Abstract
Objectives
The primary objectives of these international guidelines were to provide a global audience of clinicians with (a) a series of evidence-based recommendations for the provision of lifestyle-based mental health care in clinical practice for adults with Major Depressive Disorder (MDD) and (b) a series of implementation considerations that may be applicable across a range of settings.
Methods
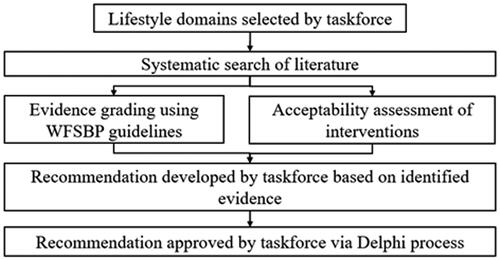
Recommendations and associated evidence-based gradings were based on a series of systematic literature searches of published research as well as the clinical expertise of taskforce members. The focus of the guidelines was eight lifestyle domains: physical activity and exercise, smoking cessation, work-directed interventions, mindfulness-based and stress management therapies, diet, sleep, loneliness and social support, and green space interaction. The following electronic bibliographic databases were searched for articles published prior to June 2020: PubMed, EMBASE, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register), CINAHL, PsycINFO. Evidence grading was based on the level of evidence specific to MDD and risk of bias, in accordance with the World Federation of Societies for Biological Psychiatry criteria.
Results
Nine recommendations were formed. The recommendations with the highest ratings to improve MDD were the use of physical activity and exercise, relaxation techniques, work-directed interventions, sleep, and mindfulness-based therapies (Grade 2). Interventions related to diet and green space were recommended, but with a lower strength of evidence (Grade 3). Recommendations regarding smoking cessation and loneliness and social support were based on expert opinion. Key implementation considerations included the need for input from allied health professionals and support networks to implement this type of approach, the importance of partnering such recommendations with behaviour change support, and the need to deliver interventions using a biopsychosocial-cultural framework.
Conclusions
Lifestyle-based interventions are recommended as a foundational component of mental health care in clinical practice for adults with Major Depressive Disorder, where other evidence-based therapies can be added or used in combination. The findings and recommendations of these guidelines support the need for further research to address existing gaps in efficacy and implementation research, especially for emerging lifestyle-based approaches (e.g. green space, loneliness and social support interventions) where data are limited. Further work is also needed to develop innovative approaches for delivery and models of care, and to support the training of health professionals regarding lifestyle-based mental health care.
1. Executive summary of recommendations
Lifestyle-based mental health care includes the assessment and intervention of the lifestyle determinants of health in the prevention, recovery and treatment of mental disorders. Both Royal Australian & New Zealand Royal College of Psychiatrists (RANZCP) (Malhi et al. Citation2021) and draft National Institutes of Health and Care Excellence (NICE) (National Institute for Health and Care Excellence Citation2022) guidelines endorse lifestyle-based approaches as important aspects of depression management. These recommendations are presented as a foundational component of care that can be used in combination with other evidence-based therapies.
Focussing on the management of Major Depressive Disorder (MDD) in adults, the current guidelines endorse this position and build on it by: (1) systematically reviewing evidence for the clinical application of specific lifestyle-based approaches in this clinical population; (2) expanding upon the four aforementioned lifestyle factors to include emerging lifestyle targets of loneliness and social support, mindfulness-based therapies and stress management, green space interaction, work-directed interventions, and; (3) providing a series of implementation considerations that may be applied across a range of settings that are applicable to a global audience. Based on available scientific evidence and supplemented with expert consensus, nine recommendations are proposed in .
Table 1. Summary of recommendations.
For all the target lifestyle behaviours recommended by the guidelines, effectiveness will be maximised when delivered in conjunction with behaviour change techniques that are appropriate for the person and their circumstances.
Informed by the evidence-base that supports these recommendations, a series of additional recommendations are provided for future research into lifestyle-based approaches to strengthen the current evidence and to inform translation and implementation into clinical settings. These include the need for utilising lessons from the field of implementation science, novel effectiveness and non-inferiority study designs, cost-effectiveness considerations, greater understanding of the optimal delivery methods, and identifying mechanisms of action.
Finally, a series of considerations are provided to assist clinicians with implementation of these recommendations, regardless of clinical setting. These include highlighting our position that lifestyle-based approaches should be considered a core component of mental health care; recognising the benefits of input from allied health professionals; engaging support networks into the delivery of the interventions; recognising the need for formal assessment of social needs; screening for substance and alcohol use; and incorporating culturally sensitive approaches and self-management strategies into the delivery of the lifestyle interventions. provides a visual summary of how these implementation considerations and recommendations sit within a continuum of care.
Figure 1. Conceptual framework for lifestyle-based mental health care. A proposed clinical flowchart for lifestyle-based mental health care using a 4 A's (Assess, Advise, Assist, Arrange) structure. For the online/colour version of this figure, each lifestyle intervention is colour coded for grade of evidence (dark green = grade B, light green = grade C, yellow = expert opinion).
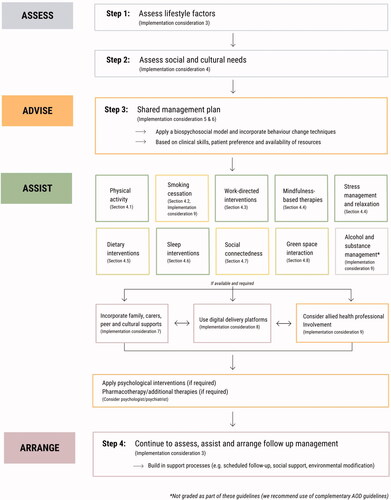
The evidence on which these guidelines are based supports the application of lifestyle-based mental health care as part of broader biopsychosocial-cultural management of MDD. Lifestyle-based mental health care is generally considered safe for most individuals (with generally low incidence of side-effects and major adverse events) when delivered alone or in conjunction with established therapies and has the potential to be provided at relatively low cost across a range of settings to adults with diverse clinical and demographic characteristics. Benefits of lifestyle-based approaches for MDD may extend to physical health outcomes (particularly cardiovascular, metabolic, and respiratory disease risk). The findings and recommendations of these guidelines encourage further research in this area, especially for those emerging lifestyle-based approaches where data are currently limited (e.g. green space, loneliness and social support interventions); greater education of health professionals regarding lifestyle-based approaches; and support for innovative approaches for the delivery of new integrative models of care for people with MDD.
2. Introduction
2.1. Rationale
Major Depressive Disorder (MDD) is a leading cause of global disability and is one of the leading causes of disease burden worldwide (GBD Mental Disorders Collaborators Citation2022). MDD is common, with approximately 4.7% of the world’s population experiencing depression in any 12-month period (Ferrari et al. Citation2013). The prevalence of MDD is also consistent across high, middle, and low income countries, emphasising the global burden of this disease (World Health Organization Citation2017). Pharmacological and psychological approaches are effective for MDD management (Leichsenring et al. Citation2022). However, meta-analyses suggest that both of these forms of therapy may have only modest benefits and are not effective for everyone for reducing depressive symptoms (Leichsenring et al. Citation2022). Moreover, antidepressant medications may be accompanied by undesirable side-effects including sexual dysfunction, sedation, cardiac dysfunction, osteoporosis, and weight gain, which may reduce treatment efficacy and diminish long-term adherence (Carvalho et al. Citation2016). Furthermore, financial and resourcing barriers to accessing mental health services are notable especially in low- and middle-income countries where there is a high prevalence of stigma to mental health care (Herrman et al. Citation2022).
Consequently, there has been considerable research and clinical interest in the role of lifestyle-based approaches for the management of mental illness. Lifestyle-based approaches can be defined as ‘the application of environmental, behavioural and motivational principles, including self-care and self-management, to the management of lifestyle-related health problems in a clinical setting’ (Sagner et al. Citation2017). This approach may present several key benefits to other approaches as they are generally considered low risk with respect to causing adverse events. Furthermore, due to the high morbidity and mortality risk associated with MDD and other mental disorders (Machado et al. Citation2018), this approach may offer a dual benefit, addressing clinical symptoms of MDD while potentially mitigating physical comorbidities – a recognised challenge for those with mental illness (Firth et al. Citation2019b).
Despite the promise and relatively low risk of using lifestyle-based mental health care, to our knowledge, there are no available clinical practice guidelines that grade the evidence for established and emerging lifestyle interventions by which to assist clinicians in providing this type of care. These guidelines are intended to address this key gap in our knowledge and to serve as an aid to clinicians. This document is intended to serve a global audience and it is acknowledged that implementation will vary across service delivery, disciplines, jurisdictional, country and regional contexts. Ultimately, lifestyle-based mental health care looks different across settings with varying resources.
2.2. Guideline objectives
A primary objective for writing these guidelines was to evaluate lifestyle-based mental health care using the best available evidence (section 4). In this document, we present the evidence and gradings before providing further discussion on clinically useful application strategies within the identified lifestyle interventions. Further, we provide an overview of the key gaps in the current evidence (section 5) and dedicate a section on implementation considerations related to contextual and practical elements of using lifestyle-based interventions for optimal mental health care (section 6).
2.3. Scope of guidelines
Using the PICO format (Population, Intervention, Comparator, Outcome), the guidelines were designed to cover the following scope.
2.3.1. Population
We acknowledge that language matters in the provision of care and in creating person-centered care for those with lived experience of mental illness. Moreover, that this language can change in its meaning or appropriateness across setting, culture, discipline and context (e.g. consumer, patient, client, service user, person). These guidelines pertain to people with current experience with a major depressive disorder, henceforth referred to as people with MDD. We recognise a move away from conventional and formal psychiatric diagnoses in mental health care, especially in clinical application for which a more transdiagnostic approach can be better suited. However, while the clinical considerations may be relevant to those with sub-clinical depression, other related mood disorders (e.g. bipolar disorder, cyclothymic disorder, or peri-partum onset depression), or depression co-occurring with anxiety, the target population was specifically people with MDD as distinct from subthreshold depressive illness or variations of mood disorders as specified in DSM-5.
2.3.2. Interventions
Although there is a wide range of interventions that may be considered as lifestyle-based mental health care, (Egger Citation2019) to ensure a feasible scope of work, these guidelines were restricted to the following approaches:
Physical activity and exercise
Smoking cessation
Work-directed interventions
Mindfulness-based therapies and stress management (including relaxation techniques and coping skills training)
Diet
Sleep
Loneliness and social support
Green space interaction
For clarity, we reviewed the eight selected lifestyle domains separately, while acknowledging that in many cases, behavioural changes recommended for people with MDD in any one domain (e.g. strategies to increase physical activity) may also have demonstrable effects in another (e.g. sleep or social connectedness). Furthermore, there are challenges in trying to artificially categorise certain lifestyle behaviours that span multiple domains under just one domain. For example, yoga is a diverse group of practices that can include exercise, stress management, relaxation, contemplative practices and breathing techniques. It is also the case that there is overlap in what constitutes some lifestyle-based approaches and those that may be used as part of psychological-based practices (e.g. mindfulness). For this activity, we consider the content as being lifestyle-based in nature, thus eligible for inclusion in these guidelines. It was considered beyond the scope of this document to include other evidence-based techniques used as part of psychological practices. We specifically excluded cessation interventions related to alcohol and illicit drugs given there are existing substance use disorder management guidelines that can be consulted for those with co-occurring mental illness (see Marel et al. Citation2016); however, this area is discussed in Section 6. We also focussed the scope of the recommendations on interventions that targeted the lifestyle factor rather than the effect of environmental and/or lifestyle factors (e.g. environmental pollution, social media use). Finally, we focussed on clinical, rather than population level interventions.
2.3.3. Comparator
Studies were not excluded based on the comparator used.
2.3.4. Outcome
The key outcome of interest was reductions in depressive symptoms of people with MDD. Other important targets of MDD treatment in this population (e.g. relapse/recurrence, length of inpatient stay, quality of life or lifestyle behaviours) were beyond the present scope.
2.4. Target audience
The document is intended for any health professional who may diagnose and/or who is part of a team providing care for adults with MDD, including allied or generalist health professionals, as well as community rehabilitation and psychosocial or peer support workers working directly with people with MDD. All recommendations should be considered with the interests, preferences and circumstances of the individual in mind and within the available clinical context with consideration of current training, expertise, and interest, of the clinician as well as the availability of related health professionals and relevant resources (Malhi et al. Citation2021).
2.5. Financial disclosure and conflicts of interest
Individual funding for each study author is included at the end of the manuscript. No funding body had any input into the design or conduct of the guidelines. Potential conflicts of interest for all taskforce members were compiled at the initiation of the guideline taskforce and declared in the relevant section of this manuscript.
3. Methods
3.1. General methods and literature search
In 2019, an internationally representative taskforce of researchers, clinicians and lived experience experts was formed and endorsed by the World Federation of Societies of Biological Psychiatry (WFSBP) and the Australasian Society of Lifestyle Medicine (ASLM). This taskforce was composed of members from nine different countries (across the Asia-Pacific, North and South America, Europe, and Africa), with representation from high-, mid-, and low-income countries. The development of these guidelines is in line with the recommendations of the WFSBP guidelines development document (see ) (Hasan et al. Citation2019).
3.2. Supporting evidence
Guideline recommendations were generated based on a series of systematic literature searches of published peer-reviewed research for each lifestyle domain as well the clinical and research expertise of the taskforce members.
3.2.1. Literature search
We searched the following electronic bibliographic databases: Pubmed, EMBASE, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register), CINAHL, PsycINFO. Search terms are included in Supplementary material. Only studies published in English were eligible for inclusion. Studies published since journal inception to June 2020 were sought. Additional eligible literature that was published after this date and that was identified by members of the taskforce was also included.
3.2.2. Eligibility criteria
Eligibility criteria were in line with the details provided in section 2.3. Although there were additional studies that evaluated the use of lifestyle-based approaches in people with sub-syndromal depression and healthy populations, and/or measured related outcomes such as stress and quality of life, these were considered beyond the scope of this work.
3.2.2.1. Types of studies included
Due to the varied level of available evidence for the included interventions, a stepwise approach was used to synthesise relevant data using the Australian National Health & Medical Research Council Evidence Hierarchy (National Health and Medical Research Council Citation2009): Initially, the search results were screened for systematic reviews and meta-analyses; if available, these data formed the basis of the guideline recommendations for that intervention. In cases where no systematic reviews and meta-analyses available, the search was expanded to randomised clinical trials (RCTs) and then non-randomised trials.
3.2.2.2. Data extraction
Titles and/or abstracts of studies retrieved using the search strategy and those from additional sources were screened independently by two reviewers to identify studies potentially meeting the inclusion criteria outlined above.
Full texts of these potentially eligible studies were retrieved and independently assessed for eligibility by two team members. Any disagreement between them over the eligibility of studies was resolved through discussion with a third author.
The reported effect sizes were extracted from the included meta-analyses or individual studies that formed the basis of each recommendation. Each effect size was categorised as Small, Moderate or Large, using standard effect size (e.g. Cohen’s d) cut offs, (Cohen Citation2013) and reported within each clinical recommendations section. Although these effect sizes provide context for the magnitude of each intervention effect, they should be viewed with caution due to limited data on treatments (e.g. stand-alone versus adjunctive to other approaches) and small sample sizes and should not be used in isolation to guide preferential treatment selection.
3.3. Risk of bias assessment
Where risk of bias assessments had already been conducted (e.g. as part of the published systematic reviews), these assessments were extracted for use in these guidelines. Where risk of bias assessment was not previously conducted, risk of bias was assessed independently by two taskforce authors, with conflicting scores resolved first through discussion; if disagreements persisted, a third author provided final judgement. Risk of bias tools were used as below:
Systematic reviews and meta-analyses were assessed using the AMSTAR-2 checklist (Shea et al. Citation2017)
RCTs were assessed using the Cochrane Risk of Bias 2 tool (Sterne et al. Citation2019)
Non-randomised and quasi-experimental studies were assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Quasi-Experimental Studies (Tufanaru et al. Citation2017)
3.4. Grading of evidence and synthesis of Evidence-Based statements
The level of evidence and strength of recommendations were graded in accordance with the WFSBP guidelines (Hasan et al. Citation2019). In summary, supporting evidence was first graded to determine the level of evidence using the matrix detailed in . For these guidelines, we amended the grading criteria for meta-analyses to consider the risk of bias of the included individual studies as well as the risk of bias of the overall meta-analysis. Acceptability of an intervention was also assessed using the following factors:
Risk–benefit ratio (e.g. adverse effects, interactions)
Cost–benefit ratio
Applicability in the target population
Ethical and legal aspects
Preferences of service users
Practicability
As described elsewhere (Hasan et al. Citation2019), the grade of recommendations was based upon the amount and quality of evidence () in conjunction with the acceptability of the intervention, resulting in strong (Grade 1), limited (Grade 2), low (Grade 3), or no evidence (Grade 4) recommendation levels. To help aid translation of these recommendations into clinical practice, recommendations were phrased as action statements where ‘should’ indicates a strong strength of evidence, ‘could’ indicates a limited strength of evidence, and ‘may’ indicates a low strength of evidence.
3.5. Taskforce consensus process
A two-stage Delphi process was used to achieve consensus from the taskforce members about each guideline recommendation. In doing so, a set of draft recommendations were developed and provided to each taskforce member via an anonymous survey for review and endorsement. This feedback was then incorporated into a revised set of recommendations, which was again disseminated for review and endorsement by the taskforce. A recommendation was finalised when >80% consensus was achieved.
3.6. Future research needs, implementation and clinical considerations
To provide further context to the recommendations and to guide their implementation, we (1) provided a ‘Clinical Advice and Tips’ Box for each guideline, (2) identified key evidence gaps and future research needs (section 5), and (3) included a series of implementation considerations for lifestyle-based approaches (section 6). These sections, along with the background and Clinical Considerations section for each domain, do not follow the previously mentioned systematic review procedure and are based on the broader current literature regarding depression (e.g. studies in subthreshold depressive symptoms as well as those in MDD).
3.7. Guideline lifecycle
Subject to availability of funding and resourcing, the taskforce intends to update these guidelines every 5 years or when developments in the research literature or clinical management warrant an update, in line with the criteria by Rosenfeld et al. (Citation2013).
3.8. External review procedure
An extensive external review process was implemented to ensure that input and feedback from relevant stakeholders was incorporated into the development of the guideline recommendations. Stakeholders included researchers who have published in relevant fields, people with lived experience of MDD, cultural experts, and mental health clinicians. The draft guidelines were disseminated for external review from November to December 2021. Submissions were reviewed by the taskforce and, where appropriate, incorporated into the final guideline document.
4. Evidence-based guideline statements
In summary, nine recommendations were developed covering the eight specific lifestyle domains. The details of the evidence on which recommendations were made are presented by lifestyle domain in this section. provides an overview of the nine recommendations, of which five received Grade B strength of evidence, two received Grade C1, and two were based on expert opinion (Grade C3). To provide support for the use of these recommendations, the sections below also include domain-specific clinical considerations, tips and advice, and further resources. For general clinical advice that applies across all domains, see Box 1. For further context and discussion of the limitations of the recommendation process, please see section 5.
Table 2. Evidence Grading System as recommended by the WFSBP (Hasan et al. Citation2019).
4.1. Physical activity and exercise interventions
4.1.1. Background literature
Physical activity, defined as any bodily movement that requires energetic expenditure, and exercise, defined as structured physical activity that aims to maintain or improve physical fitness (Caspersen et al. Citation1985), have a bidirectional relationship with MDD (Blumenthal et al. Citation1999; Schuch et al. Citation2017; Vancampfort et al. Citation2017; Ashdown-Franks et al. Citation2020). Meta-analytic evidence demonstrates that, compared to the general population, people with MDD have reduced levels of physical activity and are less likely to achieve the public health recommendations of 150 min of moderate and vigorous physical activity per week. Similarly, inactive individuals are more likely to become depressed (Schuch et al. Citation2017; Vancampfort et al. Citation2017). Although MDD is likely to be associated with reduced physical activity, there is a growing body of evidence that supports exercise and physical activity as an intervention for reducing depressive symptoms and preventing the development of MDD or the worsening of depressive symptomatology (Blumenthal et al. Citation1999; Ashdown-Franks et al. Citation2020). A recent large scale Mendelian randomisation study found that higher levels of accelerometer-based activity were causally protective against MDD (Choi et al. Citation2019). This is consistent with meta-analyses of cohort studies (Schuch et al. Citation2018). Further, Mendelian randomisation research has demonstrated that people who are more active and are genetically predisposed to MDD are less likely to develop MDD than people of equal genetic risk for MDD and low physical activity levels (Choi KW et al. Citation2020). The mechanisms underlying the potential anti-depressant effect of exercise are complex and are not fully understood. The beneficial effects may include a combination of neurobiological mechanisms (e.g. stimulation of brain-derived neurotrophic factor (Kerling et al. Citation2017), reduced inflammation, stimulation of pre-frontal cortex and hippocampus (Lin K et al. Citation2020), including volumetric changes) and psychosocial factors (e.g. increased self-efficacy, social support, improved self-esteem) (Kandola et al. Citation2019).
In a series of randomised controlled trials, aerobic exercise has been shown to be as effective as antidepressant medication (e.g. Serotonin Reuptake Inhibitors [SSRIs] such as sertraline) in reducing depressive symptoms in adults with MDD (Blumenthal et al. Citation1999; Blumenthal et al. Citation2007) and in individuals with coronary disease and MDD or elevated depressive symptoms (Blumenthal et al. Citation2012). However, the presence of depression and co-morbid anxiety may attenuate the beneficial effects of exercise on depressive symptoms (Rebar et al. Citation2015; Blumenthal et al. Citation2021).
Studies have generally been successful in recruiting people with MDD to RCTs with dropout rates being, in some cases, less than 20% (Schuch, Vancampfort, Rosenbaum, et al. Citation2016; Stubbs et al. Citation2016; Krogh et al. Citation2017). This is comparable to dropout rates found in trials of exercise in non-depressed individuals and in those receiving antidepressant medications (Schuch, Vancampfort, Rosenbaum, et al. Citation2016; Stubbs et al. Citation2016; Krogh et al. Citation2017), and lower compared to usual care controls (Stubbs et al. Citation2016). In addition, the rate of adverse events appears to be low and no worse than antidepressant medications or to control conditions (Stubbs et al. Citation2018). Furthermore, meta-analyses of RCTs have found that multiple modes of exercise improves depressive symptoms in people with MDD, including aerobic exercise (e.g. running, rowing) (Schuch, Vancampfort, Richards, et al. Citation2016), resistance training (e.g. weight lifting), (Gordon et al. Citation2018) as well as a smaller evidence base that supports yoga (Brinsley et al. Citation2021) and pilates (Fleming and Herring Citation2018) (for formal definitions of different exercise modalities, see reference (Howley Citation2001)). Meta-analyses of RCTs also have demonstrated that exercise can have significant improvements on other psychological and behavioural functions including self-esteem, various aspects of quality of life, and sleep (Schuch et al. Citation2017; Lederman et al. Citation2019).
4.1.2. Clinical recommendations
4.1.3. Clinical considerations
4.1.3.1. Type and context of intervention
Contemporary clinical trials have largely focussed on aerobic exercise; however, there is growing evidence to suggest strength-based exercise or resistance training (e.g. weight lifting) may also improve depressive symptoms (Schuch, Vancampfort, Richards, et al. Citation2016; Gordon et al. Citation2018). Given the potential complementary benefits of each mode of exercise on physical health, a regimen that combines both modes of exercise could be advantageous.
Yoga, tai chi, and qi gong are practices that incorporate physical activity as well as mindfulness, breath work, and spiritual components (Vancampfort et al. Citation2021). These practices have shown antidepressant benefits in a small number of randomised controlled trials amongst people with MDD and is supported by other international guidelines (Ravindran et al. Citation2016; Prathikanti et al. Citation2017; Sharma et al. Citation2017). There is also a wider body of evidence that suggest these interventions may benefit depressive symptoms, stress, and quality of life in non-clinical populations (Breedvelt et al. Citation2019; Sivaramakrishnan et al. Citation2019). Furthermore, due to the generally low impact on joints and, depending on the type of practice, physical intensity of mind-body interventions, they may be well-suited to individuals with physical comorbidities that prevent them from engaging in more intense forms of physical activity.
Other factors that should be considered include a person’s preference, age and physical condition – the latter is especially true in the context of a past or current COVID-19 infection and the short- and long-term implications on exercise capacity. Another consideration is the context of exercise (e.g. leisure-based physical activity vs work-related physical activity) (Teychenne et al. Citation2020). Previous observational research suggests that the context is an important factor, with leisure or transport-related physical activity showing the greatest benefits to mental health, whereas domestic and work-related activity being least beneficial (Schuch et al. Citation2021).
Some evidence suggests that exercise programs where the individual has a clear sense of autonomy may be more beneficial than prescriptive regimens (Teychenne et al. Citation2020). Hence, individualising physical activity programs to those that the individual enjoys and finds meaningful should be encouraged. The introduction of a level of accountability – whether from oneself, a clinician, peers or community – is another factor that may improve sustained adherence. This may be one reason why integration of social support into physical activity programs such as exercising with friends/family or via classes and team sports may improve efficacy and long-term adherence of the intervention. Improvements in dropout rates and effectiveness have been demonstrated when exercise is supervised (Schuch, Vancampfort, Richards, et al. Citation2016; Stubbs et al. Citation2016). In the contemporary setting, where many individuals with MDD may be recovering from COVID-19 or have ‘long COVID-19′, the importance of supervision by a recognised exercise professional (e.g. physiotherapist, exercise physiologist) is underscored (see Box 2 for further considerations).
4.1.3.2. Dose, frequency, intensity
Prescription should be based on feasibility of adherence and individualised to the person’s motivation and current levels of physical activity and fitness. A target of 150–300 min/week of moderate-intensity physical activity or 75–150 min/week of vigorous-intensity physical activity has been proposed as this aligns with the World Health Organisation 2020 guidelines on physical activity and sedentary behaviour (Bull et al. Citation2020; Teychenne et al. Citation2020). Both WHO and mental illness specific guidelines stress that these are aspirational targets for many with MDD, and the best exercise prescription is one that can be maintained. Initiating exercise of any level of duration, intensity and frequency is important and can be built up over time as an individual achieves success and an increasing sense of autonomy and internal motivation to sustain long term behaviour change (Vancampfort et al. Citation2015; Stubbs et al. Citation2018).
4.1.3.3. Assessment considerations
Caution should be taken when prescribing physical activity, especially intensive forms, to those with certain medical conditions, such as heart disease, diabetes, asthma, vertigo, osteoporosis, or joint disease (notwithstanding that exercise is recommended for many of these conditions) and the aforementioned COVID-19 related conditions. This may require consultation with physicians, possibly involving formal exercise testing, prior to initiating an exercise program. Pre-exercise screening tools and guidelines are available to support professionals, as needed (Norton and Norton Citation2011; Riebe et al. Citation2018).
4.1.3.4. Sedentary behaviour
Related to the role of increased physical activity on MDD is the association of MDD and sedentary behaviour, defined as behaviours with an energy expenditure ≤1.5 metabolic equivalents, such as sitting and reclining (Tremblay et al. Citation2017). While the evidence is based primarily on observational data (Teychenne et al. Citation2020), prospective meta-analyses suggest that higher levels of sedentary behaviour are associated with a greater risk of MDD (Zhai et al. Citation2015). Evidence from a recent prospective cohort using an objective assessment of sedentary and physical activity data with 60,235 participants found that sedentary time is a risk factor for MDD (Kandola et al. Citation2021). Importantly, the study found replacing 60 min of sedentary behaviour with light or moderate-to-vigorous activity, was associated with lower MDD symptom scores at follow-up by OR 0.75 (95% CI, 0.74–0.76) and OR 0.90 (95% CI, 0.90–0.91) respectively (Kandola et al. Citation2021). There is emerging evidence that the type of sedentary behaviour may have a differential effect, with mentally passive sedentary behaviour (e.g. watching TV) being associated with increased risk of future MDD, whereas mentally active sedentary behaviour (e.g. playing computer games, reading) is not (Hallgren et al. Citation2020; Werneck et al. Citation2021). This finding has been confirmed by a number of modestly powered randomised controlled trials, where the intervention increased sedentary behaviour in healthy populations, leading to worsening of mental health (Edwards and Loprinzi Citation2016), possibly due to increased inflammation/stress (Endrighi et al. Citation2016). Discussing strategies to reduce sedentary behaviour (e.g. using standing desks to disrupt long periods of sitting) may be incorporated into physical activity education.
4.1.4. Resources
EPA guidance on physical activity as a strategy for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organisation of Physical Therapists in Mental Health (IOPTMH) (Stubbs et al. Citation2018). Further guidelines on the use of physical activity in MDD as well as other forms of severe mental illnesses
Physical Activity factsheet (World Health Organization Citation2021). A World Health Organisation fact sheet on physical Activity guidelines across age groups
Exercise Right (Exercise Right Citation2022). A public awareness campaign run by Exercise & Sports Science Australia that provides resources and information regarding exercise
4.2. Smoking cessation interventions
4.2.1. Background literature
Smoking is a critical risk factor for several chronic diseases such as chronic obstructive pulmonary disease, lung cancer, coronary heart disease, and type II diabetes, and premature death (US Department of Health and Human Services Citation2004). People with MDD are more likely to be smokers compared to the general population, exacerbating their already elevated comorbidity and mortality risk (Weinberger et al. Citation2017). The relationship between smoking and depressive symptoms has historically been considered a form of coping or self-medication. This has resulted in a hesitancy to engage people with MDD in smoking cessation programs due to concerns that this may exacerbate depressive symptoms (Prochaska Citation2011). However, there is growing evidence to suggest that the relationship between smoking and mental illness may be bidirectional (Bjørngaard et al. Citation2013; Taylor AE et al. Citation2014; Wootton et al. Citation2020). Prospective observational studies demonstrate that smokers have increased odds of subsequent MDD later in life (Luger et al. Citation2014; Fluharty et al. Citation2017). More recently, the use of Mendelian randomisation methods have tended to corroborate this bidirectional relationship (although not consistently) (Bjørngaard et al. Citation2013; Taylor AE et al. Citation2014; Wootton et al. Citation2020). A recent Cochrane review of 34 studies concluded that there was significant, albeit very low-certainty evidence that smoking cessation is associated with reduced depressive symptoms (Taylor GM et al. Citation2021). However, there is currently limited evidence from randomised controlled trial that support smoking cessation interventions for managing depressive symptoms in people with MDD (Secades-Villa, Gonzalez-Roz, et al. Citation2017).
4.2.2. Clinical recommendations
4.2.3. Clinical considerations
4.2.3.1. Type and context of intervention
There are a wide range of pharmacological and behavioural approaches that may be beneficial to smoking cessation for people with MDD. The range of interventions is largely in line with those that can be offered to the general population. In the general population, abstinence rates for >6 months range from 3% to 5% for those unassisted through to 25–30% with combined psychological and pharmacotherapy support (Zwar et al. Citation2011). Nicotine replacement therapy is widely used both within the general population and for people with MDD. Previous meta-analyses reported that nicotine replacement therapy can provide a small but significant improvement in smoking cessation rates in those with MDD (Gierisch et al. Citation2012; Secades-Villa, González-Roz, et al. Citation2017). A recent Cochrane review of antidepressant medications for smoking cessation, in both populations with MDD and without, reported that there was high‐certainty evidence that bupropion increased long‐term smoking cessation rates. However, there was a greater risk of adverse events compared to placebo (Howes et al. Citation2020). The same review reported that varenicline has a larger effect size than bupropion, and that nortriptyline may be effective in a smaller number of studies (Howes et al. Citation2020). Commonly used psychological approaches include motivational interviewing, cognitive behavioural approaches, behavioural activation, and mindfulness-based approaches (Taylor GM et al. Citation2021). Recent meta-analytic data suggests that the use of cessation medications and greater use of behaviour change techniques was predictive of improved cessation rates (Black et al. Citation2020).
4.2.3.2. Smoking-medication interactions
Tobacco smoking can affect the metabolism of some antidepressant and antipsychotic medications (e.g. clozapine, olanzapine, fluvoxamine, duloxetine, mirtazapine, and trazodone) (Oliveira et al. Citation2017). Hence, smoking cessation may also affect metabolism and absorption of currently prescribed medications and will require appropriate monitoring. This may also affect metabolism of caffeine consumed via diet (e.g. coffee, tea, energy drinks) and therefore may potentiate stimulatory effects, resulting in restlessness and sleep disturbances (Marel et al. Citation2016). Thus, smoking status should be considered in the context of dietary- and sleep-based mental health approaches.
4.2.3.3. Ongoing support required
Smoking cessation and MDD appear to have a bidirectional relationship whereby smoking cessation can reduce depressive symptoms, but the presence of MDD can also reduce efficacy of smoking cessation efforts (Stepankova et al. Citation2017). Past diagnosis of MDD is associated with decreased abstinence rates and increased relapse rates (Stepankova et al. Citation2017). Furthermore, relevant cognitions (e.g. low self-efficacy) and behaviours (e.g. smoking as a maladaptive coping behaviour) that may reduce adherence to smoking cessation may be more prevalent amongst people with MDD. These data suggest that, to enhance the effectiveness of sustained smoking cessation interventions, people with MDD may require further support and additional long-term monitoring. There are also some concerns that the intensity of nicotine withdrawal may lead to heightened fatigue, thus smoking cessation needs to be initiated under clinical supervision.
4.2.3.4. Adjunctive lifestyle-based approaches
Physical activity has been suggested as a potential adjunctive intervention to standard smoking cessation programs. However, a Cochrane review of 24 interventions in non-clinical samples reported that there was no evidence to suggest adjunctive physical activity enhances the benefits of smoking cessation programs (Ussher et al. Citation2019). Despite the lack of evidence that physical activity facilitates smoking cessation, in addition to its antidepressant effects, physical activity may reduce symptoms of smoking withdrawal such as irritability and restlessness, be used as a coping strategy in response to cravings, and also may help minimise weight gain associated with smoking cessation (Marel et al. Citation2016). Furthermore, incorporation of exercise and other lifestyle-based approaches may improve cardio-metabolic risk factors associated with smoking (see Box 3).
Sleep disturbances are a recognised consequence of nicotine withdrawal, occurring in up to 42% of people who have quit smoking (Patterson et al. Citation2019). These symptoms can arise both from nicotine withdrawal as well as medications used for smoking cessation (e.g. varenicline). These symptoms generally subside after 3–12 months post-cessation (Patterson et al. Citation2019). Prior studies suggest that both pre-cessation and post-cessation sleep disturbances are associated with greater relapse rates, suggesting that addressing these sleep disturbances may facilitate long-term cessation (Patterson et al. Citation2019).
Similarly, weight gain is common after smoking cessation and is a predictor of smoking relapse (Tian et al. Citation2015). Lifestyle-based approaches may provide benefit in managing weight gain. A Cochrane review found limited clinical trial evidence that has evaluated such interventions but concluded that physical activity and personalised dietary interventions may be effective interventions for post-cessation weight gain (Farley et al. Citation2012). Importantly, general weight management advice only was not effective, suggesting that personalised approaches are likely to offer greater benefit.
Finally, smoking cessation and relapse appear to be highly linked to an individual’s social environment whereby the perceived reason for ongoing smoking may be linked to feelings of marginalisation or social isolation, and where smoking relapse is much higher in social networks where there are many other smokers (Blok et al. Citation2017). Within such contexts, smoking behaviour is likely to have developed and be maintained as a core vehicle for social connection with others and may be paired with other substance use behaviours (e.g. drinking) from which it is challenging to disassociate. This dynamic can include an individual’s spouse, family, friends, peers and work environment where smoking is seen as a normative behaviour (Christakis and Fowler Citation2008). It can also include mental health settings where smoking behaviours may have developed and been reinforced as part of the social milieu and to alleviate boredom (Lawn SJ et al. Citation2002). This suggests that strategies for managing ‘high risk’ social situations, and sub-cultural and peer contexts where there are many smokers, may be necessary.
4.2.4. Resources
Co-occurring alcohol and other drug and mental health conditions in alcohol and other drug treatment settings (Marel et al. Citation2016). Further guidance on management of smoking, alcohol, and other drug use in the mental health setting
Smoking and Mental Health (Mental Health Foundation Citation2021b). Resource on the connection between smoking and mental health that is suitable for general public
Supporting smoking cessation: A guide for health professionals. Second edition (Zwar et al. Citation2011). Guidelines for smoking cessation developed by The Royal Australian College of General Practitioners
4.3. Work-directed interventions
4.3.1. Background literature
In addition to providing financial benefits, employment also provides considerable social, cognitive and psychological benefits (Modini et al. Citation2016). Employment is a source of routine and structure for an individual and an avenue for social interaction. Furthermore, an individual’s employed position can be a significant source of confidence, identity, status, vocational purpose, and self-esteem. Previous prospective cohort studies suggest that employment has a protective effect on MDD and psychological distress (van der Noordt et al. Citation2014). MDD can also have a detrimental effect on work performance with studies showing increased errors and safety issues in people with MDD (Nieuwenhuijsen et al. Citation2020). A further consequence of MDD is the increased risk of absenteeism and unemployment, which may further exacerbate symptoms due to the increased isolation, financial stress, and lack of routine (Wanberg Citation2012). There is also suggestive evidence that the adverse mental health effects of unemployment may be further compounded by extended unemployment and periods outside of the workforce (Finnes et al. Citation2019). Hence, interventions that address contributing workplace-related factors and that aim to address barriers to returning to work are likely to be helpful in clinical management of depression in unemployed or underemployed individuals. Workplace culture both generally and pertaining to mental health and wellbeing, including associated stigma, are also factors that can influence the mental health of an employee and minimising mental health injury. Organisational or occupational factors may need to be considered in the context of work-directed interventions for those with MDD.
In a Cochrane review of 45 intervention studies in people with MDD, work-focussed interventions as well as clinical interventions such as psychological, pharmacological, and exercise-based interventions were investigated for their effect on a range of work-related outcomes (Nieuwenhuijsen et al. Citation2020). Interventions that used a combination of workplace changes and a clinical program had the strongest evidence and were reported to potentially reduce number of days on sick leave, reduce symptoms of depression, and improve ability to cope with work. There are certain organisational factors that have been linked to poorer mental health of employees including job strain (high demand and low control), job insecurity and precarious employment, bullying and discrimination. It has been estimated that among working men and women, 13.2%, and 17.2% of depression is attributable to job strain, respectively (LaMontagne et al. Citation2008). Protective factors include social support from colleagues and supervisors and there is meta-analytic level evidence indicating that training managers in workplace mental health may improve their mental health knowledge, attitudes towards mental health, and self-reported behaviour related to supporting employees, though the data remain in its infancy (Gayed et al. Citation2018).
4.3.3. Clinical considerations
4.3.3.1. Determine role of work-related and other factors in MDD
Determining the role of an individual’s employment in contributing or causing depressive symptoms is an important initial step in clinical management. This assessment will inform work-related management strategies such as if graded work-directed interventions are available and appropriate. This determination can be made by clinical judgement based on a comprehensive clinical assessment and may be apparent in instances where clinical care has been sought due to mental or psychological injury being reported or where work compensation claims are sought. While there is a lack of appropriately validated tools to assess this, the use of tools such as the Workplace Stressors Assessment Questionnaire and the Work Environment Scale may help guide clinical assessment (see in the Implementation Consideration section for further details on assessment tools) (Mazza et al. Citation2019). Conditions that are commonly comorbid with work-related MDD include musculoskeletal pain, trauma, and substance use (IsHak et al. Citation2018). Interventions should assess for, and where appropriate, manage these conditions as they may exacerbate and/or prolong depressive symptoms. Please see section 6.2 for further information regarding assessment of lifestyle factors in clinical care.
Table 3. Available formal assessment tools and suggested prompting questions related to specific lifestyle domainsa.
4.3.3.2. Consider partial return to work where possible
Extended unemployment increases the risk of and may exacerbate existing adverse conditions of unemployment including increased MDD, alcohol abuse, isolation, hopelessness, decreased self-esteem, suicide, financial debt, and diminished social status (Bond et al. Citation2017; Audhoe et al. Citation2018). To compound this further, research demonstrates that the probability of returning to work decreases as the length of time since employment increases (Audhoe et al. Citation2018). Therefore, partial return to work and related strategies such as temporarily reducing work hours, graded exposure to returning to full work capacity, or seeking deployment to achievable duties, should be considered to avoid extended absenteeism (Australasian Faculty of Occupational Environmental Medicine Citation2010; Mazza et al. Citation2019).
4.3.3.3. Need for work directed interventions combined with psychotherapy
A recent Cochrane review found that a combination of psychotherapy and work-targeted interventions may be more effective for the management of MDD than work-targeted interventions alone (Nieuwenhuijsen et al. Citation2020). Work-directed interventions may include modifying expected duties of the role, work routines and work environment, mentor support programs, and education regarding coping skills and compensatory work strategies (e.g. stress management strategies, memory aids) (Lerner et al. Citation2020; Nieuwenhuijsen et al. Citation2020). People with higher self-efficacy are more likely to return to work and incorporating interventions that improve self-efficacy may aid in work-directed interventions (Mazza et al. Citation2019). Additional considerations include addressing perceived work quality (Butterworth et al. Citation2013), as jobs with a high number of adverse factors (e.g. job insecurity, psychological demands) have a comparable risk of MDD relating to unemployment (Australasian Faculty of Occupational Environmental Medicine Citation2010). There are now Mental Health First Aid programs that are available in many countries around the world that are designed to assist managers, peers, friends, family and colleagues in responding to mental health concerns. They are increasingly being adapted and delivered in the workplace setting (e.g. (Mental Health First Aid International Citation2022)).
4.3.3.4. Engagement with workplace and occupational therapists
Where possible and with consent from the individual, communication with the employer can assist with treatment through management of work environment-related factors that may be exacerbating symptoms (Pomaki et al. Citation2010). Relaying concerns of the individual regarding returning to work, perceived barriers, and suggested alternative arrangements to the employer may facilitate work-directed interventions. Furthermore, collaboration with the employer may allow for additional intervention strategies that are difficult to implement without engagement from the workplace. Examples of such work-targeted interventions include partial return, temporary reduction of job demands, and delegating tasks (Finnes et al. Citation2019). Working with workplace rehabilitation providers, when available, can provide further clinical support, help coordinate, and aid in delivering individualised work-directed strategies and education (Schene et al. Citation2007; Hees et al. Citation2013). Further considerations can be seen in Box 4.
4.3.3.5. Volunteering
Volunteering may be a related avenue where paid employment may not be attainable, feasible, or necessary (e.g. post-retirement). A systematic review of health benefits of volunteering found mixed evidence of positive impacts on MDD, though a greater number of the cohort studies reported reduced levels of MDD than those reporting no benefits (Jenkinson et al. Citation2013). Heterogeneity across studies makes it difficult to synthesise clear advice, though type of volunteering did not appear to influence outcomes, nor did intensity, though sustained volunteering rather than intermittent volunteering did appear to accrue benefits for addressing MDD, particularly in older volunteers (Jenkinson et al. Citation2013). A more recent systematic review also found benefits to depressive symptoms from volunteering for older adults (Filges et al. Citation2020).
4.3.4. Resources
Realising the health benefits of work – An evidence update (Australasian Faculty of Occupational and Environmental Medicine Citation2015). Guidance document on return-to-work practices developed by The Australasian Faculty of Occupational & Environmental Medicine and The Royal Australasian College of Physicians
Best Practices for Return-to-work/Stay-at-work: Interventions for Workers with Mental Health Conditions (Pomaki et al. Citation2010). Further guidelines on return-to-work interventions for people with mental illness, developed by the Canadian Occupational Health and Safety Agency for Healthcare
Clinical guideline for the diagnosis and management of work-related mental health conditions in general practice (Mazza et al. Citation2019). Further guidelines for general practitioners for the diagnosis and management of work-related MDD as well as anxiety, post-traumatic stress disorder, acute stress disorder, adjustment disorder and substance use disorder
Returning to work after mental health issues (National Health Service Citation2021). Resource developed by the UK National Health Service on returning to work for people with mental health issues
4.4. Mindfulness-based and stress management interventions
4.4.1. Background literature
Stress and MDD have a bidirectional relationship; for example, life stressors can increase the risk of MDD, while MDD can increase susceptibility to a heightened stress response (Liu and Alloy Citation2010). Several biological systems have been implicated in the pathology of stress in MDD including hypothalamic pituitary adrenal axis, the sympathetic nervous system, genetic susceptibility, and changes in brain structure and function (Hammen Citation2015). An important aspect of addressing stressors in MDD is to improve resiliency and coping responses that help to attenuate stress responses. Stress management and mindfulness-based approaches in people with MDD can take various forms. Mindfulness-based stress reduction (MBSR) has been widely used for the purpose of targeting stress and to address MDD. Mindfulness Based Cognitive Therapy (MBCT) is a manualized, evidence-based psychological treatment for MDD, and may be preferred for prevention of relapse (Kuyken et al. Citation2016). As stated previously, mindfulness overlaps with a wide range of psychological therapies (e.g. Acceptance and commitment therapy, DBT, and positive psychology) that address similar domains and concepts but are outside the scope of these guidelines (Chakhssi et al. Citation2018; Carr et al. Citation2021). Relaxation therapies such as progressive relaxation training or autogenic training may also be beneficial for depressive symptoms (Jorm et al. Citation2008; Klainin-Yobas et al. Citation2015; Jia et al. Citation2020). A Cochrane Review and recent updated meta-analysis report that relaxation techniques can be beneficial for depressive symptoms when compared to wait-list or minimal intervention, but not compared to psychotherapy (Jorm et al. Citation2008; Jia et al. Citation2020).
Overall, evidence supports the use of stress management approaches and specifically mindfulness for MDD management (Goldberg et al. Citation2018). Mechanisms of action remain undetermined but potential cognitive mechanisms could include an improvement in mindfulness, decreases in rumination and worry, and increasing self-compassion and psychological flexibility (Gu et al. Citation2015; Alsubaie et al. Citation2017). Mindfulness practices are also associated with biological pathways relevant to MDD such as changes in hippocampal structure, autonomic nervous system function, and inflammatory pathways (Shen et al. Citation2020). Mindfulness-based interventions (MBIs) perform comparably with cognitive behaviour therapy (CBT) for the treatment of anxiety and MDD (Hofmann and Gómez Citation2017). MBCT may also be more effective for maintaining benefit compared to antidepressant medication (Zhang Z et al. Citation2018). MBCT is more effective than treatment as usual in the long-term prevention of depressive relapse and time to relapse. However, there was no statistically significant difference for the rate of relapse or time to relapse of MDD between MBCT and active treatments (e.g. CBT and antidepressants) (McCartney et al. Citation2021). MBCT and other approaches that incorporate mindfulness principles may also be superior to inactive or treatment as usual controls, in reducing symptoms of MDD, although more high-quality studies are needed to confirm the efficacy of these interventions (Seshadri et al. Citation2021).
4.4.2. Clinical recommendations
4.4.3. Clinical considerations
4.4.3.1. Considerations for delivery of mindfulness-based interventions
MBIs may be delivered by appropriately trained professionals via face to face or digital technology that includes evidence-based interventions. Training courses are available to provide upskilling opportunities and professional development for some mindfulness methods such as MBCT, where there are multiple training programs around the world. Side effects of MBIs such as discomfort, irritability, and a greater awareness of symptoms of stress or rumination, can be relatively common, especially initially (Goldberg et al. Citation2022). One systematic review found the overall prevalence of meditation-related adverse events was 8.3%, which is similar to those reported for psychotherapy practice in general (Farias et al. Citation2020). Such side effects are not necessarily an indication to terminate therapy but do require careful support and guidance as the individual learns and assimilates the skills. An individual’s religious and cultural background and preferences should also be considered with respect to the conduct of mindfulness to ensure alignment and/or integration.
4.4.3.2. Timing of mindfulness-based interventions
Learning mindfulness may not be appropriate for those experiencing acute or severe major depressive episodes or psychosis. In such situations, it may be difficult for some to engage and practice, may accentuate unpleasant symptoms, and may require close supervision in such cases. Alternatively, intervention may be delayed and safely applied when the individual is in a more stable condition. In contrast to interventions for people with elevated acuity, those experiencing mild depressive symptoms may have a lower risk of adverse events and requirement for close clinical oversight may be reduced. Thus, introductory and self-guided mindfulness training may be warranted and can be facilitated with self-guided digital health training programs and apps (e.g. Headspace, InsightTimer, Calm). See Implementation consideration #8 for further discussion on digital delivery methods.
4.4.3.3. Stress management and relaxation techniques
In addition to mindfulness-based stress reduction, stress management strategies that are individualised to the individual’s circumstance, as well as the provision of psychoeducation regarding stress-relief (e.g. addressing rest, exercise, and social support) and relaxation techniques may form a beneficial component of treatment and particularly as part of ongoing management to prevent future depressive episodes and comorbid disorders such as anxiety. There are a variety of relaxation techniques that have been investigated within clinical trials including autogenic training, guided relaxation imagery, and breathing exercises with progressive muscle relaxation being the most studied (Jorm et al. Citation2008; Jia et al. Citation2020). An advantage of relaxation techniques is that they can be relatively easily implemented without need for specialised training, which suggests that it may be particularly beneficial where health care access is limited. Further practical considerations can be seen in Box 5.
4.4.4. Resources
The Mindful Way Through Depression: Freeing Yourself from Chronic Unhappiness (Williams et al. Citation2007). A book on mindfulness practices focussed on MDD that is suitable for general public
MBCT.com (MBCT.com). Online resource for training and further information regarding MBCT
4.5. Dietary Interventions
4.5.1. Background literature:
Evidence to support the role of dietary interventions in MDD and other mental disorders has grown over the last decade (Marx et al. Citation2017). Meta-analyses of both prospective and cross-sectional observational data support an association between adherence to nutrient-dense dietary patterns such as the Mediterranean diet and a reduced risk of MDD (Firth et al. Citation2020) These associations have been reported in multiple international datasets, appear consistent across the lifespan, and persist after accounting for relevant potential confounders (O’neil, Quirk, et al. Citation2014; Lassale et al. Citation2019; Collins et al. Citation2022). Furthermore, a recent harmonised meta-analysis reported that this association persists when accounting for baseline MDD (Nicolaou et al. Citation2020). At the same time, diets high in ultra-processed foods are associated with an increased risk of depressive symptoms (Lane et al. Citation2021).
Meta-analyses of clinical trials that use whole of diet interventions have provided preliminary evidence that they significantly reduced depressive symptoms albeit in small and largely non-clinical populations (e.g. without current diagnosis of MDD) (Firth et al. Citation2019a). Several small RCTs have been conducted in people with MDD and have reported moderate-to-large improvements in depressive symptoms when randomised to receive Mediterranean-style dietary intervention compared to controls (Jacka et al. Citation2017; Parletta et al. Citation2018; Francis et al. Citation2019; Bayes et al. Citation2022). Furthermore, cost-effectiveness analysis of these dietary interventions suggests the possibility for substantial cost-savings to the individual and health system (Chatterton et al. Citation2018; Segal et al. Citation2020). Completion rates in these trials have been high (e.g. SMILES 93.9% in the diet group and 73.5% in the social support control group p = 0.024) (Jacka et al. Citation2017). The samples in all studies are small and are largely confined to Australian populations and this needs to be taken into consideration. Biological mechanisms of action are, however, plausible and include modulation of pathways involved in inflammation, oxidative stress, mitochondrial dysfunction, the gut microbiota, tryptophan–kynurenine metabolism, the HPA axis, neurogenesis and BDNF, and epigenetics (Marx et al. Citation2021).
4.5.2. Clinical recommendations
4.5.3. Clinical considerations
4.5.3.1. Type and context
While most randomised controlled trials that have used dietary interventions have used a Mediterranean style dietary pattern, this does not suggest that a Mediterranean diet is essential or superior to other healthy dietary patterns. Indeed, there is a range of healthy dietary patterns that are associated with reduced MDD risk. These include the Dietary Approaches to Stop Hypertension (DASH) diet, dietary patterns characterised by low levels of inflammation or another dietary classification, as well as healthy traditional dietary patterns (Lassale et al. Citation2019; Marx et al. Citation2021). Instead, the evidence suggests that any dietary pattern that emphasises the consumption of nutrient dense, unprocessed foods may be efficacious for reducing depressive symptoms. Therefore, dietary advice and prescription should be individualised with specific consideration of ethical, spiritual and/or religious preferences, comorbidities, food intolerances and allergies, taste preferences, and socioeconomic status. Furthermore, there is a lack of clinical trial evidence to suggest that more restrictive diets (e.g. ketogenic diet, vegan) that require exclusion of commonly consumed food groups are effective and are not currently recommended for the purpose of addressing mental health indications.
Opie et al.(Opie et al. Citation2017) provides further detail regarding dietary recommendations and can be summarised as adhering to nutrient-dense dietary patterns that include increased consumption of fruits, vegetables, legumes, wholegrain cereals, nuts, and lean meat, while reducing consumption of processed foods. The consumption of omega-3 rich foods such as fatty fish is another recommendation that is supported by previous guidelines (Opie et al. Citation2017; Guu et al. Citation2019). An expanded summary can be seen in Box 6. A common and important practical concern is the price of healthier foods. A systematic review and meta-analysis of 27 studies from 10 countries found that the healthiest diets cost on average $1.50/day more than the unhealthiest diets, or approximately $10/week (Rao et al. Citation2013). Accessibility and food security is another crucially important concern that can only be partially offset by individual or communal preparation, hence requiring greater emphasis on public health, governments, and industry to adequately respond.
4.5.3.2. Dose, frequency, intensity
An important consideration in the provision of dietary counselling for people with MDD is that full adherence to a specific dietary pattern is not essential to improve depressive symptoms and is likely an unreasonable expectation, particularly where depressive symptoms are severe, resources are limited and/or where motivation and capacity is low. Indeed, current dietary intervention studies in MDD focussed on individualised improvement in diet quality rather than full adherence (Jacka et al. Citation2017; Parletta et al. Citation2018).
Strategies to improve diet quality that were incorporated in these interventions included ‘swaps’ whereby participants were encouraged to replace currently consumed nutrient-poor food items with nutrient-dense alternatives (e.g. replacing white rice with brown rice). The SMILES trial demonstrated that participants made significant dietary changes by reducing their discretionary items by on average 21.8 items (SD16) per week (Jacka et al. Citation2017).
4.5.3.3. Assessment considerations
Screening tools have been developed that aid in identifying people with low diet quality and may assist with initial discussions regarding diet interventions. All tools have benefits and limitations, and common amongst all self-reported tools is the potential of recall bias. Where feasible, dietary assessments conducted by trained professionals (e.g. dieticians) will likely provide greater precision. The use of assessment tools and prompting questions is further discussed in the section 6 of this document.
4.5.3.4. Weight loss dietary advice
The clinical trial evidence on dietary interventions suggests that improvement in depressive symptoms can be achieved independent of weight loss (Jacka et al. Citation2017; Parletta et al. Citation2018; Francis et al. Citation2019). Therefore, ad libitum dietary advice that focuses on healthy eating strategies rather than weight loss or calorie restriction can be provided for the management of depressive symptoms. However, people with mental disorders have significantly higher levels of comorbidities and metabolic disorders compared to the general population and certain psychotropic medications may increase appetite and reduce satiety. In these circumstances, weight maintenance and management strategies may be warranted for the management of physical comorbidities where risk of metabolic disorders is present.
4.5.3.5. Dietary supplement use
The use of dietary supplements is a related area with considerable public interest. Formal clinical guidelines regarding the use of nutraceutical interventions in psychiatry have been recently published by the WFSBP and can be accessed elsewhere (Sarris et al. Citation2022). Due to the considerable benefit of a healthy diet to physical and metabolic health, dietary interventions should be prioritised over supplementation with individual nutrients. However, supplementation may be warranted in situations where dietary interventions may not be feasible (during severe acute episodes, limited access to nutrition counselling support), when treating confirmed nutrient deficiencies, or in combination with a whole of diet approach.
4.5.3.6. Therapeutic approaches in dietary counselling
MDD may affect dietary adherence, food preferences, and appetite, as a direct result of MDD or due to medications given to treat MDD. Factors such as fatigue, reduced motivation, and apathy may also reduce the effectiveness of dietary interventions that are not suited to the individual’s cognitive and motivational capacity (Kwan et al. Citation2014). Cognitive barriers, motivational difficulties, and disorganised lifestyles are additional challenges that may reduce the effectiveness of dietary interventions, particularly in those with more severe mental illness (Kwan et al. Citation2014). Furthermore, MDD (and mental illness more broadly) is associated with comorbidities such as obesity and diabetes that require specific dietetic considerations. As discussed by Kwan et al (Kwan et al. Citation2014), the use of motivational interviewing, incorporating multimodal techniques, improving awareness of nutrition requirements, and providing individualised and structured eating advice are some techniques that can be incorporated to overcome these challenges. Dieticians are trained to provide appropriate nutritional management and may help ensure sustainable dietary improvements.
4.5.4. Resources
The International Society of Nutritional Psychiatry Research (ISNPR) (ISNPR – International Society for Nutritional Research Citation2021). The ISNPR is a global network for researchers that aims to promote the generation and translation of high-quality evidence for nutritional approaches to the prevention and treatment of mental disorders.
A modified Mediterranean dietary intervention for adults with major depression: Dietary protocol and feasibility data from the SMILES trial (Opie et al. Citation2018). This publication provides a detailed guide of the dietary intervention used for the SMILES trial and may serve as a valuable reference for future clinical trial design as well as a guide to providing dietary interventions in clinical practice.
Food and Mood: Improving Mental Health Through Diet and Nutrition (FutureLearn Citation2021). A free online course available to the general public on the current evidence regarding dietary interventions in mental health.
Feeding melancholic microbes: MyNewGut recommendations on diet and mood (Dinan et al. Citation2019). Further information regarding specific components of healthy dietary patterns and current evidence for their potential role in MDD.
Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce (Sarris et al. Citation2022). Recently published clinical guidelines on dietary supplement interventions in mental disorders
4.6. Sleep-related interventions
4.6.1. Background literature
MDD and sleep have a bidirectional relationship in that poor sleep contributes to depression and depression results in poor sleep. Most people with acute MDD report difficulties initiating and/or maintaining sleep. The prevalence of insomnia symptoms in people with MDD is estimated at 80-90% (McCall et al. Citation2000; Novick et al. Citation2005), and diagnosable insomnia is present in as many as two-thirds (Geoffroy et al. Citation2018). The DSM-5 recognises sleep changes (‘insomnia or hypersomnia nearly every day’) as symptomatic of a major depressive episode (American Psychiatric Association Citation2013). Sleep disturbances in a major depressive episode correlate with MDD severity (Soehner et al. Citation2014), are a significant driver of distress and impaired quality of life (Mayers et al. Citation2003), and are independently associated with suicidal ideation and suicide attempts (Pigeon et al. Citation2012).
The presence of insomnia increases the risk of subsequent MDD onset by approximately 3 times (Breslau et al. Citation1996; Morphy et al. Citation2007; Hertenstein et al. Citation2019). Residual sleep disturbances are common after acute phase treatment for MDD (Romera et al. Citation2013) and increase the risk of future depressive relapse (Dombrovski et al. Citation2007; Franzen and Buysse Citation2008). Sleep difficulties also predict a poor response to guideline-based care for MDD such as CBT (Asarnow and Manber Citation2019). The converse is also the case: MDD predicts and can trigger insomnia disorder (Franzen and Buysse Citation2008), which will require its own independent attention once MDD is successfully treated (Sweetman et al. Citation2021). This is particularly important as residual symptoms of insomnia, despite successful treatment of MDD, are associated with an increased risk of relapse (Combs K et al. Citation2014).
Bidirectional causal relationships between insomnia and MDD have been demonstrated in a Mendelian randomisation study by Cai and colleagues (Cai et al. Citation2021). Interestingly, the genetic liability of insomnia on MDD was much larger than vice versa, with the authors concluding the disparity is consistent with the utility of sleep interventions as therapies for neurodegenerative and psychiatric disorders. These findings parallel experimental and quasi-experimental research showing that sleep deprivation increases negative affect and decreases positive affect in response to goal-enhancing events (Harvey et al. Citation2011; Konjarski et al. Citation2018).
A recent systematic review and meta-analysis of N = 65 relevant clinical trials (72 interventions, N = 8608 participants) confirms that sleep is also a modifiable risk factor for MDD (Scott et al. Citation2021). Scott et al. found improvements in depressive symptoms by sleep-focussed interventions to be mediated through improved sleep quality (g = −0.47, 95%CI [−0.57, −0.37], p < 0.001 after outliers removed to decrease heterogeneity) (Scott et al. Citation2021). Insomnia symptoms are therefore an important target for improving MDD outcomes, with MDD benefits dependent on sleep improvements (similar findings are reported elsewhere (Bei et al. Citation2018; Henry et al. Citation2021)). Effective approaches for sleep problems may augment MDD treatment in acute and maintenance phases (Freeman et al. Citation2020).
4.6.2. Clinical recommendations
4.6.3. Clinical considerations
4.6.3.1. Considerations in the management of MDD and insomnia symptoms
MDD treatment and insomnia treatment may be offered concurrently or sequentially, with choice of initial treatment depending on individual preference, presenting symptoms and severity, history, lifestyle factors and other comorbidities. If treatment does commence with a focus on MDD, CBT-I should be re-considered if the comorbid insomnia is a barrier to antidepressant treatment or appears to be maintained by insomnia-specific factors such as unproductive beliefs about sleep and poor sleep hygiene practices. Insomnia management can be accessed by referral to a CBT-I trained psychologist in some healthcare systems.
4.6.3.2. Clinically significant sleep disturbance should be treated as a common comorbidity of MDD
Current editions of both relevant international diagnostic taxonomies highlight the need for focussed clinical attention on sleep disorders, irrespective of the presence of psychiatric or medical conditions (Seow et al. Citation2018). In relation to insomnia, the legacy distinction between ‘primary’ and ‘secondary’ insomnia was removed from DSM-5 (Reynolds and O’Hara Citation2013) and the International Classification of Sleep Disorders, Third Edition (ICSD-3) (Sateia Citation2014). Clinicians are instead encouraged to recognise bidirectional relationships between sleep and MDD, and actively pursue insomnia as a potential comorbidity of MDD warranting diagnosis and attention in its own right (Seow et al. Citation2018; Grima et al. Citation2019).
4.6.3.3. Assessment and screening of comorbid sleep disturbances
Sleep disturbance is a common reason for seeking primary care treatment, and sleep interventions can be viewed as less stigmatised than mental health treatments (Aikens and Rouse Citation2005; Gee B et al. Citation2019). A recent practice review in the Australian context presents detailed clinical recommendations for optimal management of comorbid MDD and insomnia (Sweetman et al. Citation2021). Noting that the majority of people with MDD in primary care are likely to present with both MDD and insomnia, Sweetman et al.(Sweetman et al. Citation2021) recommended that people with MDD be assessed for insomnia, and vice versa. We extend this recommendation to include assessment for, and specific treatment of, any sleep problems comorbid with MDD (assessment described below) (Sarfan et al. Citation2021). Importantly, those presenting with major depressive episodes must be actively probed for an underlying bipolar diathesis, because the stimulus control and sleep restriction components of sleep interventions, such as cognitive behavioural therapy for insomnia (CBT-I), may need modification for people at risk of elevated mood states (Gottlieb et al. Citation2019; Morton and Murray Citation2020). Clinical features of bipolar diathesis are family history, history of hypomanic symptoms, history of treatment-resistant MDD, and antidepressant-related hypomania (McIntyre et al. Citation2019). Symptoms of hypomania can be probed using the Hypomanic Personality Scale (Eckblad and Chapman Citation1986) or 7 Up 7 Down inventory (Youngstrom et al. Citation2013).
4.6.3.4. Assessment considerations
Sleep parameters can be measured subjectively and objectively. Sleep variables typically include sleep onset latency (SOL; time needed to fall asleep), wake after sleep onset (WASO; periods of wakefulness after sleep onset), total sleep time (TST; the amount of time asleep in bed); time in bed (TIB; the amount of time spent in bed, including non-sleep activities); sleep efficiency (SE; TST/TIB × 100%); number of awakenings (NOA); and subjective sleep quality. Self-report sleep measures include insomnia severity measured by sleep diaries(Carney et al. Citation2012) the Insomnia Severity Index (ISI) (Bastien et al. Citation2001) and the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. Citation1989), sleep-related cognitions measured by the Dysfunctional Beliefs and Attitudes about Sleep (DBAS-16) (Morin et al. Citation2007), and daytime sleepiness measured by the Epworth Sleepiness Scale (ESS) (Johns Citation1991). The diagnosis of insomnia can be made on self-report of sleep difficulties. Actigraphic measurement may be useful to improve precision of the characterisation of sleep, and therapeutic targeting of poor sleep hygiene. Referral to a sleep specialist, and an overnight sleep study is recommended if there are queries about other causes of disturbed sleep (e.g. restless leg syndrome, sleep apnea). See Implementation Considerations section for further discussion of assessment tools.
4.6.3.5. The use of CBT-I in the context of MDD
Insomnia may be an important therapeutic target to assist management of depressive symptoms in people with diagnosed MDD (Henry et al. Citation2021). Three recent systematic reviews have investigated the antidepressant effects of CBT-I amongst people diagnosed with insomnia and subsyndromal depressive symptoms (Ballesio et al. Citation2018; Benz et al. Citation2020; Ho et al. Citation2020). A recent systematic review and meta-analysis of 8 randomised controlled trials in people with MDD and insomnia reported that CBT-I provided benefit to both depressive and insomnia symptoms with a moderate effect size (Selvanathan et al. Citation2021). Digital CBT-I, especially in fully automated format, has received significant attention over the past decade (Krystal Citation2021), and is elevated here because of its recognised translational potential (Kraepelien et al. Citation2021). There are emerging intervention studies that suggest that such interventions may be beneficial for both people with subsyndromal and MDD (Blom et al. Citation2015; Ye et al. Citation2015; Blom et al. Citation2017; Chan et al. Citation2021; Henry et al. Citation2021).
4.6.3.6. Sleep hygiene type and style
Maintaining good sleep hygiene habits can help people with MDD improve their sleep quality. Sleep education resources can be integrated into their daily routines. Despite a lack of robust evidence of the effectiveness of sleep hygiene in the management of MDD, one meta-analysis found that sleep hygiene was associated with sleep improvement, although a definitive conclusion could not be drawn (Chung K-F et al. Citation2018). Clinicians can consider verbal advice of sleep hygiene as an appropriate and easily implemented strategy to adjunct conventional interventions in general practice. Nonetheless, there is no consensus regarding the most effective elements, optimal dose, or frequency. Many versions of sleep hygiene strategies are available. Box 7 provides a sample of these strategies. A clear explanation of the underlying mechanism of each sleep hygiene recommendation may enhance treatment adherence. This can, in turn, be supported by completion of self-rated sleep hygiene tools (see in section 6).
4.6.4. Resources
How to Sleep Better (Mental Health Foundation Citation2022). Downloadable pamphlet on sleep hygiene developed by Mental Health Foundation UK
4.7. Loneliness and social support related interventions
4.7.1. Background literature
Whilst loneliness may be experienced more commonly by some groups (e.g. older adults), it can be experienced at any age (Australian Institute of Health and Welfare Citation2021). Loneliness is not exclusively an objectively measured construct (e.g. number of people living in a household); rather, it has been defined as a ‘negative psychological response to a discrepancy between the social relationships one desires (expectations) and the relationships one actually has (objective, real ones)’ (Yanguas et al. Citation2018).
Greater loneliness may predict poorer MDD outcomes (Wang J et al. Citation2018). For example, a review of 34 longitudinal quantitative studies examining the relationship between baseline measures of loneliness and poor perceived social support and outcomes at follow-up found that people with MDD who perceive their social support as poorer have worse symptoms, recovery and social functioning (Wang J et al. Citation2018). For people with MDD, the odds of being lonely may be up to 10 times greater than the general population. These odds increase for those with additional psychiatric disorders (Meltzer et al. Citation2013). Furthermore, loneliness is also associated with other psychiatric disorders including those that involve psychotic symptoms and paranoia (Solmi et al. Citation2020). Loneliness has been linked to increased suicidality with those experiencing severe loneliness being 17 times more likely to have made a suicide attempt in the past 12 months (Stickley and Koyanagi Citation2016). An absence of social support and poor social functioning can predict poor treatment response, depressive symptoms, early dropout from treatment, and risk of MDD relapse (Kawachi and Berkman Citation2001; Solmi et al. Citation2020). Conversely, membership of social groups can protect against developing MDD, alleviate existing MDD, and prevent MDD relapse (Cruwys et al. Citation2013). The relationship between loneliness and depression is also likely bidirectional, as depression commonly presents with social withdrawal.
A related concept to loneliness is social support, which can be defined as ‘the care that is either provided or perceived to be readily available in times of need’ and can be measured objectively (Haslam et al. Citation2015). An important distinction to be made is that one may have objectively many avenues for provided social support while still feeling unsupported, resulting in a low rating of perceived social support (Santini et al. Citation2015; Mann et al. Citation2017). While perceived and provided social support are interrelated concepts, they do not necessarily occur together (Mann et al. Citation2017). Indeed, previous observational studies have reported a much stronger body of evidence associating perceived social support with depressive symptoms than provided social support (Santini et al. Citation2015). Furthermore, intervention studies have investigated the effect of various interventions that address both objective and perceived social support with a recent review concluding that while these interventions had some success in improving objective measures, no intervention style was clearly effective in improving perceived social outcomes (Mann et al. Citation2017; Ma et al. Citation2020). Hence, as a result, the strength of evidence for this clinical recommendation is limited to expert opinion due to the lack of clear research on how perceived and provided social support can be genuinely implemented to lead to improvements in those with MDD (see Box 8 for clinical tips and advice). However, given the presence of evidence indicating loneliness and social support as crucial concepts to understand because of their relationship to human physical, mental and social wellbeing, especially in the contemporary COVID-19 era, this is an urgent area of future clinical research.
4.7.2. Clinical recommendations
4.7.3. Clinical considerations
4.7.3.1. Type and context of intervention
Mann et al.(Mann et al. Citation2017) recently provided an overview of currently investigated interventions for loneliness and social isolation and categorised existing interventions into four broad categories that address: (1) social skills; (2) existing social support; (3) opportunities for new social contact; and (4) maladaptive social cognitions. Their review concluded that, while cognitive interventions that address ‘maladaptive’ cognitions held the most promise, there is insufficient evidence to recommend one intervention over another. This was corroborated by Ma et al.(Ma et al. Citation2020) in a recent review of loneliness and social isolation interventions for people with mental illness where they reported that, while some interventions, particularly those that used psychoeducation and supported socialisation interventions, had some evidence in improving objective measures of social isolation, few studies had investigated subjective feelings and, of those that did, few reported positive results. Given the lack of clear evidence for a particular style of intervention to address loneliness in those with MDD, any intervention needs to be based on individualised clinical judgement until further evidence is generated.
4.7.3.2. Assessment considerations
While loneliness can be experienced by any individual, particular groups are at a higher risk. Identification of such individuals may allow for early intervention to be put in place. Individuals who have recently experienced a significant ‘transition point’ are at a greater risk of loneliness and this may present as MDD. Transition points include changes in employment (e.g. retirement, job loss), relationships (e.g. death of a partner, relationship breakup), location (moving communities), and health status (e.g. chronic disease diagnosis, new disability) (Lim, Eres, et al. Citation2020). Furthermore, older adults are at greater risk of loneliness compared to the general population due to several factors such as retirement and increased risk of physical comorbidity, which may bring changes to their usual social and community connections. Other populations include Culturally and Linguistically Diverse (CALD) and expatriate communities where social connections may not yet be established and where barriers such as differences in language and culture may be apparent.
4.7.3.3. Indirect intervention via other lifestyle factors
In addition to interventions that may directly target social isolation and loneliness, use of interventions that address other lifestyle factors may indirectly address feelings of loneliness. Increasing physical activity, for example, may help address feelings of loneliness through increased social exposure (e.g. exercise classes) and social connection (e.g. team sports). Furthermore, returning to work or volunteering opportunities may provide stable avenues for social interaction (Filges et al. Citation2020). There is also an important element to cooking, food preparation and dining that can foster both nutrition and social engagement.
4.7.3.4. Avenues and programs for social engagements
Awareness of available resources and programs that are locally available and that can improve social support can allow for individualised recommendations for those experiencing loneliness. Such avenues might include community groups such as church groups, hobbies, and support groups; sports clubs; family, friends, and spouses; and volunteering opportunities. Engaging with allied health professionals may provide additional field-specific resources for social engagement.
A related concept is ‘Social Prescribing’ which involves connecting individuals from within the primary care setting with community organisations and resources with the help of dedicated linkage workers. Despite it being recently widely adopted in countries such as the UK, there are currently limited studies that support the use of social prescribing for the treatment of MDD (Bickerdike et al. Citation2017). However, short-term improvements in MDD have been found and those participating in social prescribing interventions have reported general improvements in feelings of loneliness and social isolation (Bickerdike et al. Citation2017).
4.7.3.5. Addressing barriers to social connection
In addition to facilitating avenues for social connection, sustainable uptake is contingent on addressing the barriers to engaging with such avenues. Potential barriers to forming social connection may include factors such as limited physical access to social resources, digital literacy, geographic location, cultural norms and customs and financial constraints. Furthermore, psychosocial factors such as social anxiety may prevent social engagement despite the availability of adequate avenues for engagement. Feelings of loneliness can be accompanied by self-stigmatisation, perceived stigma from others, feelings of failure and a loss of self-esteem. One proposed strategy for addressing such stigma is through peer support where individuals can engage with people with lived experience of loneliness and/or MDD (Lim, Badcock, et al. Citation2020). It is also acknowledged that finding enjoyment, meaning and purpose in life can be challenging for those living with MDD and this may be a barrier in uptake to such programs. Clinicians should work to support the person in the context of their episode trajectory, especially where co-morbid social anxiety, or physical or psychosocial disability, is present.
4.7.3.6. Social media, social connection, and mental health
Research has started to explore the impacts of social networking and use of social media on mental health. A 2016 systematic review found that positive interactions, social support, and social connectedness on social media platforms were consistently related to lower levels of depression and anxiety, and negative interaction and social comparisons on social media platforms were related to higher levels of depression and anxiety (Seabrook et al. Citation2016). These findings suggest that it may be more important to understand how social networking is used rather than the type of social media itself. However, given the highly designed and refined nature of social media platforms to encourage use, it can be challenging for users to solely use them in a beneficial manner, especially for people experiencing MDD. A recent 2021 meta-analysis of social media and depression symptoms was the first to consider the multi-dimensional nature of social media use (time spent using social media, intensity of use, and problematic social media use) (Cunningham et al. Citation2021). It found depression symptoms were significantly, but weakly, associated with time spent using social media and intensity of use. However, the association of depression symptoms to problematic social media use was moderate (r = 0.29). ‘Problematic social media use’ is a term being increasingly used in clinical and research arenas. While definitions vary, problematic social media use reflects a pattern of use that is characterised by behavioural and psychological features of addiction, involving a compulsion to engage despite the potential negative consequences, engaging without realising, using in bed and using quickly on waking. Hence, for the clinician, these are useful habits to elicit when taking a history of social media use where either reduction, abstinence or redirection to positive uses can be offered (e.g. social connection with groups of shared and benevolent values, as an educational tool), and counselling around social comparisons and negative interactions can be offered along with relevant psychotherapy, as required.
4.7.4. Resources
A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. A detailed review of current formal interventions for addressing loneliness and provides a list of UK-based social prescribing programs (Mann et al. Citation2017).
Ending Loneliness Together (Ending Loneliness Together Citation2020). An Australian-based initiative that provides resources for addressing loneliness
British Geriatrics Society and Royal College of Psychiatrists Position Statement on Loneliness and Social Isolation (British Geriatrics Society Citation2019).
4.8. Green space interaction
4.8.1. Background literature
Although precise definitions differ, green space, or nature-based interventions, refer to interventions designed to increase an individual’s exposure to vegetation-rich environments or bodies of water (also sometimes referred to as blue space) (Taylor and Hochuli Citation2017). These environments can be urban based (e.g. parks, backyards) or natural environments (e.g. beaches, forests) (Shanahan et al. Citation2019). Increased exposure to green space is associated with reduced odds of depressive symptoms in observational studies (Gascon et al. Citation2017; Houlden et al. Citation2018). Multiple mechanisms of action have been proposed that relate to evolutionary factors, bioactive compounds within the natural environment, divergence from habitual patterns of normal experience, and promotion of physical activity (Aerts et al. Citation2018; Zhang R et al. Citation2021). Limited intervention studies largely in non-clinical samples have also reported improvements in stress, quality of life, and mood (Roberts et al. Citation2019). Furthermore, there are a small number of studies, predominantly using a single arm study design, that have reported various green space interventions improve depressive symptoms in people with MDD (Barton et al. Citation2012; Berman et al. Citation2012; Korpela et al. Citation2016; Vujcic et al. Citation2017).
Due to the limited clinical intervention data, particularly in those with a clinical diagnosis of MDD, firm recommendations regarding typical intervention factors such as dose, type, and frequency are premature. However, due to the high acceptability and low risk of green space interaction and likely benefits to other lifestyle domains (e.g. increased physical activity, social interaction), encouraging interaction with green space should be considered as a component of care (see Box 9 for practical advice and tips).
4.8.2. Clinical recommendations:
4.8.3. Clinical considerations
4.8.3.1. Type and context of intervention
There is insufficient evidence to suggest that a particular green space setting is more therapeutic than another. Due to the wide variety of green space settings (e.g. parks, gardens, forests) and modes of exposure (e.g. walking, running, sitting), recommendations should be individualised with consideration for individual level (preferences, capacity, mobility, socioeconomic status, location), and area level factors (pollution, neighbourhood or cultural safety, access). Similarly, individualised discussion regarding the mode of green space interaction is warranted to ensure sustained participation. Indeed, while some evidence suggests that passive immersion in green spaces (e.g. walking or sitting) can yield health benefits (Lovell et al. Citation2015), there is some evidence that structured programs, taking place within green spaces, may be more effective than only changing the person’s physical environment (Hunter et al. Citation2015). These include horticultural or garden therapy (Genter et al. Citation2015; Cipriani et al. Citation2017), walking groups (Hanson and Jones Citation2015), wilderness therapy (Combs KM et al. Citation2016; Harper et al. Citation2019), and outdoor sports and activities (e.g. hiking, camping, swimming, tai chi). In these settings, the presence of trained facilitators is a key component to the program’s success (Bloomfield Citation2017; Masterton et al. Citation2020). An additional benefit of participation in a structured program is that they are usually undertaken in settings that promote social interaction. Indeed, participants of green space interventions report the feeling of community was the most valuable component of the program which makes it difficult to elucidate the direct and indirect benefits of such approaches (Fieldhouse Citation2003; Hassink et al. Citation2010; Barley et al. Citation2012; Adevi and Mårtensson Citation2013).
4.8.3.2. Dose, frequency, intensity
Previous reviews have contained recommendations about the required ‘dose’ of green space exposure from which to achieve mental health benefit. Authors conclude that a minimum 10-20 minutes per exposure may be required time to provide psychological and/or physiological benefits to mental health outcomes (Meredith et al. Citation2020). A recent longitudinal study found that wellbeing increased significantly with weekly contact with nature ≥ 120 min (White et al. Citation2019). People with MDD should be encouraged to simply increase their exposure to green space above their current habitual exposure rather than prescriptive advice on a set time. A related factor is ‘nature intensity’ (also referred to as ‘vegetation complexity’), describing the level of biodiversity of a specific green space environment and how this may affect possible benefits to mental health (Lovell et al. Citation2014). The evidence supporting a positive association between nature intensity and mental wellbeing is, however, limited (Houlden et al. Citation2018). Instead, factors relevant to individual preferences, feasibility and accessibility should be prioritised (Shanahan et al. Citation2015).
4.8.3.3. Sunlight exposure and depressive symptoms
Related to the potentially beneficial effect of green space environments is the emerging evidence for sun exposure for improving depressive symptoms. Possible mechanisms relate to the increased production of vitamin D and serotonin synthesis as well as modulation of relevant immune pathways (Slominski et al. Citation2005). Regular sunlight exposure may also benefit sleep interventions through regulation of the circadian rhythm, which can be disrupted in people with MDD (McClung Citation2011). A growing number of observational and intervention studies suggest that ultraviolet light exposure may improve measures of depressive symptoms and mood (Lam et al. Citation2016; Veleva et al. Citation2018). While much of the intervention evidence relates to the use of bright light therapies within controlled environments, rather than regular sunlight exposure, this suggests that encouraging green space interaction that also incorporates sun exposure may have additive benefits to reducing depressive symptoms.
4.8.4. Resources
ParkRx (Parkrx Citation2019). Provides further resources and toolkits for green space interventions and further information on USA-based models of implementation
New Zealand’s Ministry of Health Green Script program (Ministry of Health New Zealand Citation2021). An example of a health service initiative that incorporates green space focussed interventions
5. Future research needs in lifestyle-based mental health approaches for Major Depressive Disorder
Despite a growing evidence base that supports the use of lifestyle-based approaches in mental health care, there is a clear need for further research to improve the strength of evidence, refine understanding of the most effective elements, and to inform best practice. This is evidenced by the recommendations (section 4) developed for the present guideline document where most recommendations were based on Low or Limited strength of evidence and two recommendations based on expert opinion. The primary reason for the Low or Limited strength of evidence for most domains was due to recommendations being based on evidence with a high risk of bias, preventing any recommendation receiving a Grade A strength of evidence as per the WFSBP grading recommendations (Hasan et al. Citation2019). This is a key limitation in behavioural interventions in general, where important design features such as double blinding are inherently difficult to incorporate. To help guide future research efforts and to promote further research in this field, we have identified key areas of future research needs that are applicable across lifestyle interventions.
5.1. Larger trials in people with diagnosed MDD
While many lifestyle-based approaches included in these guidelines have a larger body of evidence for subsyndromal depressive symptoms and related outcomes (e.g. quality of life, stress), there are a limited number of studies comprising people with MDD for the majority of included interventions. Further studies with people with MDD are important to ensure that interventions are feasible and effective for individuals with greater severity and acuity of symptoms and within the medical and sociodemographic context of their mental health setting. Furthermore, most studies conducted have been in relatively small sample sizes and conducted over a small timeframe (typically 1-3 months). Larger and long-term (>1 year) studies are needed to inform the sustainability and scalability of interventions. This is particularly relevant to lifestyle-based approaches where behavioural maintenance can dissipate over longer time periods. Furthermore, the use of a traditional randomised controlled trial study design to assess lifestyle intervention may be relatively resource intensive and present feasibility challenges for conducting larger trials. Researchers should consider if alternative study designs such as stepped wedge cluster randomised trials may be warranted.
5.2. Further understanding of relevant biological mechanisms of action
Identifying the biological and psychosocial mechanisms of action of lifestyle-based approaches is essential for tailoring interventions treatment response. Previous reviews of individual lifestyle domains have identified numerous potential pathways through which lifestyle-based approaches could plausibly affect mental health such as the gut microbiome and the tryptophan-kynurenine metabolism (Kandola et al. Citation2019; Marx et al. Citation2021) but much of this knowledge base comes from preclinical animal studies. To advance our understanding of optimal lifestyle-based approaches for depression, robust studies in human populations are required.
5.3. Effectiveness and implementation studies are required to inform translation
We acknowledge growing ontological (validity of traditional binary diagnoses) and epistemological (e.g. distinctions between efficacy and effectiveness trials) concerns relevant to the studies included in these guidelines. For example, the use of rigorously controlled randomised controlled trials (i.e. efficacy studies) may comprise largely homogeneous cohorts (i.e. major depressive disorder [MDD] diagnostic criteria) with homogeneous outcomes (reductions in depressive symptoms) that may not reflect ‘real world’ experience. While randomised controlled trials that investigate the efficacy of an intervention are generally considered gold-standard, a notable limitation of such designs is the limited external validity or real-world effectiveness of the study results (Rothwell Citation2005). This is largely due to the homogeneous participant population and intensive, controlled delivery of the intervention that may not be easily implemented outside of a research setting. This becomes particularly relevant to lifestyle-based approaches where, despite many studies demonstrating efficacy, the translation of such interventions requires that these interventions are also scalable and can be implemented in a ‘real-world’ setting with existing clinical care structures and more complex presentations. As such, rigorous and large-scale effectiveness trials are needed. Implementation research is also required to assess individual, environmental, and organisational level factors that enable or hinder translation of interventions into clinical practice and to assess strategies that mitigate these factors (Deenik et al. Citation2020).
While effectiveness and implementation research is often considered and evaluated as separate constructs, the use of novel effectiveness-implementation hybrid designs may help reduce the time required for research results to be translated into clinical practice (Curran et al. Citation2012). Furthermore, the use of co-design and co-production principles that come from meaningful partnerships with those with lived experience of MDD, their families, carers, clinicians, and other relevant stakeholders is required to ensure interventions are meaningful, suitable, and sustainable (Deenik et al. Citation2020).
5.4. Further assessment of cost effectiveness of lifestyle-based approaches
In addition to the need to further expand the evidence base for lifestyle-based approaches to MDD, cost effectiveness research is critical to the translation and implementation of lifestyle-based approaches. Understanding the economic and resource requirements of implementing such interventions, how and who funds these models of care and the economic benefits that they may provide is critical especially in resource poor settings. Some studies have embedded cost-effectiveness analyses into intervention studies (Chatterton et al. Citation2018; Segal et al. Citation2020); however, further data are required particularly within different health settings (e.g. primary, secondary, tertiary) and regions.
5.5. Identification of optimal dose, frequency, and delivery mode
There are limited data regarding the optimal ‘dose’, frequency, and mode of delivery (e.g. digital vs face to face, group-based vs individual) with respect to lifestyle-based interventions for MDD. Furthermore, multi-component lifestyle-based approaches have been shown to be successful models of delivery in other settings of chronic disease management (O’Neil, Hawkes, et al. Citation2014) and recent meta-analysis suggest that multi-component lifestyle interventions may also provide a small but beneficial effect on depressive symptoms (Gómez-Gómez et al. Citation2020; Wong et al. Citation2021). While there are limited studies in people with diagnosed MDD, emerging meta-analyses suggest that the effect may be greater in these populations compared to the effect seen in analyses that included other populations (Gómez-Gómez et al. Citation2020; Wong et al. Citation2021). Finally, while evidence is emerging for some lifestyle-based approaches, further non-inferiority studies are required to inform how lifestyle-based approaches perform when compared to currently recommended therapies (e.g. psychotherapy, antidepressants) given that many of the existing RCTs in this field use no-intervention passive controls (e.g. usual care) as this will inform recommendations regarding the use of lifestyle-based approaches as an adjunctive or stand-alone therapy (e.g. (Young et al. Citation2022)).
6. Implementation of lifestyle-based approaches for Major Depressive Disorder
6.1. Implementation barriers, limitations, and challenges
In addition to the future research needs that are required to address the current research gaps (discussed in section 5), there are major health system-level translational challenges that present as barriers to the implementation of lifestyle-based approaches in the clinical setting. These include, but are not limited to, the lack of training in lifestyle approaches and financial support for existing health professionals in the mental health space, the potential presence of substantial clinical and financial barriers (both provider and people with MDD), the scarcity of research that provides best practice guidance in the translation into the current mental health service delivery context, and how to develop, evaluate and scale new services. This section aims to explore these barriers in greater detail to provide an overview of the challenges to optimal service delivery and to inform future inquiry and translational research in the implementation of lifestyle-based approaches in MDD.
Health systems worldwide are highly varied and have different challenges in rolling out ‘optimal’ lifestyle approaches to depression management. We recognise that the extent to which lifestyle-based mental health care is feasible and adopted is highly variable based on a number of factors related to the individual (e.g. resources, social conditions, time, culture, personality, motivation, symptom severity/acuity, entry point into the mental health care system, past experiences), the clinician (e.g. motivation, financial reimbursement model, time, personal values or beliefs, self-efficacy, training), the setting (e.g. culture, resources and availability of staff, continuity of care, organisational will), and macro level factors (e.g. mental health as a government or area level priority, socio-economic and political context, stigma, social media and competing health messaging). Adding to these complexities is that health care systems around the world face further challenges related to changing health profiles and needs of their populations as people are living longer with chronic conditions (Calder et al. Citation2019). This is especially evident in low and middle income countries where 80% of deaths from chronic conditions occur (and where the majority of the global population resides) (World Health Organization Citation2005).
These barriers can present significant challenges to health professionals implementing lifestyle and social care interventions more broadly, and it calls for innovation in the mental health service and models of care, just as has been recommended for chronic disease management (Productivity Commission Citation2021). For example, primary care physicians, such as general practitioners in countries like Australia and UK, provide mental health care to ∼75% of those seeking such help (Australian Institute of Health Welfare Citation2021), with mental health concerns being the single most common reason individuals visit a general practitioner (The Royal Australian College of General Practitioners Citation2018). However, general practitioners manage 2.1–3.6 problems on average per short consult of ∼15 mins, leaving little time or funding-imperative for lifestyle and social approaches (Beaudoin et al. Citation2001; Stuart et al. Citation2019). Studies in the USA and Australia provide some indication of how infrequently lifestyle factors are addressed, with preventive counselling/advice about nutrition and weight, exercise, smoking, lifestyle, prevention, and/or alcohol, together given in only 8 per 100 encounters. Also, the duration of such counselling made up a small minority of the total consultation time (Ory et al. Citation2007; Britt et al. Citation2015). A 2019 systematic review examining the extent to which nutrition is taught in medical education found that ‘nutrition is insufficiently incorporated into medical education, regardless of country, setting, or year of medical education’ (Crowley et al. Citation2019). Primary care providers also cite a lack of knowledge, skill and confidence, competing pressures on time, limited referral options, lack of specific funding, and a lack of supportive organisational infrastructure (Denney-Wilson et al. Citation2010). Hence, these barriers must be proactively addressed, and health professionals must be supported with targeted training, systemic support, and funding to adapt to and overcome these significant challenges.
To overcome the significant potential barriers that service users and providers may experience, mental health services, including primary care services, are often exploring and leading in the design and implementation of new models of care. However, to date, the implementation of lifestyle-based approaches and innovation in service delivery has largely been ‘grassroots’ in nature, with new in-person and digital models of health service delivery being led by independent and relatively disconnected research bodies, clinical practices, and community organisations in response to the barriers cited, service pressures, and public and provider demand and entrepreneurism. Whilst it is outside the scope of these guidelines to evaluate such models that address the previously cited barriers across different countries, jurisdictions, and health care contexts, there exist certain common practices to optimise health provider time, financial sustainability, and referral options via:
Targeted workforce development outside the traditional roles that may be more cost and time effective in both training and service provision. This includes peer coaches utilising health coaching techniques, community or ‘link’ workers for social prescribing and social services, cultural health workers, peer support workers with an area of specialisation, peer navigators to assist in the navigation of a complex health system, and ‘peer bridgers’ who support the transition from hospital to community
Effective and efficient clinical programs such as in-person and digital group visits/shared medical appointments, and online platforms that improve outcomes whilst aiming to also enhance peer-peer support
Establishing an interdisciplinary team with complementary expertise, that emphasises skill and knowledge sharing, to achieve shared goals (Choi BC and Pak Citation2006).
Use of lower-cost technology where possible to automate, personalise, economise, and enhance care coordination of services, including virtual care, telehealth, apps, wearables, online programs, text services, decision support software and artificial intelligence
Diverse engagement with communities and professionals outside of health care (e.g. cultural and lived experience representatives, built environment designers, technology, industry, government) in service co-design, delivery, governance, and evaluation
Generating greater referral and service options and specific funding for proactive care that includes prevention and early intervention outside traditional clinical settings such as direct-to community, workplace, and school mental health and wellbeing programs
Alternative funding models including public funding, private health insurance, value-based health care, membership, and subscription models.
Harnessing and adapting the structures and mechanisms within a health care setting that already exist for individuals with chronic physical health conditions and make them available for application to mental health conditions like MDD
However, the challenge of scaling and diffusing any innovation into mainstream mental health care remains. The relatively new and rapidly growing field of implementation science provides several theories, models, and frameworks (TMFs) for the development, implementation, evaluation, and scaling of new models of care. Regardless of the size or stage of the specific program, these frameworks can be useful in identifying determinants of success or failure and matching implementation strategies to produce reproducible outcomes. One such tool that can be used to consider and justify the selection of TMFs for a given project is the Theory Comparison and Selection Tool (T-CaST) (Birken et al. Citation2018), a paper and web-enabled tool that includes 16 specific criteria. Health care systems can be highly complex and locally contextual, with various guides (e.g. (Agency for Clinical Innovation Citation2013)) indicating that those championing new models may benefit from having realistic expectations as to the time and complexity involved, developing a team, aligning with existing strategies, seeking support from leadership, and planning for long term sustainability. Hence, it is frequently recommended that new models of care should be delivered adhering as close as is practically relevant and possible to these theories, models, and frameworks. Without appropriate design, implementation, and evaluation, it is unlikely models of care will receive ongoing support and be scaled effectively, hence significantly limiting their impact.
6.2. Future implementation considerations
As demonstrated in , there are a range of clinical and implementation factors that should be considered when integrating lifestyle-based approaches into clinical care. We have devised a series of 10 considerations for the future implementation of these guidelines (Summarised in ) and will discuss each consideration individually in the following section. We acknowledge these considerations act more as principles and ideal ‘goals-posts’ to guide the long-term evolution of research and clinical practice rather than what is currently in widespread practice. We also acknowledge the vast and intersecting factors, such as the aforementioned barriers and the need to adapt to local contexts that influence the uptake of lifestyle-based approaches to mental health care into practice. As stated, these considerations were developed by taskforce consensus and did not follow the previously mentioned systematic review procedure.
Figure 3. Core implementation considerations, factors, and lifestyle interventions for lifestyle-based mental health care. To yield the greatest benefits from lifestyle-based mental health care, it requires personalised individual and group clinical approaches enabled by health service and model of care innovation including health coaching, digital technology, interdisciplinary teams, group and peer-based supports, adapted in the context of socio-economic, cultural and environmental determinants. Each lifestyle intervention is colour coded for grade of evidence (dark green = grade B, light green = grade C, yellow = expert opinion).
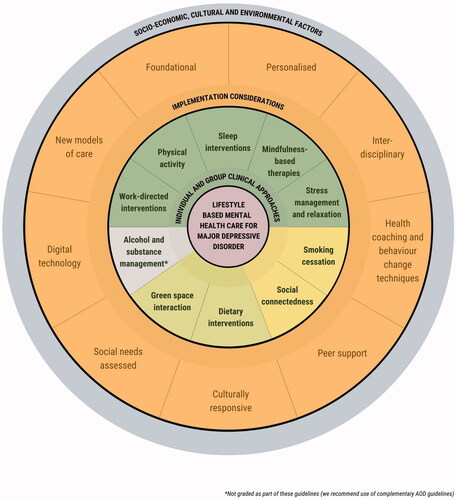
Table 4. Future implementation considerations.
Implementation consideration #1: Lifestyle assessment and interventions are foundational to mental health care and may form a starting point for treatment (sequential approach) and/or accompany psychological, pharmaceutical, or procedural interventions (adjunctive approach) to improve mental and physical health outcomes and mitigate adverse outcomes.
Given the supportive evidence for the included lifestyle interventions for managing MDD and the highly favourable safety profile, lifestyle-based approaches should be considered as a core component of mental health care with the capacity to additionally benefit comorbid physical disorders. The extent to which the lifestyle therapies of diet, exercise, sleep and substance cessation are foundational refers to care that ‘needs to be undertaken to facilitate functional recovery’ as per its definition in RANZCP guidelines (Malhi et al. Citation2021). The integration of lifestyle assessment and interventions as core components of care should not be viewed as being at the expense of pharmacotherapy or psychotherapy. Rather, this approach is intended to constitute a core component of treatment that is either initiated before commencing other therapies or to build upon other therapies where appropriate or required (see ). For some people with MDD, this might be the first step in a stepped care approach that draws upon other therapies, while, for others, it might be suitable in isolation. The feasibility of enactment of lifestyle-based approaches is also likely to be dependent upon the point in a person’s entry into the health care system, the clinician with whom they engage, the setting, and the trajectory of their journey.
Figure 4. Stepped Care Model of lifestyle-based mental health care. Lifestyle and psychological approaches are to be discussed with all people with Major Depression Disorder. Lifestyle assessment and interventions can be considered core and foundational components of care based on their strong safety profile and evidence of effect on mental, physical and social wellbeing. These approaches can be combined with other evidence-based therapies with the goal of functional recovery.
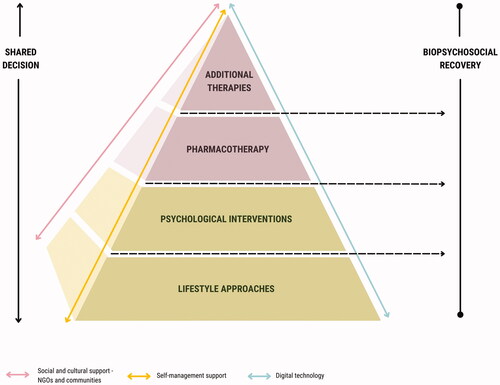
The RANZCP guidelines state that lifestyle therapies initiated during the initial phase of treatment are to be framed against a backdrop of implementing education, psychological, and social supports like addressing risk and monitoring outcomes (Malhi et al. Citation2021). Framing and applying lifestyle-based care as a key part of recovery and ongoing illness management is likely to promote uptake by people with MDD, akin to the way this approach is harnessed in more conventional chronic disease management programs. Where pharmacological or psychological therapies are already being utilised, conveying the likely cumulative benefits of initiating lifestyle changes, especially where side effects have been evident (e.g. weight gain, change in metabolic profile), is advantageous. There is also emerging evidence of productivity gains owing to lifestyle-based mental health care related to work performance and fewer absences from work (Chatterton et al. Citation2018).
Implementation consideration #2: Combining multiple lifestyle-based approaches (e.g. dietary and exercise interventions) may enhance treatment response. However, personalised considerations based on individual (e.g. motivation, acuity) and clinical (e.g. capacity, expertise) circumstances are required.
Lifestyle based mental health care is not a single intervention but rather several interacting layers that involve lifestyle domains combined with implementation. No single layer is without limitations, hence the requirement for a cumulative and diverse lifestyle and social management process. The more each of these aspects are cultivated within an individual’s life, environments, and health care, the greater the opportunity for earlier intervention, potential to prevent disease progression and relapse, enhance resilience against adverse internal or external events and improve mental, physical, and social wellbeing (see ).
Figure 5. A Swiss cheese model of Lifestyle-based mental health care. Lifestyle-based mental health care is not a single intervention but rather several mutually supportive and interacting approaches that involve lifestyle domains (covered in section 4) combined with models of care including interdisciplinary teams, peers and carers, health coaching behaviour change approaches, and digital technology (covered in section 6). The more each of these layers are cultivated and built within a person’s life and environments, the greater the likelihood of preventing disease progression, enhancing resilience against adverse internal or external events, and improving mental, physical and social wellbeing. This is illustrated by disease progression (thick black line) being mitigated by the additional layers of lifestyle approaches and implementation considerations. Each lifestyle intervention is colour coded for grade of evidence (dark green = grade B, light green = grade C, yellow = expert opinion).
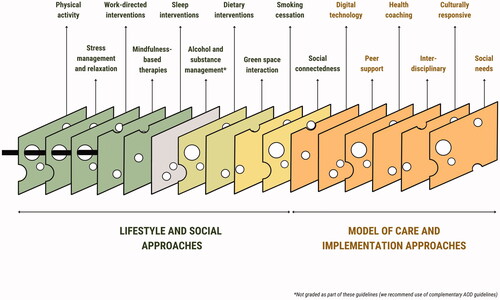
Multi-targeted, lifestyle-based programs that acknowledge the importance of a range of lifestyle factors in illness management and relapse prevention have been successfully implemented in other areas of chronic disease management including in diabetes (e.g. structured diabetes prevention programs, diabetes educator consultations) (Li et al. Citation2008) and cardiovascular disease (e.g. cardiac rehabilitation) (Medina‐Inojosa et al. Citation2021). Targeting multiple lifestyle factors in the management of MDD requires consideration of many factors. Like other conditions, these may include an individual’s capacity or receptiveness to consider multiple behavioural targets especially where other therapies, co-morbidities or intensive medication regimens are present; self-efficacy and motivation; and proficiency, confidence, health literacy or preference for a lifestyle pillar. Further considerations include those regarding potential contraindications and safety concerns including common medical comorbidities (e.g. unstable angina, heart failure, and dietary and exercise restrictions around fluid and sodium), medications (e.g. anti-glycemic and anti-hypertensives in lifestyle changes and weight loss), allergies and food intolerances, and aerobic capacity and exercise tolerance of older individuals. More specific to individuals with MDD are factors related to their illness including helplessness and hopelessness, interruptions to appetite and sleep, and the trajectory of their MDD. Examples of the synergistic effect of lifestyle approaches more commonly experienced in clinical practice have included:
Addressing sleep disorders, which subsequently improves daytime functioning and energy levels thereby facilitating increases in physical activity.
Increases in physical activity being a point of awareness for smoking-induced reduced fitness and hence motivation for smoking cessation.
Using physical activity interventions to help smoking cessation.
Increasingly, psychologists and other mental health clinicians are incorporating lifestyle targets into their therapeutic practice. The principles of CBT can readily apply to or incorporate lifestyle targets, ranging from specific CBT for insomnia programs, to sleep hygiene principles and behavioural modifications (such as sleep logs, reducing caffeine, changing habits associated with poor sleep, changing bedtimes, increasing physical activity). Moreover, applying the principles of CBT (whereby an individual’s underlying thoughts, feelings and behaviours are addressed and applied to aspects of their lifestyle) can promote long-term sustainability of those behaviours (O’Neil et al. Citation2015). These practices should be done in accordance with the discipline specific professional standards, core competencies and frameworks of that setting or in the context of a multi-disciplinary team if possible.
However, in some cases, identifying a lifestyle pillar of interest and working with the individuals to focus on one manageable target may be more achievable and feasible than addressing multiple lifestyle factors in tandem. Indeed, clinicians who support behaviour change often report ‘small and early wins’ for people with MDD to be important steps in developing confidence and self-efficacy. Hence, the individual needs, interests, self-efficacy, locus of control, intention, and motivation might guide the focus of the target behaviour(s) of interest. A practical tool for helping identify which behavioural change techniques to use with an individual with MDD can be found in the resources section of this document.
Implementation consideration #3: Assessment of lifestyle factors (using either formal tools or clinical interview) should be conducted for all people with MDD, both before and after intervention, as a means of assessing current lifestyle status and measuring progress in lifestyle changes and quality of care.
The purpose of assessing an individual’s lifestyle (e.g. diet, exercise, substance use and sleep) at treatment commencement and at a suitable follow-up period is three-fold. First, this process helps both individuals with MDD and clinician recognise the ‘baseline level’ from which change can occur and to determine which lifestyle domain(s) require focus (thereby tailoring care accordingly). Second, it provides individuals tangible evidence of improvement or change over the treatment period that can enhance confidence, self-efficacy, and motivation, which predict longer term behavioural adoption. Third, it allows for quality improvement activities to occur at clinician or service level.
There is a range of methods to address lifestyle factors that vary in their sophistication, time intensity, and user friendliness. For widely used lifestyle interventions like physical activity, there are measures that have been specifically designed for and validated with mental disorder populations such as the five-item Simple Physical Activity Questionnaire (SIMPAQ) (Rosenbaum and Ward Citation2016). There are also specific measures of sleep hygiene such as the Sleep Hygiene Index, a 13-item self-report tool used to assess the practice of sleep hygiene behaviours. Dietary assessment can be difficult and intensive to measure using existing validated tools and the suitability of a tool depends on numerous factors. However, using a 24-h recall or average dietary intake is a relatively feasible method to provide an informal overview of eating habits, though it is important to be aware of inherent recall and intention bias. It is particularly important to ask about highly processed foods and liquid calorie ingestion such as soft drinks, as these are commonly consumed, calorie dense, nutritionally devoid, and lack the ability to produce satiety. Furthermore, some medications commonly used to treat MDD, such as mirtazapine, can alter appetite; hence, asking about cravings (in particular, night-time meals) can be informative. Specifically, knowing why you are assessing diet, what it is you want to measure, demographics (age, participant literacy level), and time pressures is important. A helpful resource to make these decisions can be found in the resources section. There are also dietary recall tools that include nicotine and alcohol consumption, which can be favourable in the interest of efficiency. Where formal alcohol and other drug assessment is required, we recommend the accompanying guidelines for comorbid AOD and Mental Illness (Marel et al. Citation2016). There are ways to measure social support including the LSNS-6 tool and UCLA Loneliness scale, and mindfulness can be measured using tools like the Frieburg Mindfulness Inventory (Russell Citation1996; Lubben et al. Citation2006; Walach et al. Citation2006). However, the length of such tools should be noted. Emerging areas of lifestyle-based interventions, like use of green space, do not have formal assessment tools that have been validated or are commonly used in the mental health care setting, but prompting questions that can be integrated into clinical assessment (see ) can still be informative.
Assessment is not a ‘one-off’; sustained lifestyle behaviours are unlikely to result from episodic care but need active assessment and monitoring. Multiple guidelines in chronic diseases recommend frequent assessment and follow-up to support sustained improvements. For example, the NHMRC Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia recommend arranging fortnightly review for the first three months, and a plan for continuing monitoring for at least 12 months (National Health and Medical Research Council Citation2013). Specific timeframes must be tailored to meet individual needs and may not need to be this intensive, but this provides some reference as to the need for relatively frequent supported shared management rather than uncoordinated and reactive care. The main concern with such intensive assessment and monitoring is resource use (time, workforce, cost), access/availability and opportunity costs. In these cases, referral to an online program, group program (in-person or online), already established lifestyle program (e.g. community program, sports group), or additional health care staff (nurse, peer support worker, health coach) to provide ongoing monitoring, education, self-management, and peer support should be considered.
Implementation consideration #4: Formal assessment and consideration of social needs (e.g. housing, utilities, food, childcare) should be conducted to guide the provision of lifestyle-based approaches.
The social and environmental determinants of mental ill-health and MDD (e.g. housing, socioeconomic status, education, air pollution) are of crucial importance to assess and address, where possible. There is evidence that the distribution of depression (as well as anxiety) follows a gradient of economic disadvantage across society and that this relationship may be bidirectional (Campion et al. Citation2013). A systematic review of common mental disorders and poverty in low and middle-income countries found that over 70% of the 115 included studies reported positive associations between a variety of poverty measures and common mental disorders (Lund et al. Citation2010).
The ‘Doctors for Health Equity’ 2016 report, led by the UCL Institute of Health Equity, states that ‘Health professionals are witnesses to inequalities and see the outcomes on a daily basis. However, their potential impact on these inequalities, through action on the social determinants of health, is often under-developed’ (Institute of Health Equity and World Medical Association Citation2016). Clinicians need to be aware of the impact of these determinants, assessing and considering them in management. Involving people from diverse SES backgrounds, individuals and groups with lived experience, and social worker professionals in service design and delivery, training and research can inform the tailoring of health services to be more able to effectively address social needs (Andermann Citation2016).
The American Academy of Family Physicians provides a social needs screening tool (see ), which can be useful for clinicians working in mental health. Because of financial constraints and evidence-translation issues that can affect an individual’s ability to receive long-term lifestyle-based mental health care and, indeed, for clinicians to provide this, community linkages are critical. This is particularly true of those experiencing MDD due to socioeconomic status issues. For example, a recent study found 40% of those with mental illness are food insecure, making it more difficult for them to use lifestyle strategies to manage their mental health (Teasdale et al. Citation2021). This is where social prescribing can be employed, where available, as it may address social, economic and environmental factors related to health inequalities.
Of course, out-of-pocket expenses incurred by individuals are often a barrier to accessing mental health care and specifically physician- or allied health professional-led lifestyle-based mental health care. Economic, geographic and insurance-related considerations may present as a perceived barrier to uptake and sustainability. Until such time that this approach is offered as mainstream treatment that is subsidised, the former issues of concern will remain.
Implementation consideration #5: A person-centered, individualised approach, based on need and preference, incorporating behaviour change techniques and applying a biopsychosocial model, may aid the delivery and maintenance of lifestyle-based mental health care for people with MDD.
Behaviour change can be difficult, and clinicians are encouraged to become familiar with the range of well-researched approaches to behaviour change in health. There are a wide variety of behaviour change models, theories, and techniques including capability-motivation-opportunity-behaviour (COM-B), the transtheoretical model of change, self-determination theory, social cognitive theory, appreciative inquiry, 5 A’s (Assess, Advise, Agree, Assist, Arrange), socioecological model, and many more.
In practice, a combination of these can be used based on a biopsychosocial-cultural understanding and may include the establishment of a strong therapeutic relationship, supported self-management, SMART (specific, measurable, achievable, realistic, timely) goal setting, behavioural activation (Ekers et al. Citation2014), problem solving, self-efficacy, asset or strength-based approaches, social support, regular monitoring and identifying the driving values of the person with MDD. Environmental modification is considered in the COM-B behavioural framework, including optimising a person’s physical micro-environment context (e.g. home, workplace settings), to be more conducive to health promoting behaviours and/or creating barriers to disease promoting behaviours. There is also a growing recognition and literature on the role of built environment design in delivery of mental health care to enhance outcomes and service user satisfaction, and we recommend these aspects be strongly considered as they are often a neglected but important factors (Liddicoat et al. Citation2020).
As discussed, barriers to the adoption of lifestyle-based approaches can include individual (e.g. perceived benefits, self-efficacy), socio-economic (e.g. lack of resources, insecure environment), or clinician and/or healthcare system factors (e.g. time limits, cost, access). People with MDD may experience additional complications that reduce adherence, including mental illness–related factors (e.g. anhedonia), side effects of medication (e.g. sedation), cognitive factors (e.g. distortions, impairments), psychosocial factors (e.g. social isolation), socio-economic (e.g. poverty, access to fresh food, green space, safe neighborhoods), and clinician factors (e.g. lack of clinician training). Improving long-term adherence to treatment recommendations requires addressing factors at multiple biopsychosocial-cultural levels, shared determination of treatment plans, and detailed personalisation.
Lifestyle-based approaches may offer a novel method by which to engage typically disengaged service users in more traditional mental health treatment. They may help to overcome some of the existing barriers to their engagement with traditional mental health services such as mental health stigma. Goal setting activities that identify perceived and real threats to uptake and adherence need to be explicitly addressed. This is especially true when working with individuals who may be experiencing cognitive distortions or other cognitive impairments. As demonstrated in the diabetes prevention literature, structured and comprehensive lifestyle-based programs are especially potent while individuals are engaged, but they may wane after cessation. For example, a 15-year follow-up of one such trial found diluted effects after the initial 2.8 years of active intervention. On the other hand, long-term follow-up of the Finnish Diabetes Prevention Study (a program lasting a median 4.1 years with booster sessions) found long-term effects after 7 years (Lindström et al. Citation2013). Resource poor settings are unlikely to have capacity to cater to such an intensive and protracted schedule; however, the increasing involvement of peer workers, group modalities and health coaches may reduce financial and logistical barriers. Promisingly, since the conduct of these studies, there has been an advent in the dissemination of online programs, smart phones, and other technologies to reinforce on-going participation and promote habit formation. This is especially true in the context of COVID-19, whereby face-to-face programs may have halted or have been supplemented with telehealth and digital platforms. For example, some health coaching pilots combining face-to-face and telehealth support have developed protocols requiring only 10-15 minute follow-up consultations to adequately support behaviour change, which is a feasible in many settings (Gate et al. Citation2016).
Implementation consideration #6: Assessment and intervention plans informed by a culturally safe, culturally aware, and responsive approach may be beneficial to outcomes and improve access to high-quality lifestyle-based mental health care.
A lack of culturally aware, safe, and responsive services and information is cited as a major barrier to culturally and linguistically diverse (CALD) and Indigenous groups (Australian Institute of Health Welfare Citation2020). In Indigenous populations around the world – of which there are estimated to be up to 500 million across 90 countries – social and emotional wellbeing can include concepts beyond mental health and illness such as the importance of connection to land, culture, spirituality and ancestry (Gee G et al. Citation2014).
In the mental health setting, cultural competence represents understanding how cultural and individual beliefs and values affect perceptions and understanding of mental illness, the options and appropriateness of interventions, and the relationships with mental health service providers (Gopalkrishnan Citation2018). Genuinely understanding the local culture – or culture of origin – and concepts of mental health and wellbeing may strengthen provider-service user relationships, reduce stigma, make lifestyle-based approaches more personalised and relevant (e.g. connection to land, ‘green prescriptions’ and mindfulness), and inform and improve the health service itself. Trust is necessary to developing open therapeutic relationships that allow people from culturally diverse backgrounds to inform their practitioner about culturally grounded support mechanisms as both supportive and involved positive resources (e.g. community members, spiritual settings, practices, groups) and for harm-minimisation reasons (e.g. to avoid potentially dangerous practices).
One additional concept relevant to this consideration is Country-based or environmental therapy, which, in very broad terms, advocates healing that is conducted on Country, particularly on country to which the individual may be culturally connected. For example, in Australia there are a wide range of models of care and services within Aboriginal Community Controlled Health Organisations (ACCHOs), often with a strong focus on biopsychosocial-cultural health, interdisciplinary-community driven and strength-based approaches. Elders in the community, religious leaders, and knowledge keepers can be important contributors to mental health care (Gopalkrishnan Citation2018).
Implementation consideration #7: Including peer support workers, family, friends, and carers may aid the uptake and maintenance of lifestyle-based mental health care for people with MDD.
A key underlying premise for peer, family, and carer involvement is that these individuals and groups are in a unique position to provide support that often cannot be replicated by health-care professionals (Mental Health Foundation Citation2021a). The involvement and ongoing support of family and carers can provide an additional layer of support to people with MDD by encouraging people with MDD to access care, fostering supportive environmental change, providing encouragement, improving adherence to treatment plans, and by improving social support and reducing feelings of isolation. In addition to the need to engage with family and carers to support people with MDD, mental health services are encouraged to provide support for family and carers, who, due to the ongoing nature of mental illness, report emotional exhaustion but also report feelings of alienation and judgement from service providers (Lawn et al. Citation2015).
Peer worker support generally focuses on hope, confidence, connection, self-efficacy, and self-determination rather than illness (Orwin Citation2008), and hence, is integral to the concepts of recovery, lifestyle assessment and intervention, and wellbeing. It can promote community engagement, reduced stigmatisation, and help to address the differences in status and power imbalances between professionals and service users (Dennis Citation2003). Peer support workers are increasingly being utilised in inpatient and community-based mental health services (Pou Citation2020). Various models being explored to address gaps and enhance service access and quality, but evidence is limited (Kaleveld et al. Citation2020). Given that primary care services provide mental health care to ∼75% of those seeking such help in countries like Australia (Australian Institute of Health Welfare Citation2021), this is a service and research gap that deserves greater attention. Hence, the qualitative and quantitative outcomes of such models deserve exploration and evaluation to further optimise their meaningful integration into the diverse settings where mental health care is delivered.
There are a diverse range of important roles for peer support workers, including therapeutic roles (e.g. education, advocacy, individual therapy, facilitating groups, linking with community services, addressing social needs such as employment, education, and housing), research roles (e.g. co-design and evaluation), and throughout organisations (e.g. on management boards, recruitment panels). For example, peer-facilitated group programs used in mental health care can allow for group members to provide sympathetic understanding and provide an avenue to support or establish social networks. Within such dynamics, a variety of issues can be raised, shared, and positively responded to, such as barriers and enablers in behaviour change. Like any other mental health professional, peer support workers also need support themselves in the form of supervision and mentoring, given the complexity of mental health and healthcare systems.
Peer support has become an increasingly important strategy for individuals living with mental illness, especially in cultures where intense stigma is a major barrier to mental health care, and in healthcare systems with limited resources or limited accessibility (e.g. rural locations). This approach is popular as it can led to positive lifestyle changes, provide a sense of continuing support and connectedness and increased confidence, while also helping to combat misinformation and stigma related access to care (Pienaar and Reid Citation2021). It should be noted, however, that high level evidence for this approach to significantly improve mental health outcomes of individuals with severe mental illness is currently lacking, with a Cochrane review concluding that existing trial quality is poor and subject to bias (Chien et al. Citation2019). There is a greater need for peer support programs that are culturally tailored and provide rigorous effectiveness, economic and process evaluation data across various settings. Promisingly, the UPSIDES-RCT (2018–2022) is one such pragmatic trial being conducted across a range of high-, middle- and low-income countries (Germany, UK, Israel, India, Uganda and Tanzania), which will generate such evidence (Moran et al. Citation2020).
Implementation consideration #8: Self-management supported by digital delivery is a potentially feasible, low-cost paradigm for translation of lifestyle-based approaches into health systems worldwide
Supported self-management is a priority area for health services around the world (Crepaz-Keay Citation2010; Nichols et al. Citation2020), whereby healthcare services aim to support and empower people to manage their ongoing physical and mental health conditions themselves. Whilst it is recognised that ‘the underlying drivers and determinants of self-care capability are a range of environmental, economic and social factors that sit beyond the individual’ (Nichols et al. Citation2020), there is also an identified need to optimise how health services can enable people with MDD to be active partners in their own care. One major element of this shift is embracing the various forms of digital technology to facilitate accessible, effective and more resource-efficient supported self-management.
There are a wide variety of digital innovations that are relevant to mental health care and that are being explored in lifestyle-based mental health care (Mauriello and Artz Citation2021), including but not limited to: digital medical records (provider and user owned), telemedicine, virtual care, decision support software, use of apps with biometric and biofeedback devices, digital education programs, social media platforms, crowd-sourced digital databases (e.g. PatientsLikeMe), avatars, machine learning (Pinto et al. Citation2013), artificial intelligence and virtual reality. However, there are various barriers to digital health technologies such as digital literacy, privacy, data protection, transparency and accountability of the commercial sector, lack of consistency in technologies used across services, safety, and evidence (Cummins and Schuller Citation2020). Indeed, there can be a significant gap between the claimed and hoped benefits of certain technologies and the evidence to justify them, as can be the case for apps and wearable biometric devices (Aji et al. Citation2021).
There is growing evidence from multiple systematic reviews that digital interventions can be effective in improving depression, anxiety, and psychological well-being. However, the majority of these that are available in clinical practice are mobile- and Web-based platforms for internet cognitive behavioural therapy (iCBT) or other psychological interventions (Garrido et al. Citation2019; Lattie et al. Citation2019; Sasseville et al. Citation2021). iCBT programs include components of behavioural activation and hence, overlap with and can achieve similar goals as lifestyle-based health coaching. However, the reality is that only a select number of digital technologies currently available in mainstream clinical practice are specifically relevant to lifestyle-based approaches. Hence, there is a clear need to address this research gap and develop and evaluate digital technologies to improve the symptoms of depression via digital lifestyle-based approaches.
The extent to which digital platforms like social media groups can be used to aid lifestyle change in those with mental illness is complex. Online groups can be a motivating factor for improving physical activity with social comparison, more than the associated social support, being a mechanism promoting maintenance (Zhang J et al. Citation2016). For other areas of lifestyle, like dietary change, social comparison as a behavioural strategy is not recommended to minimise risk of disordered eating, which can be a common co-morbidity. Digital programs that employ behavioural economic strategies can effectively promote lifestyle-behaviours like healthy food purchases. In South Africa, members of a HeathyFood™ program who received a rebate of between 10% of healthy food purchases had a 6.0% rise in their ratio of healthy food to total food expenditure (Sturm et al. Citation2013).
For clinicians working in mental health, some of the more immediately available digital options include:
Digital educational programs relevant to lifestyle-based approaches, mental health, and behaviour change
Use of digital coaching platforms to register, monitor, and support lifestyle behaviours that may or may not be paired with smartphones (e.g. pedometer functions) and wearable biometric and biofeedback devices (heart rate monitors, calorie counters)
Provision of telemedicine and virtual care for health coaching for lifestyle behaviours. There is also a growing interest in the use of digital group visits/shared medical appointments in chronic disease; however, there are no studies in individuals with depression specifically
Utilising closed social media groups (e.g. Facebook) to combine professional and peer-based education, motivation and social support to improve lifestyle behaviours such as physical activity and stress management (McKeon et al. Citation2021)
Improving self-care of health professionals themselves via online course and support networks
Implementation consideration #9: If available, lifestyle interventions that involve relevant allied health professionals (e.g. exercise physiologists, dieticians) may be more beneficial for improving mental health than those that do not.
Allied health professionals are ideally placed to understand the theoretical underpinnings and clinical nuances required to execute evidence-based lifestyle-based care that comes from their training and accreditation. Indeed, meta-analyses of lifestyle-based approaches show that clinical benefit is greatest and dropout rates lowest for programs delivered by relevant health care professionals (Stubbs et al. Citation2016; Vancampfort et al. Citation2016; Firth et al. Citation2019a). Hence, including interdisciplinary allied health specialists can overcome knowledge and confidence barriers often reported by physicians (Hébert et al. Citation2012; Mörkl et al. Citation2021).
However, allied health professionals are not always accessible, available or affordable nor are all familiar with using lifestyle therapies when delivered in a mental health context. In settings where this is the case, lifestyle-based mental health care is best supervised by a qualified health professional whose expertise in this area can be supported by professional development training. For example, in the UK, ‘lifestyle medicine’ courses are now available for doctors with formal accreditation by the Royal College of General Practitioners. The capacity for allied health professional-delivered, lifestyle-based mental health care using digital platforms can bring this type of specialised care to those living with MDD, especially in settings where resources are scarce. This is increasingly important as COVID-19 places additional burden on mental health care systems around the world, particularly as global rates of MDD and anxiety have increased since 2020 (Taquet et al. Citation2021).
Implementation consideration #10: Substance use should be assessed and, where available, existing alcohol and other drug guidelines should be employed to appropriately support cessation or minimisation using established therapeutic approaches
The bidirectional relationship between mental illness and substance use is well established: substance use is a risk factor for mental illness development, symptom severity and relapse, while mental illness is a risk factor for future substance use (Davis et al. Citation2008). In addition, people with substance use disorders (SUDs) also often experience high rates of comorbid acute and chronic physical health conditions, including infections such as HIV, liver disease, and cardiac related complications (Lin WC et al. Citation2011). The exact prevalence of SUDs in mental illness and vice versa varies significantly depending on the demographics, diagnosis, geographic setting, and socioeconomic status. A 2020 systematic review and meta-analysis showed the pooled prevalence of any SUD in those with MDD was 25% (Hughes et al. Citation2020). In addition to the significant impact of substance use on an individual’s mental and physical health, there are also consequences for close relationships and society at large.
There are many barriers to accessing care, often compounded by high levels of stigma. It is not uncommon for people with a SUD and therapists to experience challenges when seeking support for people with a dual diagnosis, as treatment is often siloed and stigma around personal ‘moral’ responsibility is still frequently present. As a result, there can be substantial delays to treatment in people with SUDs, leading to preventable biopsychosocial harms (Chapman et al. Citation2015).
The role of lifestyle-based approaches in MDD may also extend into the management of substance use disorders, though there is currently limited evidence across lifestyle domains (Firth et al. Citation2020). Physical activity is one of the more commonly studied areas of lifestyle and may affect alcohol and/or substance use through various biopsychosocial mechanisms (Wang D et al. Citation2014; Linke and Ussher Citation2015), such as an increase in positive affect, acute reduction in cravings and urges, and an improvement in co-morbid physical disease, MDD, and anxiety. These mechanisms are pertinent as substance use can be a means of ‘self-medicating’ psychological distress, past and ongoing trauma, and mental illness (Smith et al. Citation2017).
It is important to consider that individuals presenting with substance use may have many other mental, medical and socioeconomic challenges. Hence, if available, an interdisciplinary approach that may include medical, psychological, allied health, social work, legal, financial, and peer support are often required. Peer support and group programs as Alcoholics Anonymous, Narcotics Anonymous, and SMART recovery (Self-Management And Recovery Training), that focus on the addictive behaviour as opposed to the substance, are available online and in person, and are run by trained facilitators, have shown promise (Beck et al. Citation2017). Further guidance can be gained from the dedicated clinical guidelines for the management of substance use in the mental health setting (see Marel et al. Citation2016).
6.3. Resources
The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders (Malhi et al. Citation2021). Clinical guidelines for mood disorders, developed by the Royal Australian and New Zealand College of Psychiatrists, that provides a model of care where lifestyle factors and interventions are a core component of clinical management.
The Lift Project (The Lift Project Citation2022). An online interdisciplinary intervention that aims to improve wellbeing
Mindfulness for Wellbeing and Peak Performance (Hassed and Chambers Citation2016). An online course on mindfulness via Monash University
The Theory and Techniques Tool (Human Behaviour Change Project Citation2022). An online tool that explores the links between 74 Behaviour Change Techniques (BCTs) and 26 Mechanisms of Action (MoAs).
Food & Mood Centre Dietary Assessment Overview (The Food & Mood Centre Citation2021). Further guidance on dietary assessment tools in the research and clinical setting
The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness (Firth et al. Citation2019b). A recent Lancet commission report on physical comorbidities present with mental illness and possible interventions for clinical management and further research.
Statement of interest
See funding section.
Box 1 Clinical advice and tips for lifestyle-based mental health care.
Delivery of lifestyle lifestyle-based mental health care is suggested to be in line with our proposed conceptual framework ()
Explore individual factors (e.g. financial, geographical, medical, and social considerations) when initiating behaviour change to promote uptake and sustainability
Explore the individual’s capability, opportunity, and motivation for initiating and maintaining behaviour change
Encourage the individual to seek out formal programs relevant to lifestyle interventions that provide supervision and structured activity.
Encourage the individual to incorporate social components (e.g. clubs, community groups, friends and/or family) to interventions.
Clinicians are encouraged to engage with relevant allied health professionals and specialists, where warranted
Consider the integration of digital and online tools to lifestyle interventions to assist with adherence and self-management
Box 2 Clinical advice and tips for increasing physical activity and exercise
Inquire about and encourage individuals to engage in modes of physical activity that they enjoy and at a frequency and intensity that they can sustain
Discuss the use of supervised physical activity options such as group classes, use of personal trainers, and team sports as these may improve adherence in some individuals
Engaging with an accredited exercise physiologist may be warranted especially where physical comorbidities exist for individuals with MDD (e.g heart conditions, COVID-19) to overcome barriers to participating in physical activity
Pairing exercise with enjoyable activities can increase motivation e.g. listening to music, socialising, or exercising with a partner, watching television, or exercising in a pleasant environment
Positive mental, physical, and social experiences acknowledged during physical activity (in real-time as opposed to retrospectively) can guide attention and increase motivation
Encourage exercise routines that are feasible to implement most days rather than sporadically and that are effortful but not too difficult, exhausting, or painful. Examples may include commencing with initially low-moderate intensity exercise (e.g. walking/cycling short distances) rather than commencing with high intensity exercise routines (e.g. sprints, long distance running)
Where feasible and appropriate, work with individuals to gradually incorporate bouts of higher intensity exercise to gain maximal antidepressant benefits
Provide examples of ways to reduce sedentary behaviour as well as improving physical activity (e.g. digital apps and push notifications, reminders, standing/walking meetings, environmental modifications such as standing desks)
Box 3 Clinical advice and tips for smoking cessation
Provide individualised education on the physical, mental, social, and financial benefits of smoking cessation
Encourage social support from family and friends and local smoking cessation support groups, including ‘social quitting’ when multiple people quit at the same time
If required, refer to a smoking cessation specialist
Discuss alternative coping strategies for stressors
Assess and develop strategies with individuals for managing urges, noting that the strategy intensity should often match the urge intensity to be effective
Acronyms like ‘DEaDS’ may be helpful in managing urges: Delay (by 10 minutes), Escape/avoid (change environment), Distract (e.g. call someone, physical activity), Substitute (e.g. nicotine replacement therapy, water)
Discuss management strategies for triggers, such as environmental cues, linked to smoking
Formal smoking cessation interventions should be discussed with an appropriate medical practitioner including:
Nicotine replacement therapy
Specific smoking cessation medications e.g. varenicline
Antidepressant medications e.g. bupropion
Talking therapies including cognitive behavioural therapy
Provide education related to withdrawal symptoms and relapse management
Box 4 Clinical advice and tips related to employment and work-directed interventions
Where available, an interdisciplinary approach, including the use of a trained workplace rehabilitation provider, may facilitate work-directed interventions
Consideration should be given to whether the individual can engage in work, either at full or reduced capacity. Consideration needs to incorporate individual related factors (e.g. symptom severity, personal motivation, comorbidities) and work-related factors (e.g. work environment, support from management, ongoing stressors)
Ongoing management should identify and address personal factors (e.g. personal relationships, finances, housing arrangements), health behaviours and attitudes, employment factors, and medical factors that may impair or delay recovery
Work-directed interventions should incorporate the individual’s functional capacity rather than considering only improvement in depressive symptoms
Individualised volunteer activities can be encouraged where employment is not feasible or appropriate
Further information provided by Mazza et al. (Citation2019).
Box 5 Clinical advice and tips to address stress.
Identify and address the underlying causes of stress if possible, including administering a social needs screening tool (see Implementation consideration #4). Examples include the provision of education regarding problem solving skills and/or assertiveness training and communication skills or linkages with community and other support workers based on the specific stressor(s)
Provide preventative and therapeutic interventions to enhance resilience to stressors. Where feasible, these can include identifying and addressing negative cognitions that may be exacerbating and reinforcing external stressors, positive psychology practices, loci of control, self-efficacy, social support, lifestyle measures, or insecure environmental factors (e.g. housing, employment)
Educate and reinforce that regular practice of mind-body techniques (e.g. MBSR, relaxation techniques) improves the skill to evoke these benefits when needed as well as increasing resilience to stressors
Mindfulness-based techniques that are delivered by well-trained professionals are likely to be more effective and mitigate mindfulness-related adverse events
Simple techniques include box breathing (breath for 4 seconds in, hold for 4 seconds, exhale over 4 seconds, hold for 4 seconds), progressive muscle relaxation, and basic mindfulness exercises
Discuss ways to prioritise more time to activities that can reduce stress, such as time with family, friends, and hobbies
Box 6 Clinical advice and tips for diet.
Encourage adherence to nutrient-dense, minimally processed dietary patterns, such as the Mediterranean diet
Incorporate joy, social connection, and mindfulness into the ‘food experience’ where possible
Where required and available, refer to a trained dietician
Increase consumption of fruits, vegetables, legumes, wholegrains, nuts, seeds, herbs and spices as tolerated
Cooking in bulk and freezing, planning meals in advance, and buying frozen vegetables, canned and dried legumes, and tinned fish can be affordable, convenient, and nutrient dense
Include a high consumption of foods rich in omega-3 polyunsaturated fatty acids and fibre
Limit intake of ultra-processed foods and discretionary foods, and replace ultra-processed foods with minimally processed nutritious foods
Consume red meat in moderation and opt for lean sources rather than processed and/or fatty cuts, considering the individual’s cultural-religious background
Include extra virgin olive oil as the main source of cooking and added oil
Consume the daily recommended water intake
Avoid excessive alcohol consumption
Partially adapted from Opie et al. (Citation2017)
Box 7 Clinical advice and tips for sleep hygiene.
Where required and available, refer to a sleep specialist
Individuals should avoid going to bed unless tired
Establish a consistent sleep schedule on both weeknights and weekends
Aim for at least 7 h of sleep
Reduce screen time and other sources of bright/artificial lighting before bedtime
Reduce fluid intake before bedtime particularly, caffeinated and/or alcoholic beverages
Introduce relaxing activities prior to going to bed (e.g. mindfulness-based meditation)
Individuals should ensure the place of sleep is relaxing, dark, and is at a comfortable temperature
Box 8 Clinical advice and tips to address loneliness and social support.
Explore options for using digital platforms to generate positive social connections, role models, peer support, and generating in-person social connections
Use behaviour change techniques to support reconnecting with past and present beneficial social connections that have gone dormant, and establishing new social connections through shared values and interests
Assess for and address negative cognitions related to social engagement
Assess social media use and related behaviours. Assessment tools related to problematic internet use may aid this (Tiego et al. Citation2021)
Specific populations (e.g. older adults, CALD communities) and people experiencing major life events (e.g. retirement, job loss) are at a greater risk of loneliness
Social prescribing models, where available, may be beneficial in addressing loneliness
Interventions should be personalised to individual circumstances and preferences (e.g religiosity, spirituality) and may incorporate other lifestyle domains (e.g. team sport)
Box 9 Clinical advice and tips for green space interaction.
Explore individual and area level factors when initiating green space-related behaviour change to promote uptake and sustainability
Even small levels of green space exposure may provide benefit and so encouraging small bouts of green space exposure may help aid in establishing new routines.
Encourage forms of green space exposure that the individual finds enjoyable and relaxing
Seek out formal programs where available such as walking groups, garden tours, and outdoor mindfulness and exercise programs
Encourage incorporating social components to green space exposure such as engaging with friends and/or family members
Incorporating natural elements into current living environment can increase green space exposure where outdoor exposure is not possible or overwhelming
Supplementary Material
Download MS Word (13.3 KB)Acknowledgments
We wish to thank the following individuals for their valuable feedback and review of this manuscript: Dr Mats Hallgren PhD (Department of Global Public Health Sciences, Karolinska Institute, Sweden); Simon Matthews FASLM MAPS DipIBLM (Wellcoaches Australia/Singapore and Avondale University Lifestyle Medicine and Health Research Centre); A/Prof Megan Teychenne (Deakin University, Geelong, Australia, Institute for Physical Activity and Nutrition (IPAN)); Professor Bob Morgan (University of Newcastle); Dr Neil Bailey (Epworth Centre for Innovation in Mental Health); Murat Yucel (BrainPark, Turner Institute for Brain and Mental Health, School of Psychological Sciences, and Monash Biomedical Imaging Facility, Monash University, Melbourne, Victoria, Australia); Dr. Jeroen Deenik (GGz Centraal and the School for Mental Health and Neuroscience, Maastricht University); Heather M. Francis (Macquarie University, Australia); Dr Dan Siskind (Metro South Addiction and Mental Health Service, Brisbane, Australia; School of Clinical Medicine, University of Queensland, Brisbane, Australia); Nicola Veronese (University of Palermo, Department of Internal Medicine, Geriatrics Section, Palermo, Italy); Beny Lafer, MD, PhD (Department of Psychiatry, University of São Paulo Medical School, Brazil); Evan Matthews, PhD (Centre for Health Behaviour Research, The School of Health Sciences, Waterford Institute of Technology, Ireland); Joseph Firth (Division of Psychology and Mental Health, University of Manchester); Dr Rebecca Segrave (BrainPark, Turner Institute for Brain and Mental Health & Monash Biomedical Imaging, Monash University, Australia); Sam Hughes (BrainPark, Monash University); Dr Farhana Mann (University College London, UK); Joep van Agteren (Mental Health and Wellbeing Program, South Australian Health and Medical Research Institute (SAHMRI)).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Adevi AA, Mårtensson F. 2013. Stress rehabilitation through garden therapy: the garden as a place in the recovery from stress. Urban Forestry Urban Greening. 12(2):230–237.
- Aerts R, Honnay O, Van Nieuwenhuyse A. 2018. Biodiversity and human health: mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br Med Bull. 127(1):5–22.
- Agency for Clinical Innovation 2013. Understanding the process to develop a Model of Care in the ACI: an ACI framework. Chatswood (Australia): NSW Health.
- Aikens JE, Rouse ME. 2005. Help-seeking for insomnia among adult patients in primary care. J Am Board Fam Pract. 18(4):257–261.
- Aji M, Gordon C, Stratton E, Calvo RA, Bartlett D, Grunstein R, Glozier N. 2021. Framework for the design engineering and clinical implementation and evaluation of mHealth apps for sleep disturbance: systematic review. J Med Internet Res. 23(2):e24607.
- Alsubaie M, Abbott R, Dunn B, Dickens C, Keil TF, Henley W, Kuyken W. 2017. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: a systematic review. Clin Psychol Rev. 55:74–91.
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. New York (NY): American Psychiatric Association.
- Andermann A. 2016. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 188(17–18):E474–E483.
- Asarnow LD, Manber R. 2019. Cognitive behavioral therapy for insomnia in depression. Sleep Med Clin. 14(2):177–184.
- Ashdown-Franks G, Firth J, Carney R, Carvalho AF, Hallgren M, Koyanagi A, Rosenbaum S, Schuch FB, Smith L, Solmi M, et al. 2020. Exercise as medicine for mental and substance use disorders: a meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 50(1):151–170.
- Audhoe SS, Nieuwenhuijsen K, Hoving JL, Sluiter JK, Frings-Dresen MH. 2018. Perspectives of unemployed workers with mental health problems: barriers to and solutions for return to work. Disabil Rehabil. 40(1):28–34.
- Australasian Faculty of Occupational Environmental Medicine. 2010. Realising the health benefits of work: position statement. Sydney: Australasian Faculty of Occupational & Environmental Medicine.
- Australasian Faculty of Occupational and Environmental Medicine. 2015. Realising the health benefits of work – an evidence update. Melbourne: Royal Australasian College of Physicians.
- Australian Institute of Health Welfare. 2020. Indigenous health and wellbeing. Canberra: AIHW.
- Australian Institute of Health Welfare. 2021. Mental health services in Australia. Canberra: AIHW.
- Ballesio A, Aquino M, Feige B, Johann AF, Kyle SD, Spiegelhalder K, Lombardo C, Rucker G, Riemann D, Baglioni C. 2018. The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: a systematic review and network meta-analysis. Sleep Med Rev. 37:114–129.
- Barley EA, Robinson S, Sikorski J. 2012. Primary-care based participatory rehabilitation: users’ views of a horticultural and arts project. Br J Gen Pract. 62(595):e127–e134.
- Barton J, Griffin M, Pretty J. 2012. Exercise-, nature-and socially interactive-based initiatives improve mood and self-esteem in the clinical population. Perspect Public Health. 132(2):89–96.
- Bastien CH, Vallières A, Morin CM. 2001. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2(4):297–307.
- Bayes J, Schloss J, Sibbritt D. 2022. The effect of a Mediterranean diet on the symptoms of depression in young males (the “AMMEND” study): a randomized control trial. Am J Clin Nutr. 116(2):572–580.
- Beaudoin C, Lussier M-T, Gagnon R, Brouillet M-I, Lalande R. 2001. Discussion of lifestyle-related issues in family practice during visits with general medical examination as the main reason for encounter: an exploratory study of content and determinants. Patient Educ Couns. 45(4):275–284.
- Beck A, Forbes E, Baker A, Kelly P, Deane F, Shakeshaft A, Hunt D, Kelly J. 2017. Systematic review of SMART Recovery: outcomes, process variables, and implications for research. Psychol Addict Behav. 31:1–20.
- Bei B, Asarnow LD, Krystal A, Edinger JD, Buysse DJ, Manber R. 2018. Treating insomnia in depression: insomnia related factors predict long-term depression trajectories. J Consult Clin Psychol. 86(3):282–293.
- Benz F, Knoop T, Ballesio A, Bacaro V, Johann AF, Rucker G, Feige B, Riemann D, Baglioni C. 2020. The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: a systematic review and network meta-analysis. Clin Psychol Rev. 80:101873.
- Berman MG, Kross E, Krpan KM, Askren MK, Burson A, Deldin PJ, Kaplan S, Sherdell L, Gotlib IH, Jonides J. 2012. Interacting with nature improves cognition and affect for individuals with depression. J Affect Disord. 140(3):300–305.
- Bickerdike L, Booth A, Wilson PM, Farley K, Wright K. 2017. Social prescribing: less rhetoric and more reality. A systematic review of the evidence. BMJ Open. 7(4):e013384.
- Birken SA, Rohweder CL, Powell BJ, Shea CM, Scott J, Leeman J, Grewe ME, Alexis Kirk M, Damschroder L, Aldridge WA, et al. 2018. T-CaST: an implementation theory comparison and selection tool. Implement Sci. 13(1):1–10.
- Bjørngaard JH, Gunnell D, Gabrielsen ME, Davey Smith G, Skorpen F, Krokan HE, Vatten LJ, Romundstad PR. 2013. The causal role of smoking in anxiety and depression: a Mendelian randomization analysis of the HUNT study. Psychol Med. 43:1–9.
- Black N, Eisma MC, Viechtbauer W, Johnston M, West R, Hartmann‐Boyce J, Michie S, Bruin M. 2020. Variability and effectiveness of comparator group interventions in smoking cessation trials: a systematic review and meta‐analysis. Addiction. 115(9):1607–1617.
- Blok DJ, de Vlas SJ, van Empelen P, van Lenthe FJ. 2017. The role of smoking in social networks on smoking cessation and relapse among adults: a longitudinal study. Prev Med. 99:105–110.
- Blom K, Jernelov S, Kraepelien M, Bergdahl MO, Jungmarker K, Ankartjarn L, Lindefors N, Kaldo V. 2015. Internet treatment addressing either insomnia or depression, for patients with both diagnoses: a randomized trial. Sleep. 38(2):267–277.
- Blom K, Jernelov S, Ruck C, Lindefors N, Kaldo V. 2017. Three-year follow-up comparing cognitive behavioral therapy for depression to cognitive behavioral therapy for insomnia, for patients with both diagnoses. Sleep. 40(8).
- Bloomfield D. 2017. What makes nature-based interventions for mental health successful? BJPsych Int. 14(4):82–85. eng.
- Blumenthal JA, Babyak MA, Craighead WE, Davidson J, Hinderliter A, Hoffman B, Doraiswamy PM, Sherwood A. 2021. The role of comorbid anxiety in exercise and depression trials: secondary analysis of the SMILE‐II randomized clinical trial. Depress Anxiety. 38(2):124–133.
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, et al. 2007. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 69(7):587–596.
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, et al. 1999. Effects of exercise training on older patients with major depression. Arch Intern Med. 159(19):2349–2356.
- Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, O’Hayer CVF, Mabe S, Johnson J, Doraiswamy PM, et al. 2012. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol. 60(12):1053–1063.
- Bond G, Lerner D, Drake R. 2017. Work-focused interventions for depression (final report). Washington, DC: Assistant Secretary for Planning and Evaluation, Health and Human Services. https://aspehhsgov/basic-report/work-focused-interventions-depression-final-report
- Borland R, Yong H-H, O’connor R, Hyland A, Thompson M. 2010. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 12(Suppl 1):S45–S50.
- Breedvelt JJ, Amanvermez Y, Harrer M, Karyotaki E, Gilbody S, Bockting CL, Cuijpers P, Ebert DD. 2019. The effects of meditation, yoga, and mindfulness on depression, anxiety, and stress in tertiary education students: a meta-analysis. Front Psychiatry. 10:193.
- Breslau N, Roth T, Rosenthal L, Andreski P. 1996. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 39(6):411–418.
- Brinsley J, Schuch F, Lederman O, Girard D, Smout M, Immink MA, Stubbs B, Firth J, Davison K, Rosenbaum S. 2021. Effects of yoga on depressive symptoms in people with mental disorders: a systematic review and meta-analysis. Br J Sports Med. 55(17):992–1000.
- British Geriatrics Society 2019. Position statement on loneliness and social isolation. London: British Geriatrics Society.
- Britt H, Miller GC, Henderson J, Bayram C, Harrison C, Valenti L, Wong C, Gordon J, Pollack AJ, Pan Y. 2015. General practice activity in Australia 2014–15. Sydney: Sydney University Press.
- Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput J-P, Chastin S, Chou R, et al. 2020. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 54(24):1451–1462.
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, Project ACQI. 1998. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 158(16):1789–1795.
- Butterworth P, Leach L, McManus S, Stansfeld S. 2013. Common mental disorders, unemployment and psychosocial job quality: is a poor job better than no job at all? Psychol Med. 43(8):1763–1772.
- Buysse DJ, Reynolds IC, Monk TH, Berman SR, Kupfer DJ. 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28(2):193–213.
- Cai L, Bao Y, Fu X, Cao H, Baranova A, Zhang X, Sun J, Zhang F. 2021. Causal links between major depressive disorder and insomnia: a Mendelian randomisation study. Gene. 768:145271. eng.
- Calder R, Dunkin R, Rochford C, Nichols T. 2019. Australian health services: too complex to navigate: a review of the national reviews of Australia’s health service arrangements. Mitchell Institute.
- Campion J, Bhugra D, Bailey S, Marmot M. 2013. Inequality and mental disorders: opportunities for action. Lancet. 382(9888):183–184.
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. 2012. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 35(2):287–302.
- Carr A, Cullen K, Keeney C, Canning C, Mooney O, Chinseallaigh E, O’Dowd A. 2021. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Posit Psychol. 16(6):749–769.
- Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. 2016. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 85(5):270–288.
- Caspersen CJ, Powell KE, Christenson GM. 1985. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 100(2):126–131.
- Chakhssi F, Kraiss JT, Sommers-Spijkerman M, Bohlmeijer ET. 2018. The effect of positive psychology interventions on well-being and distress in clinical samples with psychiatric or somatic disorders: a systematic review and meta-analysis. BMC Psychiatr. 18(1):1–17.
- Chan CS, Wong CY, Branda Y, Hui VK, Ho FY, Cuijpers P. 2021. Treating depression with a smartphone-delivered self-help cognitive behavioral therapy for insomnia: a parallel-group randomized controlled trial. Psychol Med. 1–15.
- Chapman C, Slade T, Hunt C, Teesson M. 2015. Delay to first treatment contact for alcohol use disorder. Drug Alcohol Depend. 147:116–121.
- Chatterton ML, Mihalopoulos C, O’Neil A, Itsiopoulos C, Opie R, Castle D, Dash S, Brazionis L, Berk M, Jacka F. 2018. Economic evaluation of a dietary intervention for adults with major depression (the “SMILES” trial). BMC Public Health. 18(1):1–11.
- Chien WT, Clifton AV, Zhao S, Lui S. 2019. Peer support for people with schizophrenia or other serious mental illness. Cochrane Database Syst Rev. 4:CD010880.
- Choi BC, Pak AW. 2006. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research and policy: 1. Definitions, objectives, and evidence of effectiveness. Clin Investig Med. 29:351–364.
- Choi KW, Chen C-Y, Stein MB, Klimentidis YC, Wang M-J, Koenen KC, Smoller JW, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. 2019. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiatry. 76(4):399–408.
- Choi KW, Zheutlin AB, Karlson RA, Wang MJ, Dunn EC, Stein MB, Karlson EW, Smoller JW. 2020. Physical activity offsets genetic risk for incident depression assessed via electronic health records in a biobank cohort study. Depress Anxiety. 37(2):106–114.
- Christakis NA, Fowler JH. 2008. The collective dynamics of smoking in a large social network. N Engl J Med. 358(21):2249–2258.
- Chung F, Abdullah HR, Liao P. 2016. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 149(3):631–638.
- Chung K-F, Lee C-T, Yeung W-F, Chan M-S, Chung EW-Y, Lin W-L. 2018. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 35(4):365–375.
- Cipriani J, Benz A, Holmgren A, Kinter D, McGarry J, Rufino G. 2017. A systematic review of the effects of horticultural therapy on persons with mental health conditions. Occup Ther Mental Health. 33(1):47–69.
- Cohen J. 2013. Statistical power analysis for the behavioral sciences. San Diego (CA): Academic press.
- Cohen S, Kamarck T, Mermelstein R. 1983. Perceived stress scale (PSS). J Health Soc Beh. 24:385.
- Collins S, Dash S, Allender S, Jacka F, Hoare E. 2022. Diet and mental health during emerging adulthood: a systematic review. Emerging Adulthood. 10(3):645–659.
- Combs K, Smith PJ, Sherwood A, Hoffman B, Carney RM, Freedland K, Craighead WE, Blumenthal JA. 2014. Impact of sleep complaints and depression outcomes among participants in the standard medical intervention and long-term exercise study of exercise and pharmacotherapy for depression. J Nerv Mental Dis. 202(2):167–171.
- Combs KM, Hoag MJ, Javorski S, Roberts SD. 2016. Adolescent self-assessment of an outdoor behavioral health program: longitudinal outcomes and trajectories of change. J Child Fam Stud. 25(11):3322–3330.
- Connor KM, Davidson JR. 2003. Development of a new resilience scale: the Connor‐Davidson resilience scale (CD‐RISC). Depress Anxiety. 18(2):76–82.
- Crepaz-Keay D. 2010. Self-management of mental health problems. Empowerment in mental health-working together towards leadership. Leuven (Belgium): World Health Organisation.
- Crowley J, Ball L, Hiddink GJ. 2019. Nutrition in medical education: a systematic review. Lancet Planet Health. 3(9):e379–e389.
- Cruwys T, Dingle GA, Haslam C, Haslam SA, Jetten J, Morton TA. 2013. Social group memberships protect against future depression, alleviate depression symptoms and prevent depression relapse. Soc Sci Med. 98:179–186.
- Cummins N, Schuller BW. 2020. Five crucial challenges in digital health. Front Digit Health. 2:38.
- Cunningham S, Hudson CC, Harkness K. 2021. Social media and depression symptoms: a meta-analysis. Res Child Adolesc Psychopathol. 49(2):241–253.
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. 2012. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 50(3):217–226.
- Davis L, Uezato A, Newell JM, Frazier E. 2008. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 21(1):14–18.
- Deenik J, Czosnek L, Teasdale SB, Stubbs B, Firth J, Schuch FB, Tenback DE, van Harten PN, Tak ECPM, Lederman O, et al. 2020. From impact factors to real impact: translating evidence on lifestyle interventions into routine mental health care. Transl Behav Med. 10(4):1070–1073.
- Denney-Wilson E, Fanaian M, Wan Q, Vagholkar S, Schütze H, Mark M. 2010. Lifestyle risk factors in general practice: routine assessment and management. Aust Fam Physician. 39(12):950–953.
- Dennis C-L. 2003. Peer support within a health care context: a concept analysis. Int J Nurs Stud. 40(3):321–332.
- Dinan TG, Stanton C, Long-Smith C, Kennedy P, Cryan JF, Cowan CS, Cenit MC, van der Kamp J-W, Sanz Y. 2019. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin Nutr. 38(5):1995–2001.
- Dombrovski AY, Mulsant BH, Houck PR, Mazumdar S, Lenze EJ, Andreescu C, Cyranowski JM, Reynolds CF. 3rd. 2007. Residual symptoms and recurrence during maintenance treatment of late-life depression. J Affect Disord. 103(1-3):77–82.
- Eckblad M, Chapman LJ. 1986. Development and validation of a scale for hypomanic personality. J Abnorm Psychol. 95(3):214–222.
- Edwards MK, Loprinzi PD. 2016. Experimentally increasing sedentary behavior results in increased anxiety in an active young adult population. J Affect Disord. 204:166–173.
- Egger G. 2019. Lifestyle medicine: the ‘why’, ‘what’ and ‘how’ of a developing discipline. Aust J Gen Pract. 48(10):665–668.
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. 2014. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLoS One. 9(6):e100100.
- Ending Loneliness Together. 2020. Pyrmont (Australia): ending loneliness together. [accessed 2021 October 19]. https://endingloneliness.com.au
- Endrighi R, Steptoe A, Hamer M. 2016. The effect of experimentally induced sedentariness on mood and psychobiological responses to mental stress. Br J Psychiatry. 208(3):245–251.
- Exercise Right. 2022. Mental health. Queensland, Australia: Exercise & Sports Science Australia.
- Farias M, Maraldi E, Wallenkampf K, Lucchetti G. 2020. Adverse events in meditation practices and meditation‐based therapies: a systematic review. Acta Psychiatr Scand. 142(5):374–393.
- Farley AC, Hajek P, Lycett D, Aveyard P. 2012. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Syst Rev. 1:CD006219.
- Ferrari A, Somerville A, Baxter A, Norman R, Patten S, Vos T, Whiteford H. 2013. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 43(3):471–481.
- Ferris JA, Wynne HJ. 2001. The Canadian problem gambling index. Ottawa (ON): Canadian Centre on Substance Abuse.
- Fieldhouse J. 2003. The impact of an allotment group on mental health clients’ health, wellbeing and social networking. Br J Occup Ther. 66(7):286–296.
- Filges T, Siren A, Fridberg T, Nielsen BC. 2020. Voluntary work for the physical and mental health of older volunteers: a systematic review. Campbell Syst Rev. 16(4):e1124.
- Finnes A, Ghaderi A, Dahl J, Nager A, Enebrink P. 2019. Randomized controlled trial of acceptance and commitment therapy and a workplace intervention for sickness absence due to mental disorders. J Occup Health Psychol. 24(1):198–212.
- Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, Stubbs B, Schuch FB, Carvalho AF, Jacka F, et al. 2019a. The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom Med. 81(3):265–280.
- Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, Allan S, Caneo C, Carney R, Carvalho AF, et al. 2019b. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 6(8):675–712. eng.
- Firth J, Solmi M, Wootton RE, Vancampfort D, Schuch FB, Hoare E, Gilbody S, Torous J, Teasdale SB, Jackson SE, et al. 2020. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 19(3):360–380..
- Fleming KM, Herring MP. 2018. The effects of pilates on mental health outcomes: a meta-analysis of controlled trials. Complement Ther Med. 37:80–95.
- Fluharty M, Taylor AE, Grabski M, Munafò MR. 2017. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 19(1):3–13.
- Francis HM, Stevenson RJ, Chambers JR, Gupta D, Newey B, Lim CK. 2019. A brief diet intervention can reduce symptoms of depression in young adults–a randomised controlled trial. PLoS ONE. 14(10):e0222768.
- Franzen PL, Buysse DJ. 2008. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 10(4):473–481.
- Freeman D, Sheaves B, Waite F, Harvey AG, Harrison PJ. 2020. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. 7(7):628–637.
- FutureLearn. 2021. Improving mental health through diet – online course. FutureLearn. [accessed 2021 Oct 19]. https://www.futurelearn.com/courses/food-and-mood.
- Gadinger M, Schilling O, Litaker D, Fischer J. 2012. The Work-Health-Check (WHC): a brief new tool for assessing psychosocial stress in the workplace. Work. 43(3):345–360.
- Garrido S, Millington C, Cheers D, Boydell K, Schubert E, Meade T, Nguyen QV. 2019. What works and what doesn’t work? A systematic review of digital mental health interventions for depression and anxiety in young people. Front Psychiatry. 10:759.
- Gascon M, Zijlema W, Vert C, White MP, Nieuwenhuijsen MJ. 2017. Outdoor blue spaces, human health and well-being: a systematic review of quantitative studies. Int J Hyg Environ Health. 220(8):1207–1221.
- Gate L, Warren-Gash C, Clarke A, Bartley A, Fowler E, Semple G, Strelitz J, Dutey P, Tookman A, Rodger A. 2016. Promoting lifestyle behaviour change and well-being in hospital patients: a pilot study of an evidence-based psychological intervention. J Public Health (Oxf). 38(3):e292–e300.
- Gayed A, Milligan-Saville JS, Nicholas J, Bryan BT, LaMontagne AD, Milner A, Madan I, Calvo RA, Christensen H, Mykletun A, et al. 2018. Effectiveness of training workplace managers to understand and support the mental health needs of employees: a systematic review and meta-analysis. Occup Environ Med. 75(6):462–470.
- GBD Mental Disorders Collaborators. 2022. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 9:137–150.
- Gee B, Orchard F, Clarke E, Joy A, Clarke T, Reynolds S. 2019. The effect of non-pharmacological sleep interventions on depression symptoms: a meta-analysis of randomised controlled trials. Sleep Med Rev. 43:118–128.
- Gee G, Dudgeon P, Schultz C, Hart A, Kelly K. 2014. Aboriginal and Torres Strait Islander social and emotional wellbeing. Working Together Aborig Torres Strait Isl Mental Health Wellbeing Princ Pract. 2:55–68.
- Genter C, Roberts A, Richardson J, Sheaff M. 2015. The contribution of allotment gardening to health and wellbeing: a systematic review of the literature. Br J Occup Ther. 78(10):593–605.
- Geoffroy PA, Hoertel N, Etain B, Bellivier F, Delorme R, Limosin F, Peyre H. 2018. Insomnia and hypersomnia in major depressive episode: prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J Affect Disord. 226:132–141.
- Gierisch JM, Bastian LA, Calhoun PS, McDuffie JR, Williams JW. 2012. Smoking cessation interventions for patients with depression: a systematic review and meta-analysis. J Gen Intern Med. 27(3):351–360.
- Goldberg SB, Lam SU, Britton WB, Davidson RJ. 2022. Prevalence of meditation-related adverse effects in a population-based sample in the United States. Psychother Res. 32(3):215–291.
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, Simpson TL. 2018. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 59:52–60.
- Gómez-Gómez I, Bellón JÁ, Resurrección DM, Cuijpers P, Moreno-Peral P, Rigabert A, Maderuelo-Fernández JÁ, Motrico E. 2020. Effectiveness of universal multiple-risk lifestyle interventions in reducing depressive symptoms: systematic review and meta-analysis. Prev Med. 134:106067.
- Gopalkrishnan N. 2018. Cultural diversity and mental health: considerations for policy and practice. Front Public Health. 6:179.
- Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. 2018. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry. 75(6):566–576.
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J. 1995. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction. 90(5):607–614.
- Gottlieb JF, Benedetti F, Geoffroy PA, Henriksen TEG, Lam RW, Murray G, Phelps J, Sit D, Swartz HA, Crowe M, et al. 2019. The chronotherapeutic treatment of bipolar disorders: a systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 21(8):741–773.
- Grima NA, Bei B, Mansfield D. 2019. Insomnia theory and assessment. Aust J Gen Pract. 48(4):193–197.
- Group I. 2003. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 4(2):121–132.
- Group WAW. 2002. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 97(9):1183–1194.
- Gu J, Strauss C, Bond R, Cavanagh K. 2015. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin Psychol Rev. 37:1–12.
- Guu T-W, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Hamazaki K, Freeman MP, Maes M, Matsuoka YJ, Belmaker RH, et al. 2019. International Society for Nutritional Psychiatry Research Practice Guidelines for omega-3 fatty acids in the treatment of major depressive disorder. Psychother Psychosom. 88(5):263–273.
- Hallgren M, Nguyen T-T-D, Owen N, Stubbs B, Vancampfort D, Lundin A, Dunstan D, Bellocco R, Lagerros YT. 2020. Cross-sectional and prospective relationships of passive and mentally active sedentary behaviours and physical activity with depression. Br J Psychiatry. 217(2):413–419.
- Hammen CL. 2015. Stress and depression: old questions, new approaches. Curr Opin Psychol. 4:80–85.
- Hanson S, Jones A. 2015. Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br J Sports Med. 49(11):710–715.
- Harper NJ, Mott AJ, Obee P. 2019. Client perspectives on wilderness therapy as a component of adolescent residential treatment for problematic substance use and mental health issues. Child Youth Serv Rev. 105:104450.
- Harvey AG, Murray G, Chandler RA, Soehner A. 2011. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 31(2):225–235.
- Hasan A, Bandelow B, Yatham LN, Berk M, Falkai P, Möller H-J, Kasper S, Chairs WGTF, WFSBP Guideline Task Force Chairs 2019. WFSBP guidelines on how to grade treatment evidence for clinical guideline development. World J Biol Psychiatry. 20(1):2–16.
- Haslam C, Cruwys T, Haslam SA, Jetten J. 2015. Social connectedness and health. In: Pachana, N. editor. Encyclopedia of geropsychology. Singapore: Springer.
- Hassed C, Chambers R. 2016. Mindfulness for well-being and peak performance. Melbourne, Australia: Monash University.
- Hassink J, Elings M, Zweekhorst M, van den Nieuwenhuizen N, Smit A. 2010. Care farms in the Netherlands: attractive empowerment-oriented and strengths-based practices in the community. Health Place. 16(3):423–430.
- Australian Institute of Health and Welfare. 2021. Social isolation and loneliness. Canberra: AIHW.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. 1991. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 86(9):1119–1127.
- Hébert ET, Caughy MO, Shuval K. 2012. Primary care providers’ perceptions of physical activity counselling in a clinical setting: a systematic review. Br J Sports Med. 46(9):625–631.
- Hees HL, de Vries G, Koeter MW, Schene AH. 2013. Adjuvant occupational therapy improves long-term depression recovery and return-to-work in good health in sick-listed employees with major depression: results of a randomised controlled trial. Occup Environ Med. 70(4):252–260.
- Henry AL, Miller CB, Emsley R, Sheaves B, Freeman D, Luik AI, Littlewood DL, Saunders KEA, Kanady JC, Carl JR, et al. 2021. Insomnia as a mediating therapeutic target for depressive symptoms: a sub‐analysis of participant data from two large randomized controlled trials of a digital sleep intervention. J Sleep Res. 30(1):e13140.
- Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, Furukawa TA, Kessler RC, Kohrt BA, Maj M, et al. 2022. Time for united action on depression: a Lancet–World Psychiatric Association Commission. The Lancet. 399(10328):957–1022.
- Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Frojmark M, Palagini L, Rucker G, Riemann D, et al. 2019. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 43:96–105.
- Ho FY, Chan CS, Lo WY, Leung JC. 2020. The effect of self-help cognitive behavioral therapy for insomnia on depressive symptoms: an updated meta-analysis of randomized controlled trials. J Affect Disord. 265:287–304.
- Hofmann SG, Gómez AF. 2017. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 40(4):739–749.
- Holtgraves T. 2009. Evaluating the problem gambling severity index. J Gambl Stud. 25(1):105–120.
- Horne JA, Östberg O. 1976. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 4:97–110.
- Houlden V, Weich S, Porto de Albuquerque J, Jarvis S, Rees K. 2018. The relationship between greenspace and the mental wellbeing of adults: a systematic review. PLoS One. 13(9):e0203000.
- Howes S, Hartmann‐Boyce J, Livingstone‐Banks J, Hong B, Lindson N. 2020. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2020(4):CD000031.
- Howley ET. 2001. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 33(6 Suppl):S364–S369. discussion S419.
- Hughes C, Devine RT, Foley S, Ribner AD, Mesman J, Blair C. 2020. Couples becoming parents: trajectories for psychological distress and buffering effects of social support. J Affect Disord. 265:372–380.
- Human Behaviour Change Project. 2022. Theory & techniques tool. [accessed 2022]. https://theoryandtechniquetool.humanbehaviourchange.org/
- Hunter RF, Christian H, Veitch J, Astell-Burt T, Hipp JA, Schipperijn J. 2015. The impact of interventions to promote physical activity in urban green space: a systematic review and recommendations for future research. Soc Sci Med. 124:246–256.
- Institute of Health Equity and World Medical Association. 2016. Doctors for Health Equity. The role of the World Medical Association, national medical associations and doctors in addressing the social determinants of health and health equity. http://www.instituteofhealthequity.org/Content/FileManager/wma-ihe-report_-doctors-for-health-equity-2016.pdf.
- IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, Dascal J, Marcia L, Gohar Y, Eskander L, et al. 2018. Pain and depression: a systematic review. Harv Rev Psychiatry. 26(6):352–363.
- [ISNPR] International Society for Nutritional Research. 2021. International Society for Nutritional Research. [accessed 2021 October 19]. http://www.isnpr.org.
- Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, et al. 2017. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’trial). BMC Med. 15(1):1–13.
- Jenkinson CE, Dickens AP, Jones K, Thompson-Coon J, Taylor RS, Rogers M, Bambra CL, Lang I, Richards SH. 2013. Is volunteering a public health intervention? A systematic review and meta-analysis of the health and survival of volunteers. BMC Public Health. 13(1):1–10.
- Jia Y, Wang X, Cheng Y. 2020. Relaxation therapy for depression: an updated meta-analysis. J Nerv Ment Dis. 208(4):319–328.
- Johns MW. 1991. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 14(6):540–545.
- Jorm AF, Morgan AJ, Hetrick SE. 2008. Relaxation for depression. Cochrane Database Syst Rev. 4:CD007142.
- Kaleveld L, Bock C, Seivwright A. 2020. Increasing and Improving Community Mental Health Supports in Western Australia: the findings of a co-design process led by the Western Australian Association for Mental Health in partnership with the Centre for Social Impact. Perth, Australia: The University of Western Australia.
- Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. 2019. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 107:525–539.
- Kandola A, del Pozo Cruz B, Osborn D, Stubbs B, Choi K, Hayes J. 2021. Impact of replacing sedentary behaviour with other movement behaviours on depression and anxiety symptoms: a prospective cohort study in the UK Biobank. BMC Med. 19(1):1–12.
- Kawachi I, Berkman LF. 2001. Social ties and mental health. J Urban Health. 78(3):458–467.
- Kerling A, Kück M, Tegtbur U, Grams L, Weber-Spickschen S, Hanke A, Stubbs B, Kahl K. 2017. Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. J Affect Disord. 215:152–155.
- Klainin-Yobas P, Oo WN, Suzanne Yew PY, Lau Y. 2015. Effects of relaxation interventions on depression and anxiety among older adults: a systematic review. Aging Ment Health. 19(12):1043–1055.
- Konjarski M, Murray G, Lee VV, Jackson ML. 2018. Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Med Rev. 42:47–58. eng.
- Korpela KM, Stengård E, Jussila P. 2016. Nature walks as a part of therapeutic intervention for depression. Ecopsychology. 8(1):8–15.
- Kraepelien M, Forsell E, Blom K. 2021. Large-scale implementation of insomnia treatment in routine psychiatric care: patient characteristics and insomnia-depression comorbidity. J Sleep Res. 31:e13448.
- Krogh J, Hjorthøj C, Speyer H, Gluud C, Nordentoft M. 2017. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open. 7(9):e014820.
- Krystal A. 2021 Aug 12–15. Advances in digital cognitive behavioral therapy for the treatment of insomnia. Psychiatric Times.
- Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, Hayes R, Huijbers M, Ma H, Schweizer S, et al. 2016. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry. 73(6):565–574.
- Kwan CL, Gelberg HA, Rosen JA, Chamberlin V, Shah C, Nguyen C, Pierre JM, Erickson ZD, Mena SJ, King M Jr, et al. 2014. Nutritional counseling for adults with severe mental illness: key lessons learned. J Acad Nutr Diet. 114(3):369–374. eng.
- Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, Ramasubbu R, Yatham LN, Tam EM. 2016. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 73(1):56–63.
- LaMontagne AD, Keegel T, Vallance D, Ostry A, Wolfe R. 2008. Job strain—attributable depression in a sample of working Australians: assessing the contribution to health inequalities. BMC Public Health. 8(1):1–9.
- Lane MM, Davis JA, Beattie S, Gómez‐Donoso C, Loughman A, O’Neil A, Jacka F, Berk M, Page R, Marx W, et al. 2021. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta‐analysis of 43 observational studies. Obesity Rev. 22(3).
- Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M, Akbaraly T. 2019. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. 24(7):965–986.
- Lattie EG, Adkins EC, Winquist N, Stiles-Shields C, Wafford QE, Graham AK. 2019. Digital mental health interventions for depression, anxiety, and enhancement of psychological well-being among college students: systematic review. J Med Internet Res. 21(7):e12869.
- Lawn S, McNaughton D, Fuller L. 2015. What carers of family members with mental illness say, think and do about their relative’s smoking and the implications for health promotion and service delivery: a qualitative study. Int J Mental Health Promot. 17(5):261–277.
- Lawn SJ, Pols RG, Barber JG. 2002. Smoking and quitting: a qualitative study with community-living psychiatric clients. Soc Sci Med. 54(1):93–104.
- Lederman O, Ward PB, Firth J, Maloney C, Carney R, Vancampfort D, Stubbs B, Kalucy M, Rosenbaum S. 2019. Does exercise improve sleep quality in individuals with mental illness? A systematic review and meta-analysis. J Psychiatr Res. 109:96–106.
- Leichsenring F, Steinert C, Rabung S, Ioannidis JP. 2022. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta‐analytic evaluation of recent meta‐analyses. World Psychiatry. 21(1):133–145.
- Lerner D, Adler DA, Rogers WH, Ingram E, Oslin DW. 2020. Effect of adding a work-focused intervention to integrated care for depression in the Veterans Health Administration: a randomized clinical trial. JAMA Netw Open. 3(2):e200075-e200075.
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, et al. 2008. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. The Lancet. 371(9626):1783–1789.
- Liddicoat S, Badcock P, Killackey E. 2020. Principles for designing the built environment of mental health services. Lancet Psychiatry. 7(10):915–920.
- Lim MH, Badcock J, Smith B, Engel L, Brophy L, McGrath K, Newton-Palmer T, Tebbey N, Karzis S, Mound F. 2020. Ending loneliness together in Australia. New South Wales, Australia: Ending Loneliness Together.
- Lim MH, Eres R, Vasan S. 2020. Understanding loneliness in the twenty-first century: an update on correlates, risk factors, and potential solutions. Soc Psychiatry Psychiatr Epidemiol. 55(7):793–810.
- Lin K, Stubbs B, Zou W, Zheng W, Lu W, Gao Y, Chen K, Wang S, Liu J, Huang Y, et al. 2020. Aerobic exercise impacts the anterior cingulate cortex in adolescents with subthreshold mood syndromes: a randomized controlled trial study. Transl Psychiatry. 10(1):1–7.
- Lin WC, Zhang J, Leung GY, Clark RE. 2011. Chronic physical conditions in older adults with mental illness and/or substance use disorders. J Am Geriatr Soc. 59(10):1913–1921.
- Lindström J, Peltonen M, Eriksson J, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, Uusitupa M, Tuomilehto J, Finnish Diabetes Prevention Study (DPS). 2013. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 56(2):284–293.
- Linke SE, Ussher M. 2015. Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am J Drug Alcohol Abuse. 41(1):7–15.
- Liu RT, Alloy LB. 2010. Stress generation in depression: a systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev. 30(5):582–593.
- Lovell R, Husk K, Cooper C, Stahl-Timmins W, Garside R. 2015. Understanding how environmental enhancement and conservation activities may benefit health and wellbeing: a systematic review. BMC Public Health. 15(1):1–18.
- Lovell R, Wheeler BW, Higgins SL, Irvine KN, Depledge MH. 2014. A systematic review of the health and well-being benefits of biodiverse environments. J Toxicol Environ Health B Crit Rev. 17(1):1–20.
- Lovibond PF, Lovibond SH. 1995. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 33(3):335–343.
- Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, Stuck AE. 2006. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 46(4):503–513.
- Luger TM, Suls J, Vander Weg MW. 2014. How robust is the association between smoking and depression in adults? A meta-analysis using linear mixed-effects models. Addict Behav. 39(10):1418–1429.
- Lund C, Breen A, Flisher AJ, Kakuma R, Corrigall J, Joska JA, Swartz L, Patel V. 2010. Poverty and common mental disorders in low and middle income countries: a systematic review. Soc Sci Med. 71(3):517–528.
- Ma R, Mann F, Wang J, Lloyd-Evans B, Terhune J, Al-Shihabi A, Johnson S. 2020. The effectiveness of interventions for reducing subjective and objective social isolation among people with mental health problems: a systematic review. Soc Psychiatry Psychiatr Epidemiol. 55(7):839–876.
- Machado MO, Veronese N, Sanches M, Stubbs B, Koyanagi A, Thompson T, Tzoulaki I, Solmi M, Vancampfort D, Schuch FB, et al. 2018. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med. 16(1):1–13.
- Mahmood MH, Coons SJ, Guy MC, Pelletier KR. 2010. Development and testing of the workplace stressors assessment questionnaire. J Occup Environ Med. 52(12):1192–1200.
- Malhi GS, Bell E, Bassett D, Boyce P, Bryant R, Hazell P, Hopwood M, Lyndon B, Mulder R, Porter R, et al. 2021. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 55(1):7–117.
- Mann F, Bone JK, Lloyd-Evans B, Frerichs J, Pinfold V, Ma R, Wang J, Johnson S. 2017. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 52(6):627–638.
- Marel C, Mills KL, Kingston R, Gournay K, Deady M, Kay-Lambkin F, Baker A, Teesson M. 2016. Guidelines on the management of co-occurring alcohol and other drug and mental health conditions in alcohol and other drug treatment settings. 2nd ed. Sydney: Centre of Research Excellence in Mental Health and Substance Use, National Drug and Alcohol Research Centre, University of New South Wales.
- Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, Borsini A, Firth J, Pariante CM, Berding K, et al. 2021. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 26(1):117–134.
- Marx W, Moseley G, Berk M, Jacka F. 2017. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. 76(4):427–436.
- Marx W, Veronese N, Kelly JT, Smith L, Hockey M, Collins S, Trakman GL, Hoare E, Teasdale SB, Wade A, et al. 2021. The dietary inflammatory index and human health: an umbrella review of meta-analyses of observational studies. Adv Nutr. 12(5):1681–1690.
- Masterton W, Carver H, Parkes T, Park K. 2020. Greenspace interventions for mental health in clinical and non-clinical populations: What works, for whom, and in what circumstances? Health Place. 64:102338. eng.
- Mastin DF, Bryson J, Corwyn R. 2006. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 29(3):223–227.
- Mauriello L, Artz K. 2021. Digital lifestyle medicine: designing, delivering, and scaling for impact. Am J Lifestyle Med.
- Mayers AG, Van Hooff JC, Baldwin DS. 2003. Quantifying subjective assessment of sleep and life‐quality in antidepressant‐treated depressed patients. Hum Psychopharmacol. 18(1):21–27.
- Mazza D, Brijnath B, Chakraborty S. 2019. Clinical guideline for the diagnosis and management of work-related mental health condition in general practice. Melbourne: Monash University.
- MBCT.com. MBCT.com. [accessed 2021 October 19]. https://www.mbct.com.
- McCall WV, Reboussin BA, Cohen W. 2000. Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res. 9(1):43–48.
- McCartney M, Nevitt S, Lloyd A, Hill R, White R, Duarte R. 2021. Mindfulness‐based cognitive therapy for prevention and time to depressive relapse: systematic review and network meta‐analysis. Acta Psychiatr Scand. 143(1):6–21.
- McClung CA. 2011. Circadian rhythms and mood regulation: insights from pre-clinical models. Eur Neuropsychopharmacol. 21:S683–S693.
- McIntyre RS, Zimmerman M, Goldberg JF, First MB. 2019. Differential diagnosis of major depressive disorder versus bipolar disorder: current status and best clinical practices. J Clin Psychiatry. 80(3):ot18043ah2.
- McKeon G, Steel Z, Wells R, Newby J, Hadzi-Pavlovic D, Vancampfort D, Rosenbaum S. 2021. A mental health–informed physical activity intervention for first responders and their partners delivered using facebook: mixed methods pilot study. JMIR Form Res. 5(4):e23432.
- Medina‐Inojosa JR, Grace SL, Supervia M, Stokin G, Bonikowske AR, Thomas R, Lopez‐Jimenez F. 2021. Dose of cardiac rehabilitation to reduce mortality and morbidity: a population‐based study. J Am Heart Assoc. 10(20):e021356.
- Meltzer H, Bebbington P, Dennis MS, Jenkins R, McManus S, Brugha TS. 2013. Feelings of loneliness among adults with mental disorder. Soc Psychiatry Psychiatr Epidemiol. 48(1):5–13.
- Mental Health First Aid International. 2022. Mental Health First Aid International. [accessed 2022 April 21]. https://mhfainternational.org/
- Mental Health Foundation. 2022. How to sleep better. London: Mental Health Foundation.
- Mental Health Foundation. 2021a. Peer support. London (UK): Mental Health Foundation. [accessed 2021 October 19]. https://www.mentalhealth.org.uk/a-to-z/p/peer-support.
- Mental Health Foundation. 2021b. Smoking and mental health. London (UK): Mental Health Foundation. [accessed 2021 Oct 19]. https://www.mentalhealth.org.uk/a-to-z/s/smoking-and-mental-health.
- Meredith GR, Rakow DA, Eldermire ER, Madsen CG, Shelley SP, Sachs NA. 2020. Minimum time dose in nature to positively impact the mental health of college-aged students, and how to measure it: A scoping review. Front Psychol. 10:2942.
- Ministry of Health New Zealand. 2021. Green prescriptions – information for health professionals. Wellington: Ministry of Health New Zealand. [accessed 2021 Oct 19]. https://www.health.govt.nz/our-work/preventative-health-wellness/physical-activity/green-prescriptions.
- Modini M, Joyce S, Mykletun A, Christensen H, Bryant RA, Mitchell PB, Harvey SB. 2016. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 24(4):331–336.
- Moran GS, Kalha J, Mueller-Stierlin AS, Kilian R, Krumm S, Slade M, Charles A, Mahlke C, Nixdorf R, Basangwa D, et al. 2020. Peer support for people with severe mental illness versus usual care in high-, middle-and low-income countries: study protocol for a pragmatic, multicentre, randomised controlled trial (UPSIDES-RCT). Trials. 21(1):1–15.
- Morin CM, Vallières A, Ivers H. 2007. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 30(11):1547–1554.
- Mörkl S, Stell L, Buhai DV, Schweinzer M, Wagner-Skacel J, Vajda C, Lackner S, Bengesser SA, Lahousen T, Painold A, et al. 2021. ‘An Apple a Day’?: psychiatrists, psychologists and psychotherapists report poor literacy for nutritional medicine: international survey spanning 52 countries. Nutrients. 13(3):822.
- Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. 2007. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 30(3):274–280.
- Morton E, Murray G. 2020. Assessment and treatment of sleep problems in bipolar disorder-a guide for psychologists and clinically focused review. Clin Psychol Psychother. 27(3):364–377.
- National Health and Medical Research Council. 2009. NHMRC levels of evidence and grades for recommendations for developers of guidelines. Canberra: National Health and Medical Research Council.
- National Health and Medical Research Council. 2013. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Canberra: Health Do Canberra.
- National Health Service. 2021. Returning to work after mental health issues. London (UK): National Health Service. [accessed 2021 October 19]. https://www.nhs.uk/mental-health/advice-for-life-situations-and-events/return-to-work-after-mental-health-issues/.
- National Institute for Health and Care Excellence. 2022. Depression in adults: treatment and management (update).
- Nichols T, Calder R, Morgan M, Lawn S, Beauchamp A, Trezona A, Byambasuren O, Bowman J, Clinton-McHarg T, Willis K. 2020. Self-care for health: a national policy blueprint: policy paper 2020-01. Melbourne: Mitchell Institute, Victoria University.
- Nicolaou M, Colpo M, Vermeulen E, Elstgeest LEM, Cabout M, Gibson-Smith D, Knuppel A, Sini G, Schoenaker DAJM, Mishra GD, et al. 2020. Association of a priori dietary patterns with depressive symptoms: a harmonised meta-analysis of observational studies. Psychol Med. 50(11):1872–1883.
- Nieuwenhuijsen K, Verbeek JH, Neumeyer-Gromen A, Verhoeven AC, Bültmann U, Faber B. 2020. Interventions to improve return to work in depressed people. Cochrane Database of Syst Rev. 10:CD006237.
- Norton K, Norton L. 2011. Pre-exercise screening. Guide to the Australian adult pre-exercise screening system. Queensland Australia: Exercise and Sports Science Australia, Fitness Australia and Sports Medicine Australia.
- Novick JS, Stewart JW, Wisniewski SR, Cook IA, Manev R, Nierenberg AA, Rosenbaum JF, Shores-Wilson K, Balasubramani GK, Biggs MM, et al. 2005. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. J Clin Psychiatry. 66(8):1002–1011.
- O’Gurek DT, Henke C. 2018. A practical approach to screening for social determinants of health. Fam Pract Manag. 25(3):7–12.
- O’Neil A, Hawkes AL, Atherton JJ, Patrao TA, Sanderson K, Wolfe R, Taylor CB, Oldenburg B. 2014. Telephone-delivered health coaching improves anxiety outcomes after myocardial infarction: the ‘ProActive Heart’trial. Eur J Prev Cardiol. 21(1):30–38.
- O’Neil A, Quirk SE, Housden S, Brennan SL, Williams LJ, Pasco JA, Berk M, Jacka FN. 2014. Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health. 104(10):e31–e42.
- O’Neil A, Taylor B, Hare DL, Sanderson K, Cyril S, Venugopal K, Chan B, Atherton JJ, Hawkes A, Walters DL, et al. 2015. Long-term efficacy of a tele-health intervention for acute coronary syndrome patients with depression: 12-month results of the MoodCare randomized controlled trial. Eur J Prev Cardiol. 22(9):1111–1120.
- Oliveira P, Ribeiro J, Donato H, Madeira N. 2017. Smoking and antidepressants pharmacokinetics: a systematic review. Ann Gen Psychiatry. 16(1):17–18.
- Opie RS, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, Jacka FN. 2017. Dietary recommendations for the prevention of depression. Nutr Neurosci. 20(3):161–171.
- Opie RS, O’Neil A, Jacka FN, Pizzinga J, Itsiopoulos C. 2018. A modified Mediterranean dietary intervention for adults with major depression: dietary protocol and feasibility data from the SMILES trial. Nutr Neurosci. 21(7):487–501. eng.
- Orwin D. 2008. Thematic review of peer supports: literature review and leader interviews. Wellington, New Zealand: Mental Health Commission.
- Ory MG, Peck BM, Browning C, Forjuoh SN. 2007. Lifestyle discussions during doctor-older patient interactions: the role of time in the medical encounter. Medscape General Med. 9(4):48.
- Parkrx. 2019. Parkrx. San Francisco (CA): Institute at the Golden Gate; [accessed 2021 Oct 19]. https://www.parkrx.org.
- Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, Itsiopoulos C, Niyonsenga T, Blunden S, Meyer B. 2018. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomised controlled trial (HELFIMED). J Austral Coll Nutr Environ Med. 37(1):6–18.
- Patterson F, Grandner MA, Malone SK, Rizzo A, Davey A, Edwards DG. 2019. Sleep as a target for optimized response to smoking cessation treatment. Nicotine Tob Res. 21(2):139–148.
- Pienaar MA, Reid M. 2021. A diabetes peer support intervention: patient experiences using the Mmogo-method®. Health SA. 26:1512.
- Pigeon WR, Pinquart M, Conner K. 2012. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 73(9):e1160-1167–e1167.
- Pinto MD, Hickman RL, Jr Clochesy J, Buchner M. 2013. Avatar-based depression self-management technology: promising approach to improve depressive symptoms among young adults. Appl Nurs Res. 26(1):45–48.
- Pomaki G, Franche R-L, Khushrushahi N, Murray E, Lampinen T, Mah P. 2010. Best practices for return-to-work/stay-at-work interventions for workers with mental health conditions. Vancouver (BC): Occupational Health and Safety Agency for HealthCare in BC.
- Pou T. 2020. Mental health & addiction consumer, peer support & lived experience: workforce development strategy 2020 to 2025. Auckland: Te Pou.
- Prathikanti S, Rivera R, Cochran A, Tungol JG, Fayazmanesh N, Weinmann E. 2017. Treating major depression with yoga: a prospective, randomized, controlled pilot trial. PLoS One. 12(3):e0173869.
- Prochaska JJ. 2011. Smoking and mental illness—breaking the link. N Engl J Med. 365(3):196–198.
- Productivity Commission. 2021. Innovations in care for chronic health conditions. Canberra: Productivity Commission.
- Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C. 1994. Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction. 89(5):563–572.
- Rao M, Afshin A, Singh G, Mozaffarian D. 2013. Do healthier foods and diet patterns cost more than less healthy options? A systematic review and meta-analysis. BMJ Open. 3(12):e004277.
- Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, Ravindran L, Yatham LN, Kennedy SH, Lam RW, et al. 2016. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. Complementary and alternative medicine treatments. Can J Psychiatry. 61(9):576–587.
- Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. 2015. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 9(3):366–378.
- Reynolds CF, 3rd, O’Hara R. 2013. DSM-5 sleep-wake disorders classification: overview for use in clinical practice. Am J Psychiatry. 170(10):1099–1101.
- Riebe D, Ehrman JK, Liguori G, Magal M. 2018. ACSM’s guidelines for exercise testing and prescription. Philadelphia (PA): Wolters Kluwer.
- Roberts H, van Lissa C, Hagedoorn P, Kellar I, Helbich M. 2019. The effect of short-term exposure to the natural environment on depressive mood: a systematic review and meta-analysis. Environ Res. 177:108606.
- Romera I, Perez V, Ciudad A, Caballero L, Roca M, Polavieja P, Gilaberte I. 2013. Residual symptoms and functioning in depression, does the type of residual symptom matter? A post-hoc analysis. BMC Psychiatry. 13(1):51.
- Rosenbaum S, Ward PB. 2016. The simple physical activity questionnaire. Lancet Psychiatry. 3(1):e1.
- Rosenfeld RM, Shiffman RN, Robertson P, Department of Otolaryngology State University of New York Downstate. 2013. Clinical practice guideline development manual: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 148(1 Suppl):S1–S55.
- Rossouw PJ, Rossouw JG. 2016. The predictive 6-factor resilience scale: neurobiological fundamentals and organizational application. Int J Neuropsychother. 4(1):31–45.
- Rothwell PM. 2005. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet. 365(9453):82–93.
- Russell DW. 1996. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 66(1):20–40.
- Sagner M, Egger G, Binns A, Rossner S. 2017. Lifestyle medicine: lifestyle, the environment and preventive medicine in health and disease. London: Academic Press.
- Santini ZI, Koyanagi A, Tyrovolas S, Mason C, Haro JM. 2015. The association between social relationships and depression: a systematic review. J Affect Disord. 175:53–65.
- Sarfan LD, Hilmoe HE, Gumport NB, Gasperetti CE, Zieve GG, Harvey AG. 2021. Outcomes of the Transdiagnostic Intervention for Sleep and Circadian Dysfunction (TranS-C) in a community setting: unpacking comorbidity. Behav Res Ther. 145:103948.
- Sarris J, Ravindran A, Yatham LN, Marx W, Rucklidge JJ, McIntyre RS, Akhondzadeh S, Benedetti F, Caneo C, Cramer H, et al. 2022. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J Biol Psychiatry. 1–32.
- Sasseville M, LeBlanc A, Boucher M, Dugas M, Mbemba G, Tchuente J, Chouinard M-C, Beaulieu M, Beaudet N, Skidmore B, et al. 2021. Digital health interventions for the management of mental health in people with chronic diseases: a rapid review. BMJ Open. 11(4):e044437.
- Sateia MJ. 2014. International classification of sleep disorders-third edition: highlights and modifications. Chest. 146(5):1387–1394.
- Schene AH, Koeter MW, Kikkert MJ, Swinkels JA, McCrone P. 2007. Adjuvant occupational therapy for work-related major depression works: randomized trial including economic evaluation. Psychol Med. 37(3):351–362.
- Schuch F, Vancampfort D, Firth J, Rosenbaum S, Ward P, Reichert T, Bagatini NC, Bgeginski R, Stubbs B. 2017. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 210:139–150.
- Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, Hallgren M, Ponce De Leon A, Dunn AL, Deslandes AC, et al. 2018. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 175(7):631–648.
- Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. 2016. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 77:42–51.
- Schuch FB, Vancampfort D, Rosenbaum S, Richards J, Ward PB, Veronese N, Solmi M, Cadore EL, Stubbs B. 2016. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Braz J Psychiatry. 38(3):247–254.
- Schuch FB, Werneck AO, Vancampfort D, Stubbs B, Teychene M, Lotufo PA, Benseñor I, Brunoni AR. 2021. Cross-sectional associations of leisure and transport related physical activity with depression and anxiety. J Psychiatr Res. 140:228–234.
- Scott AJ, Webb TL, Martyn-St James M, Rowse G, Weich S. 2021. Improving sleep quality leads to better mental health: a meta-analysis of randomised controlled trials. Sleep Med Rev. 60:101556.
- Seabrook EM, Kern ML, Rickard NS. 2016. Social networking sites, depression, and anxiety: a systematic review. JMIR Ment Health. 3(4):e5842.
- Secades-Villa R, Gonzalez-Roz A, García-Pérez Á, Becona E. 2017. Psychological, pharmacological, and combined smoking cessation interventions for smokers with current depression: a systematic review and meta-analysis. PLoS One. 12(12):e0188849.
- Segal L, Twizeyemariya A, Zarnowiecki D, Niyonsenga T, Bogomolova S, Wilson A, O’Dea K, Parletta N. 2020. Cost effectiveness and cost-utility analysis of a group-based diet intervention for treating major depression–the HELFIMED trial. Nutr Neurosci. 23(10):770–778.
- Selvanathan J, Pham C, Nagappa M, Peng PW, Englesakis M, Espie CA, Morin CM, Chung F. 2021. Cognitive behavioral therapy for insomnia in patients with chronic pain–a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 60:101460.
- Seow LSE, Verma SK, Mok YM, Kumar S, Chang S, Satghare P, Hombali A, Vaingankar J, Chong SA, Subramaniam M. 2018. Evaluating DSM-5 insomnia disorder and the treatment of sleep problems in a psychiatric population. J Clin Sleep Med. 14(2):237–244.
- Seshadri A, Orth SS, Adaji A, Singh B, Clark MM, Frye MA, McGillivray J, Fuller-Tyszkiewicz M. 2021. Mindfulness-based cognitive therapy, acceptance and commitment therapy, and positive psychotherapy for major depression. APT. 74(1):4–12.
- Shanahan D, Astell–Burt T, Barber E, Brymer E, Cox D, Dean J, Depledge M, Fuller R, Hartig T, Irvine K, et al. 2019. Nature–based interventions for improving health and wellbeing: the purpose, the people and the outcomes. Sports. 7(6):141.
- Shanahan DF, Fuller RA, Bush R, Lin BB, Gaston KJ. 2015. The health benefits of urban nature: how much do we need? BioScience. 65(5):476–485.
- Sharma A, Barrett MS, Cucchiara AJ, Gooneratne NS, Thase ME. 2017. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 78(01):e59–0.
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. 2017. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017:j4008.
- Shen H, Chen M, Cui D. 2020. Biological mechanism study of meditation and its application in mental disorders. Gen Psych. 33(4):e100214.
- Sivaramakrishnan D, Fitzsimons C, Kelly P, Ludwig K, Mutrie N, Saunders DH, Baker G. 2019. The effects of yoga compared to active and inactive controls on physical function and health related quality of life in older adults-systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 16(1):1–22.
- Slominski A, Wortsman J, Tobin DJ. 2005. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB j. 19(2):176–194.
- Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. 2008. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 15(3):194–200.
- Smith LL, Yan F, Charles M, Mohiuddin K, Tyus D, Adekeye O, Holden KB. 2017. Exploring the link between substance use and mental health status: what can we learn from the self-medication theory? J Health Care Poor Underserved. 28(2S):113–131.
- Soehner AM, Kaplan KA, Harvey AG. 2014. Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J Affect Disord. 167:93–97.
- Solmi M, Veronese N, Galvano D, Favaro A, Ostinelli EG, Noventa V, Favaretto E, Tudor F, Finessi M, Shin JI, et al. 2020. Factors associated with loneliness: an umbrella review of observational studies. J Affect Disord. 271:131–138.
- Spoormaker VI, Verbeek I, van den Bout J, Klip EC. 2005. Initial validation of the SLEEP-50 questionnaire. Behav Sleep Med. 3(4):227–246.
- Stepankova L, Kralikova E, Zvolska K, Pankova A, Ovesna P, Blaha M, Brose LS. 2017. Depression and smoking cessation: evidence from a smoking cessation clinic with 1-year follow-up. Ann Behav Med. 51(3):454–463.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, et al. 2019. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 366:l4898.
- Stickley A, Koyanagi A. 2016. Loneliness, common mental disorders and suicidal behavior: Findings from a general population survey. J Affect Disord. 197:81–87.
- Stockwell T, Murphy D, Hodgson R. 1983. The severity of alcohol dependence questionnaire: its use, reliability and validity. Br J Addict. 78(2):145–155.
- Stuart B, Leydon G, Woods C, Gennery E, Elsey C, Summers R, Stevenson F, Chew-Graham C, Barnes R, Drew P, et al. 2019. The elicitation and management of multiple health concerns in GP consultations. Patient Educ Couns. 102(4):687–693.
- Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, Brand S, Cordes J, Malchow B, Gerber M, et al. 2018. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry. 54:124–144.
- Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Soundy A, Veronese N, Solmi M, Schuch FB. 2016. Dropout from exercise randomized controlled trials among people with depression: a meta-analysis and meta regression. J Affect Disord. 190:457–466.
- Sturm R, An R, Segal D, Patel D. 2013. A cash-back rebate program for healthy food purchases in South Africa: results from scanner data. Am J Prev Med. 44(6):567–572.
- Sweetman A, Lack L, Van Ryswyk E, Vakulin A, Reed RL, Battersby MW, Lovato N, Adams RJ. 2021. Co-occurring depression and insomnia in Australian primary care: recent scientific evidence. Med J Aust. 215(5):230–236.
- Taquet M, Holmes EA, Harrison PJ. 2021. Depression and anxiety disorders during the COVID-19 pandemic: knowns and unknowns. Lancet. 398(10312):1665–1666.
- Taylor AE, Fluharty ME, Bjørngaard JH, Gabrielsen ME, Skorpen F, Marioni RE, Campbell A, Engmann J, Mirza SS, Loukola A, et al. 2014. Investigating the possible causal association of smoking with depression and anxiety using Mendelian randomisation meta-analysis: the CARTA consortium. BMJ Open. 4(10):e006141.
- Taylor GM, Lindson N, Farley A, Leinberger-Jabari A, Sawyer K, te Water Naudé R, Theodoulou A, King N, Burke C, Aveyard P, et al. 2021. Smoking cessation for improving mental health. Cochrane Database Syst Rev. 3:CD013522.
- Taylor L, Hochuli DF. 2017. Defining greenspace: multiple uses across multiple disciplines. Landscape Urban Plann. 158:25–38.
- Teasdale SB, Müller-Stierlin AS, Ruusunen A, Eaton M, Marx W, Firth J. 2021. Prevalence of food insecurity in people with major depression, bipolar disorder, and schizophrenia and related psychoses: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 1–18.
- Teychenne M, White RL, Richards J, Schuch FB, Rosenbaum S, Bennie JA. 2020. Do we need physical activity guidelines for mental health: what does the evidence tell us? Mental Health Phys Act. 18:100315.
- The Food & Mood Centre. 2021. Dietary assessment resource. [accessed 2022]. https://foodandmoodcentre.com.au/resources/
- The Lift Project. 2022. The Lift Project. [accessed 2022 April 20]. https://www.theliftproject.global/.
- The Royal Australian College of General Practitioners. 2018. General practice: health of the nation. East Melbourne: RACGP.
- Tian J, Venn A, Otahal P, Gall S. 2015. The association between quitting smoking and weight gain: a systemic review and meta‐analysis of prospective cohort studies. Obes Rev. 16(10):883–901.
- Tiego J, Lochner C, Ioannidis K, Brand M, Stein DJ, Yücel M, Grant JE, Chamberlain SR. 2021. Measurement of the problematic usage of the Internet unidimensional quasitrait continuum with item response theory. Psychol Assess. 33(7):652–671.
- Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SF, Altenburg TM, Chinapaw MJ, on behalf of SBRN Terminology Consensus Project Participants 2017. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 14(1):1–17.
- Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. 2017. Chapter 3: systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Adelaide, South Australia, Australia: Joanna Briggs Institute.
- US Department of Health and Human Services. 2004. The health consequences of smoking: a report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- Ussher MH, Faulkner GEJ, Angus K, Hartmann-Boyce J, Taylor AH. 2019. Exercise interventions for smoking cessation. Cochrane Database Syst Rev (10). Art. No.: CD002295.
- van der Noordt M, IJzelenberg H, Droomers M, Proper KI. 2014. Health effects of employment: a systematic review of prospective studies. Occup Environ Med. 71(10):730–736.
- Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, Probst M, Ward PB, Gaughran F, De Hert M, et al. 2017. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta‐analysis. World Psychiatry. 16(3):308–315.
- Vancampfort D, Madou T, Moens H, De Backer T, Vanhalst P, Helon C, Naert P, Rosenbaum S, Stubbs B, Probst M. 2015. Could autonomous motivation hold the key to successfully implementing lifestyle changes in affective disorders? A multicentre cross sectional study. Psychiatry Res. 228(1):100–106.
- Vancampfort D, Rosenbaum S, Schuch FB, Ward PB, Probst M, Stubbs B. 2016. Prevalence and predictors of treatment dropout from physical activity interventions in schizophrenia: a meta-analysis. Gen Hosp Psychiatry. 39:15–23.
- Vancampfort D, Stubbs B, Van Damme T, Smith L, Hallgren M, Schuch F, Deenik J, Rosenbaum S, Ashdown-Franks G, Mugisha J, et al. 2021. The efficacy of meditation-based mind-body interventions for mental disorders: a meta-review of 17 meta-analyses of randomized controlled trials. J Psychiatr Res. 134:181–191.
- Veleva BI, van Bezooijen RL, Chel VG, Numans ME, Caljouw MA. 2018. Effect of ultraviolet light on mood, depressive disorders and well‐being. Photodermatol Photoimmunol Photomed. 34(5):288–297.
- Vujcic M, Tomicevic-Dubljevic J, Grbic M, Lecic-Tosevski D, Vukovic O, Toskovic O. 2017. Nature based solution for improving mental health and well-being in urban areas. Environ Res. 158:385–392.
- Wagnild G. 2009. A review of the Resilience Scale. J Nurs Meas. 17(2):105–113.
- Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S. 2006. Measuring mindfulness—the Freiburg mindfulness inventory (FMI). Pers Individual Differ. 40(8):1543–1555.
- Wanberg CR. 2012. The individual experience of unemployment. Annu Rev Psychol. 63:369–396.
- Wang D, Wang Y, Wang Y, Li R, Zhou C. 2014. Impact of physical exercise on substance use disorders: a meta-analysis. PLoS One. 9(10):e110728.
- Wang J, Mann F, Lloyd-Evans B, Ma R, Johnson S. 2018. Associations between loneliness and perceived social support and outcomes of mental health problems: a systematic review. BMC Psychiatry. 18(1):1–16.
- Weinberger AH, Kashan RS, Shpigel DM, Esan H, Taha F, Lee CJ, Funk AP, Goodwin RD. 2017. Depression and cigarette smoking behavior: a critical review of population-based studies. Am J Drug Alcohol Abuse. 43(4):416–431.
- Werneck AO, Hoare E, Stubbs B, van Sluijs EM, Corder K. 2021. Associations between mentally-passive and mentally-active sedentary behaviours during adolescence and psychological distress during adulthood. Prev Med. 145:106436.
- White MP, Alcock I, Grellier J, Wheeler BW, Hartig T, Warber SL, Bone A, Depledge MH, Fleming LE. 2019. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci Rep. 9(1):1–11.
- Williams MG, Teasdale JD, Segal ZV, Kabat-Zinn J. 2007. The mindful way through depression: freeing yourself from chronic unhappiness. New York (NY): Guilford Press.
- Wong VW-H, Ho FY-Y, Shi N-K, Sarris J, Chung K-F, Yeung W-F. 2021. Lifestyle medicine for depression: a meta-analysis of randomized controlled trials. J Affect Disord. 284:203–216.
- Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, Hemani G, Jones HJ, Zammit S, Davey Smith G, et al. 2020. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. 50(14):2435–2443.
- World Health Organization. 2005. Chronic diseases in low and middle income countries. Geneva: WHO.
- World Health Organization. 2017. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization.
- World Health Organization. 2021. Physical activity fact sheet. Geneva: WHO. https://www.who.int/news-room/fact-sheets/detail/physical-activity.
- Yanguas J, Pinazo-Henandis S, Tarazona-Santabalbina FJ. 2018. The complexity of loneliness. Acta Biomed. 89(2):302.
- Ye YY, Zhang YF, Chen J, Liu J, Li XJ, Liu YZ, Lang Y, Lin L, Yang XJ, Jiang XJ. 2015. Internet-Based Cognitive Behavioral Therapy for Insomnia (ICBT-i) improves comorbid anxiety and depression-a meta-analysis of randomized controlled trials. PLoS One. 10(11):e0142258.
- Young LM, Moylan S, John T, Turner M, Opie R, Hockey M, Saunders D, Bruscella C, Jacka F, Teychenne M, et al. 2022. Evaluating telehealth lifestyle therapy versus telehealth psychotherapy for reducing depression in adults with COVID-19 related distress: the curbing anxiety and depression using lifestyle medicine (CALM) randomised non-inferiority trial protocol. BMC Psychiatry. 22(1):1–12.
- Youngstrom EA, Murray G, Johnson SL, Findling RL. 2013. The 7 up 7 down inventory: a 14-item measure of manic and depressive tendencies carved from the General Behavior Inventory. Psychol Assess. 25(4):1377–1383.
- Zhai L, Zhang Y, Zhang D. 2015. Sedentary behaviour and the risk of depression: a meta-analysis. Br J Sports Med. 49(11):705–709.
- Zhang J, Brackbill D, Yang S, Becker J, Herbert N, Centola D. 2016. Support or competition? How online social networks increase physical activity: a randomized controlled trial. Prev Med Rep. 4:453–458.
- Zhang R, Zhang C-Q, Rhodes RE. 2021. The pathways linking objectively-measured greenspace exposure and mental health: a systematic review of observational studies. Environ Res. 198:111233.
- Zhang Z, Zhang L, Zhang G, Jin J, Zheng Z. 2018. The effect of CBT and its modifications for relapse prevention in major depressive disorder: a systematic review and meta-analysis. BMC Psychiatry. 18(1):1–14.
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. 1988. The multidimensional scale of perceived social support. J Personal Assess. 52(1):30–41.
- Zwar N, Richmond R, Borland R, Peters M, Litt J, Bell J, Caldwell B, Ferretter I. 2011. Supporting smoking cessation: a guide for health professionals. Melbourne: The Royal Australian College of General Practitioners.