom of depression and addiction, was measured using the Snaith-Hamilton Pleasure Scale (SHAPS). Alcohol use and dependency were measured with the Alcohol Use Disorders Identification Test (AUDIT). Craving was assessed using the Obsessive Compulsive Drinking Scale (OCDS). The Social Adaptation Self-evaluation Scale (SASS) was used to measure the level of social adaptation. We used common Russian translations of the originals (verified by back translation into English) (see Levchuk et al. Citation2020 for more details). The psychometric tests were performed during the first week of admission, after remission of withdrawal or intoxication symptoms.
Biomarkers
Peripheral venous blood was collected from each subject at 8.00–9.00 a.m. on the morning after hospital admission, after eight hours of overnight fasting before intake of any food or medication. Blood was sampled in BD Vacutainer tubes with coagulation activator and centrifuged at 2000 rcf at 4 °C for 20 min. Serum samples were stored at −80 °C until analysed. Concentrations of cytokines were determined on the MAGPIX multiplex analyser (Luminex, Austin, Texas, USA) using xMAP® Technology based on the Core Facility «Medical genomics», Tomsk NRMC. Panel HCYTMAG-60K-PX41 by MILLIPLEX® MAP (Merck, Darmstadt, Germany) was used to determine the levels of the markers. The detected information is processed by special Luminex xPONENT® software, with subsequent export of data to the MILLIPLEX® Analyst 5.1 program. For a list of the cytokines measured and the abbreviations used, see .
Table 1. Demographics and clinical characteristics of the study population.
Statistical analysis
Data analysis was carried out using SPSS Statistics software (version 23) for Windows. The Shapiro-Wilk test was used to determine if the data followed a normal distribution. Nonparametric Kruskal-Wallis and Mann-Whitney U-tests were used to assess the significance of intergroup differences. The Chi-square test was used to analyse the categorical variables. Statistically significant differences were considered p-values less than 0.05. Bonferroni correction was used for multiple comparisons.
Results
Demographics and clinical characteristics of the study population
The demographics and clinical characteristics of the study population are summarised in and . In the groups of MD and healthy individuals, the majority were women (p = 0.0001 and p = 0.0001 respectively compared with AUD group; p = 0.0001 and p = 0.048 respectively compared with AUD + MD group). The depression subtype significantly differed between MD as comorbidity of AUD in comparison to the group of MD only patients for Depressive Episode (F32) and Dysthymia (F34.1).
Table 2. Nosological structure of MD in the study groups.
The cytokine levels in the entire study population
The cytokine levels as measured in the study population are shown in .
Table 3. The cytokine levels in study population.
In MD group we found increased IFN-2α (p = 0.0001), IL-6 (p = 0.004), IL-7 (p = 0.0001) levels, and decreased IL-1β (p = 0.017) and TNFα (p = 0.0001) levels in comparison to those in healthy controls. The level of anti-inflammatory cytokines (IL-1RA and IL-10) was lower than that in healthy individuals (p = 0.0001; p = 0.001 respectively).
Patients with AUD showed an elevated level of IFN-2α (p = 0.0001), IL-2 (p = 0.016), IL-7 (p = 0.0001), IL-8 (р=0.0001) and IL-4 (р=0.0001) and decreased level of IL-12p40 (p = 0.004) and IL-1RA (р=0.008) compared with healthy individuals.
MD + AUD group demonstrated an increase in IFN-2α (p = 0.0001) and IL-8 (p = 0.012) levels against the background of a decrease in IL-1RA (p = 0.0001) and IL-10 (p = 0.019) in comparison to healthy persons.
Serum cytokine levels in MD and comorbidity MD + AUD
In MD group we found increased IL-6 (p = 0.001), IL-7 (p = 0.001) and IL-13 (p = 0.006) levels, and decreased TNFα (р=0.0001), IL-1RA (p = 0.012), IL-10 (p = 0.002) compared with group MD + AUD ().
Serum cytokine levels in AUD and comorbidity MD + AUD
Patients with AUD showed elevated levels of IL-1β (p = 0.046), IL-2 (p = 0.004), IL-7 (p = 0.0001), IL-4 (p = 0.049) and IL-13 (p = 0.015) in contrast with MD + AUD group ().
Correlation of cytokine levels with psychometric measures
Spearman's correlation analyses were provided between the cytokine levels and psychometric measures (HAMD-17, HARS, SHAPS, AUDIT-C, OCDS and SASS) in the three groups. The results are given in .
Discussion
The present study examined cytokine levels in the serum of newly admitted patients with MD and AUD in comparison to their levels in persons with comorbidity MD + AUD and in healthy individuals. The serum levels of fifteen cytokines (11 pro-inflammatory and 4 anti-inflammatory) were measured in the morning after admission in fasting state and before the start of any medication. For 8 of the 11 pro-inflammatory and for all 4 anti-inflammatory cytokines, significant differences existed between the levels measured in members of the four groups of individuals. Most consistent were the increase in IFN-2α and the decrease in IL-1RA in patients compared to healthy volunteers. The levels of IL-7 and IL-13 were significantly higher in both patients with pure AUD and those with pure MD compared to patients with a combination of both. There was no significant correlation between the levels of any of the cytokines measured and the duration of AUD illness.
Demographics and baseline parameters
There were significantly more women in the MD group than in the groups AUD and MD + AUD. These differences are consistent with literature data. Adult women are about twice as likely to be diagnosed with major depression as men (Kessler Citation2003) and the gender difference continues in subjects of 60 year and older (Girgus et al. Citation2017). In this meta-analysis of Girgus et al. (Citation2017), a small but significant difference in symptomatology between male and female depressed patients became evident. Men with depression were more likely to report alcohol/drug abuse and risk taking/poor impulse control, while depressed women reported more intensely depressed mood, appetite disturbance/weight change, and sleep disorders (Cavanagh et al. Citation2017). The large number of men in the group comorbidity MD + AUD is explained by the fact that patients were predominantly recruited from the department of addictive disorders. Relevant gender differences were also observed in a longitudinal study of 12–21 year old scholars (Marmorstein Citation2009). The largest association has been found between alcohol use and symptoms of depression in adolescent girls, but gender inequality decreases with age and levels off in adulthood. Evidence suggests that AUD increases the risk of MD, rather than vice versa which is probably attributable to pharmacological and metabolic changes as a result of long-term alcohol exposure (Boden and Fergusson Citation2011). In our study, dysthymia was the most common MD associated with AUD. However, among individuals of an European population with AUD, nearly 33% met the criteria for major depressive disorder, and only 11% - dysthymia (Grant et al. Citation2004; McHugh and Weiss Citation2019). However, in the aforementioned study, meeting during the preceding 12 months the criteria of the fourth edition of the diagnostic and statistical manual of mental disorders (DSM-IV) (American Psychiatric Association Citation1994) is applied much more strictly, resulting in a much lower prevalence of dysthymia than in our case. In addition, the ICD-10 diagnostic criteria for dysthymia may allow including probable cases of "double depression”, when dysthymia was in fact a manifestation of "underrecovery" from a previous depressive episode.
Cytokines in MD
In MD patients, several pro-inflammatory cytokines were higher (IFN-2α, IL-6 and IL-7) or lower (IL-1β and TNFα) than in the control group. At the same time, the level of anti-inflammatory cytokines such as IL-1RA were reduced compared to healthy individuals. The imbalance between pro- and anti-inflammatory cytokines in patients with depressive disorder is supported by the results of other studies (Song et al. Citation2009; Pandey et al. Citation2018). In the meta-analysis of Dowlati et al. (Citation2010) who included data from 24 studies measuring TNFα, IL-1β, IL-6, IL-4, IL-2, IL-8, IL-10, and IFN-γ in patients with major depression, only the levels of TNFα and IL-6 were significantly higher in depressed subjects in comparison to healthy control subjects. However, in a more recent meta-analysis of 82 studies by Köhler et al. (Citation2017) the peripheral levels of IL-6, TNF⍺, IL-10, IL-13, and IL-1RA were significantly higher in depressed patients in comparison to healthy individuals, while the levels of IL-1β, and transforming growth factor-beta 1 were not significantly different. Another meta-analysis showed that treatment with antidepressants significantly reduced TNFα levels only in responders, and that responders had significantly lower TNFα levels than nonresponders (Liu et al. Citation2020). For an overview of the various findings, please refer to Petralia et al. (Citation2020). We should definitely consider that our results may be biased by the nosological heterogeneity of our MD patient population. IL-1β has been considered as a marker of resistant depression (Uint et al. Citation2019), the decrease in this cytokine in our study may be due to the fact that almost all patients responded well to therapy. In the development of depression, a role has recently been attributed to activation of both the inflammatory response system (IRS) and the Compensatory Immune Response System (CIRS) (Debnath et al. Citation2021). Here, an important role must be assigned to neuroglia (Jia et al. Citation2021), which, incidentally, is probably mediated in part through astrocytes (Dossi et al. Citation2018; Petralia et al. Citation2020; Jia et al. Citation2021).
Cytokines in AUD
In our patients with AUD, we measured significantly elevated levels of IFN-2α, IL-2 IL-7, IL-8 and IL-4 and decreased levels of IL-12p40 and IL-1RA in comparison to the levels in healthy individuals. Although the elevated levels of pro-inflammatory cytokines TNFα and IL-6 had a particularly large effect size in such patient groups (next to IL-7 and IL-8) (Adams et al. Citation2020), we did not observe significant differences for these cytokines between subjects with AUD and healthy persons. The relationship between the use of alcohol in AUD and activation of the immune system is rather complex as alcohol has several peripheral effects that may play a role. This may include influencing the intestinal epithelium to increase permeability to bacterial products, which may then penetrate into the general circulation (Leclercq et al. Citation2014). In addition, alcohol has toxic effects in various organ systems (liver, heart, lungs) where the cellular damage may result in immune activation, which incidentally may itself also be a mechanism of the damage (Crews et al. Citation2006; Fujimoto et al. Citation2000). In addition, alcohol has an acute effect on the levels of interleukins (increase IL-8 and decrease TNF⍺) (Hillmer et al. Citation2020). In experiments in mice, it has been demonstrated that alcohol consumption contributes to a decrease in proinflammatory function (Starkenburg et al. Citation2001) and this does correlate with the higher risk of infection of people with AUD (Szabo and Mandrekar Citation2009). Altogether, many possibilities exist for a peripheral mechanism for the cytokine changes in AUD, which may obscure a possible cerebral component.
Cytokines in MD + AUD
Patients in the MD + AUD group demonstrated elevated IFN-2α and IL-8 levels and decreased IL-1RA and IL-10 levels in comparison to healthy individuals. In hepatitis C patients, treatment with IFN-2α often leads to the development of depression (Udina et al. Citation2012; Machado et al. Citation2017). The mechanism is not entirely known, but has often been associated with impaired serotonergic neurotransmission (Schaefer et al. Citation2003; Prather et al. Citation2009; Hoyo-Becerra et al. Citation2014). The prophylactic efficacy of selective serotonin reuptake inhibitors (SSRIs) has been sufficiently proven (Baraldi et al. Citation2012; Jiang et al. Citation2014; Ehret and Sobieraj Citation2014; Sarkar and Schaefer Citation2014). In any case, it has been found that depression due to treatment with INF⍺ is primarily related to biological factors and less or not psychological factors related to neuroticisms (Małyszczak et al. Citation2019). IL-6 also appears to play a role in susceptibility to IFN⍺-induced depression (Cassidy et al. Citation2002; Prather et al. Citation2009; Udina et al. Citation2012; Machado et al. Citation2017). In alcohol-dependent-subjects IL-10 level was negatively associated with depression, anxiety and alcohol-craving (Leclercq et al. Citation2012).
In our study patients with MD + AUD showed a decreased level of IL-1, IL-2, IL-7, IL-4 and IL-13 in comparison to the AUD group (). These findings differ from those of Neupane and colleagues (Neupane et al. Citation2014) who found higher levels of the IL-6, TNF and IFN-γ in Nepalese AUD patients with (a history of) MD than individuals without MD. Because the differences were greatest in individuals who drank little, these authors suggest that frequent and intense drinking may attenuate the difference. This may also be a reason for the discrepancy with our results. This fact may also explain the previously mentioned not increased levels of TNFα and IL-6 in our population with AUD.
In the study of Archer et al. (Citation2019) patients with major depressive disorder (MDD) and AUD showed higher levels of IL-6, IL-8, high sensitivity C-reactive protein (hs-CRP), and Chitinase-3-like protein 1 (YKL-40 or CHI3L1), than in MDD patients without AUD. We obtained opposite results for IL-6, but used an entirely different design. In our study we found increased IL-6, IL-7 and IL-13 levels, and decreased TNFα, IL-1RA and IL-10 levels in the MD group in comparison to the MD + AUD group ().
Possible involvement of dorsal diencephalic connection system (habenula)
In recent years we have become very interested in the possible role of the evolutionarily very old dorsal diencephalic connection (DDC) system that allows the forebrain to influence the midbrain via the habenuloid complex (Loonen and Ivanova Citation2018). The habenula's connections, well preserved during evolution, to the dopaminergic, serotonergic, and (indirectly) adrenergic centres in the midbrain regulate the activity of these ascending monoaminergic pathways and thus play an important role in depression and addiction (Loonen and Ivanova Citation2016b; Batalla et al. Citation2017). Within this system, the interaction between microglia, astrocytes and neurons may play an important role (Zhao et al. Citation2018; Guan et al. Citation2020). It has been suggested that the release of cytokines from microglia and other immunocompetent cells in the lateral habenula results in remodelling of the extracellular matrix – and therefore modulating the function – of this structure (Ito et al. Citation2021). The majority of the habenuloid output of the habenula to the midbrain is glutamatergic, either primary or as a co-transmitter with, for example, acetylcholine (Antolin-Fontes et al. Citation2015). It is tempting to speculate about what role matrix changes might play in bringing about a shift in the neurotransmitter output of the habenula.
Possible future avenues of cytokines study
Given the undoubted role of immune system dysregulation in the pathophysiology of affective disorders and addictions, we suggest the need for an integrated approach to study the mechanisms of the influence of alcohol on mood disorders. Genes encoding cytokines are highly polymorphic and data on the existence of a genetic profile associated with pro-inflammatory cytokines in patients suffering from mood disorders and genes involved in innate immunity in addicted people are described (Clerici et al. Citation2009; Crews Citation2012). Stress events are also associated with activation of the inflammatory immune system as well as changes in inflammatory cytokines in the brain (Audet et al. Citation2014). Transcriptome profiling enables has revealed involvement of the brain’s neuroimmune system in regulating alcohol abuse and dependence as well as psychiatric disorders (Warden et al. Citation2016; Chen et al. Citation2022). Combining the results of studies of the above approaches with the measurement of the serum cytokine profile and immunophenotyping, in our opinion, may be promising in further studies. At the same time, complex research requires more serious approaches to data processing. Modern deep and machine learning methods can help interpret complex relationships between clinical and biological parameters (Durstewitz et al. Citation2019). For example, the new nomothetic network psychiatry approach, which uses machine learning methods to build data-driven causal models of mental illness by assembling risk-resilience, adverse outcome pathways, cognitome, brainome, staging, symptomatome, and phenomenome latent scores in a causal model (Stoyanov and Maes Citation2021).
Given the wide variability in cytokine levels from various external and internal factors, it is promising to comprehensively study the genetic, transcriptomic and proteomic information related to cytokines. Also, future lines of research should focus on the application of sophisticated analysis methods such as machine learning and deep learning to understand the complex mechanisms of interaction between psychiatric diseases and the immune system.
Limitations
The main limitations of our research are the discrepancy between the studied groups in terms of gender and structure of MD. However, since our study design was cross-sectional, this reflects clinical reality. It seems inappropriate to us to stratify for gender or diagnostic category of mood disorders in an exploratory study, as there may be an interaction between these variables and possibly causal activation of the immune system. Another important limitation of our study is that our findings regarding TNFα and IL-6 in individuals with AUD are not consistent with literature data.
Conclusion
Although both depressive mood disorders and alcohol-related disorders involve changes within the immune system, it is extremely difficult to get a grip on the biological mechanisms involved. Because alcohol exhibits a broad palette of cerebral and peripheral effects, it is difficult to work out how changes in serum levels of cytokines relate to the biological changes that determine how alcohol causes mental disorders characterised by mood change or alcohol-seeking behaviour. Therefore, even with our study, we cannot reach unequivocal conclusions. Hence, in our opinion, it is better to focus this type of research on a well-established influence of certain cytokines on the functioning of specific intracerebral circuits found in other types of research. Promising, in our opinion, is a comprehensive assessment of the dysregulation of the immune system in affective and addictive diseases using machine and deep learning methods. We do think that our findings, together with the results of others, may provide an impetus to look with greater emphasis at the interaction of the immune system with the functioning of the basic behavioural regulatory dorsal diencephalic connection system. Then the results of our study can be reinterpreted and more successfully.
Supplementary Tables
Download MS Word (27.9 KB)Acknowledgements
Dutch universities have suspended all forms of cooperation with Russian educational and research institutions due to the conflict with Russia over Ukraine. The Board of the Faculty of Science and Engineering of the University of Groningen exempted the manuscript of this article because the data collection was already completed and the results were described in this manuscript.
Disclosure statement
The authors report there are no competing interests to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Data availability statement
The datasets generated for this study will not be made publicly available, but they are available on reasonable request to Svetlana A. Ivanova ([email protected]), following approval of the Board of Directors of the MHRI, in line with local guidelines and regulations.
Additional information
Funding
References
- Adams C, Conigrave JH, Lewohl J, Haber P, Morley KC. 2020. Alcohol use disorder and circulating cytokines: a systematic review and meta-analysis. Brain Behav Immun. 89:501–512. 10.1016/j.bbi.2020.08.002.
- American Psychiatric Association 1994. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington (DC): American Psychiatric Association.
- Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I. 2015. The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology. 96(Pt B):213–222.
- Archer M, Niemelä O, Luoto K, Kultti J, Hämäläinen M, Moilanen E, Koivukangas A, Leinonen E, Kampman O. 2019. Status of inflammation and alcohol use in a 6-month follow-up study of patients with major depressive disorder. Alcohol. 81:21–26.
- Audet MC, McQuaid RJ, Merali Z, Anisman H. 2014. Cytokine variations and mood disorders: influence of social stressors and social support. Front Neurosci. 8:416.
- Baraldi S, Hepgul N, Mondelli V, Pariante CM. 2012. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 32(4):531–543.
- Batalla A, Homberg JR, Lipina TV, Sescousse G, Luijten M, Ivanova SA, Schellekens AFA, Loonen AJM. 2017. The role of the habenula in the transition from reward to misery in substance use and mood disorders. Neurosci Biobehav Rev. 80:276–285.
- Boden JM, Fergusson DM. 2011. Alcohol and depression. Addiction. 106(5):906–914.
- Cassidy EM, Manning D, Byrne S, Bolger E, Murray F, Sharifi N, Wallace E, Keogan M, O'Keane V. 2002. Acute effects of low-dose interferon-alpha on serum cortisol and plasma interleukin-6. J Psychopharmacol. 16(3):230–234.
- Cavanagh A, Wilson CJ, Kavanagh DJ, Caputi P. 2017. Differences in the expression of symptoms in men versus women with depression: a systematic review and meta-analysis. Harv Rev Psychiatry. 25(1):29–38.
- Chen Y, Dai J, Liang Q, Tang L, Mikhailova T, Li M, Liu C. 2022. The neuroimmune changes captured by transcriptome in brains of psychiatric and neurological disorders. medRxiv. 2022.02.14.22269692.
- Clerici M, Arosio B, Mundo E, Cattaneo E, Pozzoli S, Dell'Osso B, Vergani C, Trabattoni D, Altamura AC. 2009. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectr. 14(8):419–425.
- Costello EJ, Copeland WE, Shanahan L, Worthman CM, Angold A. 2013. C-reactive protein and substance use disorders in adolescence and early adulthood: a prospective analysis. Drug Alcohol Depend. 133(2):712–717.
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. 2006. Cytokines and alcohol. Alcohol Clin Exp Res. 30(4):720–730.
- Crews FT. 2012. Immune function genes, genetics, and the neurobiology of addiction. Alcohol Res: Curr Rev. 34(3):355.
- Debnath M, Berk M, Maes M. 2021. Translational evidence for the inflammatory response system (IRS)/compensatory immune response system (CIRS) and neuroprogression theory of major depression. Prog Neuropsychopharmacol Biol Psychiatry. 111:110343.
- Dossi E, Vasile F, Rouach N. 2018. Human astrocytes in the diseased brain. Brain Res Bull. 136:139–156.
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry. 67(5):446–457.
- Durstewitz D, Koppe G, Meyer-Lindenberg A. 2019. Deep neural networks in psychiatry. Mol Psychiatry. 24(11):1583–1598.
- Ehret M, Sobieraj DM. 2014. Prevention of interferon-alpha-associated depression with antidepressant medications in patients with hepatitis C virus: a systematic review and meta-analysis. Int J Clin Pract. 68(2):255–261.
- Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, et al. 2000. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 24(4 Suppl):48S–54S.
- Girgus JS, Yang K, Ferri CV. 2017. The gender difference in depression: are elderly women at greater risk for depression than elderly men? Geriatrics (Basel). 2(4):35.
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. 2004. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 61(8):807–816.
- Guan YF, Huang GB, Xu MD, Gao F, Lin S, Huang J, Wang J, Li YQ, Wu CH, Yao S, et al. 2020. Anti-depression effects of ketogenic diet are mediated via the restoration of microglial activation and neuronal excitability in the lateral habenula. Brain Behav Immun. 88:748–762.
- Hillmer AT, Nadim H, Devine L, Jatlow P, O'Malley SS. 2020. Acute alcohol consumption alters the peripheral cytokines IL-8 and TNF-α. Alcohol. 85:95–99.
- Hoyo-Becerra C, Schlaak JF, Hermann DM. 2014. Insights from interferon-α-related depression for the pathogenesis of depression associated with inflammation. Brain Behav Immun. 42:222–231.
- Hunt GE, Malhi GS, Lai HMX, Cleary M. 2020. Prevalence of comorbid substance use in major depressive disorder in community and clinical settings, 1990–2019: systematic review and meta-analysis. J Affect Disord. 266:288–304.
- Ito H, Nozaki K, Sakimura K, Abe M, Yamawaki S, Aizawa H. 2021. Activation of proprotein convertase in the mouse habenula causes depressive-like behaviors through remodeling of extracellular matrix. Neuropsychopharmacology. 46(2):442–454.
- Jia X, Gao Z, Hu H. 2021. Microglia in depression: current perspectives. Sci China Life Sci. 64(6):911–925.
- Jiang HY, Deng M, Zhang YH, Chen HZ, Chen Q, Ruan B. 2014. Specific serotonin reuptake inhibitors prevent interferon-α-induced depression in patients with hepatitis C: a meta-analysis. Clin Gastroenterol Hepatol. 12(9):1452–1460.e3.
- Kessler RC. 2003. Epidemiology of women and depression. J Affect Disord. 74(1):5–13.
- Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, et al. 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 135(5):373–387.
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. 2000. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 22(4):370–379. 10.1016/S0893-133X(99)00134-7.
- Leclercq S, Cani PD, Neyrinck AM, Stärkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P. 2012. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 26(6):911–918.
- Leclercq S, De Saeger C, Delzenne N, de Timary P, Stärkel P. 2014. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry. 76(9):725–733.
- Levchuk LA, Meeder EMG, Roschina OV, Loonen AJM, Boiko AS, Michalitskaya EV, Epimakhova EV, Losenkov IS, Simutkin GG, Bokhan NA, et al. 2020. Exploring brain derived neurotrophic factor and cell adhesion molecules as biomarkers for the transdiagnostic symptom anhedonia in alcohol use disorder and comorbid depression. Front Psychiatry. 11:296.
- Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, Que J, Gadad BS, Trivedi MH, Kelsoe JR, et al. 2020. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. 25(2):339–350.
- Loonen AJM, Ivanova SA. 2016a. Circuits regulating pleasure and happiness-mechanisms of depression. Front Hum Neurosci. 10:571.
- Loonen AJM, Ivanova SA. 2016b. Circuits regulating pleasure and happiness in major depression. Med Hypotheses. 87:14–21.
- Loonen AJM, Ivanova SA. 2018. Circuits regulating pleasure and happiness: evolution and role in mental disorders. Acta Neuropsychiatr. 30(1):29–42.
- Machado MO, Oriolo G, Bortolato B, Köhler CA, Maes M, Solmi M, Grande I, Martín-Santos R, Vieta E, Carvalho AF. 2017. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: a critical systematic review. J Affect Disord. 209:235–245.
- Małyszczak K, Inglot M, Frydecka D, Hadryś T, Pawłowski T. 2019. Biological and psychological components of depression in patients receiving IFN-alpha therapy for hepatitis C. Adv Clin Exp Med. 28(9):1217–1222.
- Marmorstein NR. 2009. Longitudinal associations between alcohol problems and depressive symptoms: early adolescence through early adulthood. Alcohol Clin Exp Res. 33(1):49–59.
- Martinez P, Lien L, Zemore S, Bramness JG, Neupane SP. 2018. Circulating cytokine levels are associated with symptoms of depression and anxiety among people with alcohol and drug use disorders. J Neuroimmunol. 318:80–86.
- McHugh RK, Weiss RD. 2019. Alcohol use disorder and depressive disorders. ARCR. 40(1):arcr.v40.1.01.
- Miller AH, Maletic V, Raison CL. 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 65(9):732–741.
- Neupane SP, Lien L, Martinez P, Aukrust P, Ueland T, Mollnes TE, Hestad K, Bramness JG. 2014. High frequency and intensity of drinking may attenuate increased inflammatory cytokine levels of major depression in alcohol-use disorders. CNS Neurosci Ther. 20(10):898–904.
- Neupane SP. 2016. Neuroimmune interface in the comorbidity between alcohol use disorder and major depression. Front Immunol. 7:655.
- Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X. 2018. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J Psychiatry Neurosci. 43(6):376–385.
- Petralia MC, Mazzon E, Fagone P, Basile MS, Lenzo V, Quattropani MC, Di Nuovo S, Bendtzen K, Nicoletti F. 2020. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun Rev. 19(5):102504.
- Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. 2009. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 23(8):1109–1116.
- Rehm J, Shield KD. 2019. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 21(2):10.
- Saha S, Lim CC, Degenhardt L, Cannon DL, Bremner M, Prentis F, Lawrence Z, Heffernan E, Meurk C, Reilly J, et al. 2022. Comorbidity between mood and substance-related disorders: a systematic review and meta-analysis. Aust N Z J Psychiatry. 5648674211054740. (7):757–770.
- Sarkar S, Schaefer M. 2014. Antidepressant pretreatment for the prevention of interferon alfa-associated depression: a systematic review and meta-analysis. Psychosomatics. 55(3):221–234.
- Schaefer M, Schwaiger M, Pich M, Lieb K, Heinz A. 2003. Neurotransmitter changes by interferon-alpha and therapeutic implications. Pharmacopsychiatry. 36(Suppl 3):S203–S206.
- Song C, Halbreich U, Han C, Leonard BE, Luo H. 2009. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 42(5):182–188.
- Starkenburg S, Munroe ME, Waltenbaugh C. 2001. Early alteration in leukocyte populations and Th1/Th2 function in ethanol-consuming mice. Alcohol Clin Exp Res. 25(8):1221–1230.
- Stoyanov D, Maes MH. 2021. How to construct neuroscience-informed psychiatric classification? Towards nomothetic networks psychiatry. World J Psychiatry. 11(1):1–12.
- Sullivan LE, Fiellin DA, O'Connor PG. 2005. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 118(4):330–341.
- Szabo G, Mandrekar P. 2009. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 33(2):220–232.
- Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Langohr K, Solà R, Vieta E, Martín-Santos R. 2012. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 73(8):1128–1138.
- Uint L, Bastos GM, Thurow HS, Borges JB, Hirata TDC, França JID, Hirata MH, Sousa AGMR. 2019. Increased levels of plasma IL-1b and BDNF can predict resistant depression patients. Rev Assoc Med Bras. (1992). 65(3):361–369.
- Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Dwork AJ, Mann JJ, Arango V. 2018. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl Psychiatry. 8(1):279.
- Vasilieva SN, Simutkin GG, Schastnyy ED, Bokhan NA. 2021. Clinical-dynamic features of affective disorders comorbid with alcohol dependence. Int J Ment Health Addiction. 19(5):1443–1451.
- Walther A, Rice T, Kufert Y, Ehlert U. 2016. Neuroendocrinology of a male-specific pattern for depression linked to alcohol use disorder and suicidal behavior. Front Psychiatry. 7:206. 2017
- Warden A, Erickson E, Robinson G, Harris RA, Mayfield RD. 2016. The neuroimmune transcriptome and alcohol dependence: potential for targeted therapies. Pharmacogenomics. 17(18):2081–2096.
- World Health Organization 2004. International statistical classification of diseases and health related problems ICD-10. World Health Organization: Geneva (CH).
- Zhao YW, Pan YQ, Tang MM, Lin WJ. 2018. Blocking p38 signaling reduces the activation of pro-inflammatory cytokines and the phosphorylation of p38 in the habenula and reverses depressive-like behaviors induced by neuroinflammation. Front Pharmacol. 9:511.