Abstract
We evaluate the spectrum of ordered three-node substructures in food webs taking self-links (cannibalism) into account. If the order of nodes in the network cannot be neglected, 512 substructures can be distinguished. Simple statistical models of networks impose constraints on the structure that prohibit a large number of substructures completely. We analyse two variants of the widely used niche model, the original niche model and the generalised niche model, and show analytically and numerically that they exclude 344 and 320 substructures, respectively. The prohibition rules for three-node substructures in the two niche-model variants are further contrasted with a large set of empirical food webs, which reveals that up to about 30% of the three-node substructures that occur in empirical food webs are prohibited by the model algorithms.
Introduction
Networks are a powerful tool to describe systems as diffe-rent as transportation and power systems, social communities, or the World Wide Web (Albert & Barabási Citation2002; Newman Citation2003). In the biological sciences, networks are used to analyse e.g. metabolic pathways in cells (Jeong et al. Citation2000), neural systems (Varshney et al. Citation2011), or the interactions of species in an ecosystem (Dunne et al. Citation2002a). In this article, we are interested in the structure of food webs, which are the networks of predator–prey interactions in ecological communities (Drossel & McKane Citation2005). Understanding the structure of these networks is of great importance to unravel the factors that determine the resistance or vulnerability of ecosystems against accelerating species loss (Dunne et al. Citation2002b; Binzer et al. Citation2011). The analysis of substructures has been used successfully to identify important building blocks or functional units of larger networks. We follow the convention of Milo et al. (Citation2002) and call such a building block that occurs significantly more often than in suitably randomised networks a motif. In theoretical ecology, the dynamics of interconnected populations that form a motif (or module, if the population size associated with a node in the motif is considered as a dynamic quantity) is a frequently studied subject (McCann et al. Citation1998). While in most types of networks self-links are not important, they naturally occur in food webs as cannibalistic interactions within species (Fox Citation1975; Polis Citation1981). They are, however, usually neglected even in the ecological literature on motif analysis (Stouffer et al. Citation2007), despite the intricate effects cannibalism can have on the population dynamics in food-web modules (Persson et al. Citation2003; Claessen et al. Citation2004).
Several models exist that generate artificial food-web structures using stochastic algorithms. These artificial networks resemble certain aspects of natural food webs, but usually also impose constraints on the links among the species. Often the existence of an order of the nodes (species) is assumed, e.g. induced by the mean body masses of the species, such that species with a higher index have a higher mean body mass. In the cascade model (Cohen & Newman Citation1985) and the generalised cascade model (Stouffer et al. Citation2005), the species form a completely ordered set, and predators are prohibited from having prey with a higher index (body mass) than themselves. This has obvious effects on the number of substructures that may occur in these types of networks. The niche model (Williams & Martinez Citation2000) is one of the most widely used models for food-web structures. It also assumes that the species form a completely ordered set. It constrains predators to take their prey from a contiguous interval on the niche axis, but up to half of the interval may extend above the niche position of the predator. This makes links that point in the direction of the node order possible, but the contiguity of the feeding ranges imposes constraints regarding the presence of cannibalistic links. The generalised niche model (Stouffer et al. Citation2006) relaxes the contiguity constraint of the feeding ranges by assigning predators a certain number of prey species with a lower index at random. We use here the convention that links point from predator to prey (Milo et al. Citation2002; Newman Citation2003; Camacho et al. Citation2007; Stouffer et al. Citation2007), while part of the literature about food webs uses another convention, where the links point in the direction of the resource flow (Drossel & McKane Citation2005).
The structure of the article is as follows. In the second section, we describe the full set of ordered three-node substructures including cannibalism, we present the simple statistical model allowing us to define the spectrum of substructures (i.e., the frequencies of their occurrence) analytically, and we compare it with the results obtained with our numerical algorithms. In the third section we build analytically the binary spectra of three-node substructures for food webs generated with the (generalised) niche model and we compare them with a data set of empirical food webs. We further determine the quantitative importance of prohibited substructures in the empirical food webs. The results are discussed in the last section.
The spectrum of ordered three-node substructures
In this article, we consider only simple networks that are characterised by one type of nodes (biological species in the context of food webs) and one type of links (feeding interactions among species). This is indeed a simplification, as non-trophic interactions can be very common in food webs (Kéfi et al. Citation2012). A link between two species i and j is considered directed. Furthermore, the networks are ordered, i.e., between any two arbitrarily chosen nodes i and j, we can define a relation such that one of the nodes is higher than the other. In food webs, the average adult body mass of the species can be used to define such a relation.
In a recent paper (Paulau et al. Citation2015), the motif analysis of food webs has been extended by taking the anisotropy of ecological niche space and the order of species into account. In the simplest case, the niche space (a niche axis) is one-dimensional (Cohen and Stephens Citation1978) and, following Elton (Citation1927), the position on this axis defines the feeding interactions of a species. If this one-dimensional niche space is anisotropic, species with a high position on the niche axis feed on those with a low position with a different probability than vice versa. Since in food webs it has been reported that predators are commonly larger than their prey (at least when parasites are excluded), body mass is indeed a useful proxy for niche position (Brose et al. Citation2006).
In such an anisotropic situation, the members of the 13 isomorphism classes of connected three-node substructures (Milo et al. Citation2002) that are formed by cyclic and mirror permutations of nodes are no longer statistically equivalent and the extended spectrum of three-node substructures must be analysed (). In each substructure, the nodes are ordered in such a way that the lowest index corresponds to the bottom node, the intermediate index corresponds to the middle node, and the highest index to the top node. A three-node substructure can be fully described by a 3 × 3 adjacency matrix M with elements Mij that are 1 if i is a predator of j and 0 else. The six non-diagonal elements correspond to links between the nodes and there are 26 = 64 different three-node substructures, as shown in
Figure 1. The 64 ordered three-node substructures without self-links. The index (rank or niche position) of a node increases from bottom to top for each substructure. The horizontal displacement of nodes is only for illustrative purposes. Shaded rectangles mark the substructures that are always prohibited in both the original and the generalised niche model, irrespective of the cannibalistic configuration. The integer index m ∈ [0; 63] is a decimal form of the binary number formed by the off-diagonal elements of the adjacency matrix.
![Figure 1. The 64 ordered three-node substructures without self-links. The index (rank or niche position) of a node increases from bottom to top for each substructure. The horizontal displacement of nodes is only for illustrative purposes. Shaded rectangles mark the substructures that are always prohibited in both the original and the generalised niche model, irrespective of the cannibalistic configuration. The integer index m ∈ [0; 63] is a decimal form of the binary number formed by the off-diagonal elements of the adjacency matrix.](/cms/asset/8640d716-ea38-4d8d-99eb-cea832a2a31f/tiee_a_1157304_f0001_b.gif)
A lot of literature about networks is focused only on connected substructures (Milo et al. Citation2002; Stouffer et al. Citation2007). If one considers a network of only three nodes and one of them (i) is isolated, i.e., Mij = Mji = 0 ∀ j / = i, then this isolated node has no effect on the others and only the connected nodes are important. But the consideration of only three-node substructures of larger networks is a rather rough limitation. If we would study substructures with one more node, then some of the previously ignored isolated nodes could be not isolated anymore and could play an important role in the functioning of the network. Therefore, we include substructures with isolated nodes in our analysis, because their frequencies are also a relevant measure of ordered networks.
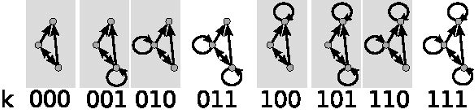
Usually, the diagonal elements of M are ignored, too, but as we have pointed out in the introduction, self-links are important in food webs. We use the diagonal elements of M to distinguish eight configurations, corresponding to three cannibalistic links that can be either present or absent (). This leads to a total of 512 different three-node substructures. We index them using (k, m) indices, where k (given in binary format for convenience, see ) is the cannibalistic configuration and m (given in decimal format) refers to the configuration of links between the species ().
Figure 2. All substructures of have 8 possible configurations describing the presence and absence of self-links. It is illustrated for substructure 15. Shaded rectangles mark the substructures that are prohibited in both the original and the generalised niche model.
To illustrate the properties of the spectrum of ordered substructures and to test our numerical algorithms, we consider here a modified version of the directed random graph discussed in more detail by Paulau et al. (Citation2015). Assume that we have a set of N indexed nodes and the directed link between each two of them has the probability p↑, if the source node index is smaller than target node index, and p↓ for an inversely directed link. In addition each node is cannibalistic (self-linked) with probability ps. Analogously to (Paulau et al. Citation2015), one can define the conditional probabilities for any ordered three-node substructure. For example, the probability Pk,m of substructure k = 011 and m = 15 in is
(1) where the first two multipliers describe the link between middle and top nodes, the third and fourth multipliers describe the link between bottom and top nodes, the fifth and sixth multipliers describe the double link between bottom and middle nodes. Finally, the last three multipliers describe the presence or absence of cannibalistic links. The number of all possible different combinations of ordered triplets in a network with N nodes is the binomial coefficient
and hence the mean frequency of each substructure can be defined as
(2)
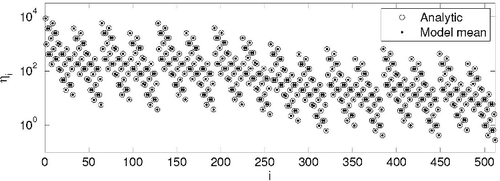
We numerically generated a large number of initialisations of the directed random graph with cannibalistic links. Our counting algorithm allows us to compute the mean spectrum of ordered substructures numerically. The last is in perfect agreement with the analytical prediction, EquationEquation (2)(2) (see , this figure is given to demonstrate the agreement of analytical and numerical results, rather than to distinguish densities of every separate substructure). We would like to note here, that in the directed random graph the probabilities Pk,m do not depend on the size of the network, while frequencies ηk,m do depend.
Figure 3. The spectrum of the directed random graph with cannibalistic links. Circles – analytical prediction, dots – numerical results obtained from 40,000 initialisations of the random graph with N = 100, p↑ = 0.3, p↓ = 0.4, and ps = 0.1. The index i is the decimal form of binary number formed by 0s and 1s of the adjacency matrix.
Three-node substructures of food-web models and empirical data
One of the prerequisites of food-web models to be able to reproduce empirical food-web topologies is that the niche values of the species form a totally ordered set (Stouffer et al. Citation2005). Very simple early models like the cascade model (Cohen & Newman Citation1985) comply with this, but also more recent and complex models like the niche model (Williams & Martinez Citation2000) assume the ordering of the species. The cascade model constrains predators to feed only on prey with a lower niche index than itself, which prohibits every substructure with an upwards arrow in , i.e., there are only eight allowed substructures (the first and fifth columns of the figure). One of the main improvements of the niche model over the cascade model is that it allows for the possibility of upward links. However, the niche model assumes that a predator species preys on all species from a contiguous interval on the niche axis, and the centre of this interval is constrained to be not higher than the predator's niche value. Due to this constraint, a predator that feeds on a prey with higher niche index is for example always assumed to be a cannibal. For three species i, j, k with ordering i < j < k, a substructure is prohibited due to the intervality constraint if at least one of the following conditions is fulfilled:
(C1)
(C2)
(C3)
(C4)
The first condition prohibits substructures where the bottom species feeds on the top species but not on the intermediate species or itself, the second condition prohibits substructures where the bottom species feeds on the intermediate species but not on itself, the third condition prohibits substructures where the intermediate species feeds on the top species but not on itself, and the fourth condition prohibits substructures where the top species preys on the bottom species and on itself but not on the intermediate species. The generalised niche model (Stouffer et al. Citation2006) was introduced as an interpolation between the niche model and the generalised cascade model that, in contrast to the niche model, has no constraints regarding diet contiguity, but prohibits upward feeding links. To this end, a parameter c ∈ [0, 1] is introduced that reduces the contiguous feeding range of a predator. To correct for the decreased expected number of prey, a corresponding number of prey species is selected at random from the pool of species with a lower niche index than the predator that are not already prey of the predator. This removes the diet-contiguity constraint for downward links, i.e., condition (EquationC4(C4) ), but substructures that fulfil either of the conditions (EquationC1
(C1) )–(EquationC3
(C3) ) are prohibited in the generalised niche model, too.
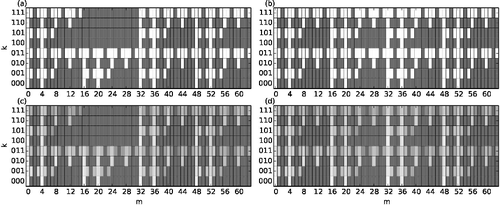
With these conditions, we can compute the binary spectra of allowed and prohibited three-node substructures in the niche model and the generalised niche model ((a) and 4(b)). Of the 512 potential substructures, only 168 are allowed in the niche model and only 192 are allowed in the generalised niche model. Numerical simulations of the two models ((c) and (d)) confirmed the analytical predictions for the binary spectra, but several of the allowed substructures were found to occur extremely rarely in the niche model (e.g. substructure (k = 111, m = 11)).
Figure 4. Spectra of three-node substructures in the niche model (a) and (c) and the generalised niche model (b) and (d). (a) and (b) Substructures that appear with nonzero (zero) probability are marked with white (grey) boxes. (c) and (d) Cumulative spectra in logarithmic representation (log(1 + ηkm)) of 10,000 initialisations of both niche model variants with N = 100 species and connectance C = 0.1. In the generalised niche model, the factor that reduces the contiguous feeding range of predators is set to c = 0.8. Dark grey corresponds to zero probability, white corresponds to maximal probability.
Natural feeding relations also underly some constraints, although they may be not as strict as in the niche model algorithms. To determine whether the substructures that are prohibited by the two niche model variants occur in empirical food webs, we evaluated 63 food webs from different habitat types that we obtained from a large food-web database (Riede et al. Citation2010; Digel et al. Citation2014). Only food webs for which body masses of all species were available were used. Species numbers in these food webs ranged from 26 to 492. The food webs in our analysis included 21 river or stream food webs, 19 lake food webs, 15 terrestrial food webs, 5 marine food webs, and 3 estuary food webs.
Prior to computing the spectrum of three-node substructures, we ordered the food webs according to the body masses of the species (with the lowest index for the lightest species). This was not always possible unambiguously because in some webs species with identical body masses (within the limits of experimental accuracy) were present. Of all possible three-node substructures we found that all but one occur in the empirical food webs (the exception is substructure (101,45)). Three-hundred and forty three (319) of the 511 present substructures are prohibited in the (generalised) niche model (see ). The niche model provides qualitatively good agreement with experimental webs for some properties such as the fraction of top species or omnivore species, as well as for Z-scores of motifs (Stouffer et al. Citation2007); however, the frequencies of substructures of empirical food webs are often (e.g. for the most frequently occurring connected substructure (000, 48), see and Paulau et al. (Citation2015)) more than two standard deviations away from the mean of the niche-model prediction. Some limitations of the niche model are discussed in (Williams & Martinez Citation2008), and more recently other variants of the niche model have been proposed that relax the assumption of diet contiguity. The latter include the extended generalised niche model (Capitán et al. Citation2013) and the probabilistic niche model (Williams & Purves Citation2011), which both allow for non-contiguous diets both below and above a predator's niche position. This will apparently resolve the problem of prohibited substructures, while it maintains to be a challenge to have all frequencies of substructure within ±2 standard deviations around the mean of the model prediction.
Figure 5. Cumulative spectrum of empirical food webs in logarithmic representation (log(1 + ηkm)). Substructures that are prohibited in both variants of the niche model but present in the empirical data are emphasised. Substructures that are allowed in the niche model or that occur with zero density are marked by dark grey colour, and substructures that are allowed in the generalised niche model but prohibited in the niche model are crossed out with a single line. The only substructure that is not observed in the empirical data-set, (101,45), is emphasised with a white frame. Dark grey corresponds to zero occurrence ηk,m, white corresponds to maximal occurrence ηk,m. All substructures that are allowed in the models are also observed in the empirical data (cf. ).
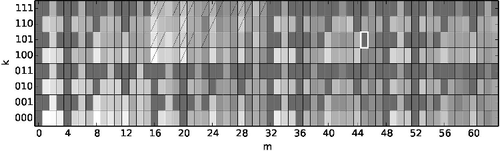
Figure 6. Quantitative spectra of connected three-node substructures in (a) the entire data-set of 63 natural food webs, (b) lake food webs, (c) marine food webs, (d) estuary food webs, (e) terrestrial food webs, and (f) river or stream food webs. Substructures that are prohibited in the niche model and in the generalised niche model are marked with black symbols, substructures that are allowed in the generalised niche model but prohibited in the niche model are marked with grey symbols, and substructures that are allowed in both models are marked with open symbols. Substructures that appear with relative frequency < 0.02 are omitted in this plot. Frequencies are normalised to the occurrence of the most frequent connected substructure, (000,20) in estuary food webs and (000,48) in all other cases.
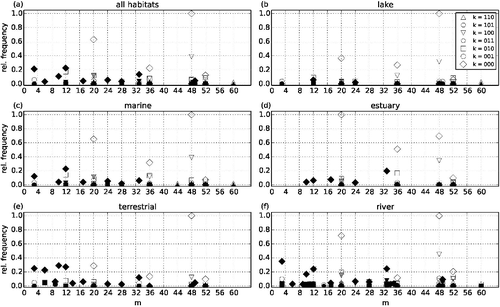
The empirical food webs have relatively small connectance (median C ≈ 0.1); therefore, the substructures with isolated nodes have very high frequencies. The trivial substructure (k = 000, m = 0) occurs approximately 10 times more often than any other substructure and nearly all connected substructures may look like quantitatively unimportant. Therefore, we discuss quantitative details only for connected substructures. In total, 3,702,652 connected substructures exist in the empirical food webs. Of these, 31.3% are prohibited in the niche model and 27.9% are prohibited in the generalised niche model. The three quantitatively most important of them, (000,48), (000,20), and (100,48), are allowed in both niche model variants, but of the 12 substructures that appear with a frequency of at least 0.1 relative to substructure (000,48), 5 (4) are prohibited in the (generalised) niche model (a). The only connected substructure of high quantitative importance that is allowed by the generalised niche model but prohibited by the original niche model is (100,20).
When the empirical food webs are grouped according to their habitat type, considerable differences can be found in the fraction of substructures that are prohibited by the two model algorithms ((b)–6(f)). It ranges from 8.9% (7.2%) in lake food webs up to 37.7% (33.9%) in river food webs and even 45.0% (44.0%) in terrestrial food webs (numbers in parentheses refer to the generalised niche model).
Discussion
In ecological networks such as food webs, self-links have a very specific meaning that cannot be ignored. This has consequences for the spectrum of three-node substructures in these networks, which consists of 512 different elements, including 432 connected substructures. The niche model is a simple, but nevertheless highly successful and widely accepted model for the prediction of food web structures. Here we have shown that due to the assumption of contiguous feeding intervals, which is a central building block of the niche model, most of the sub structures cannot occur in the niche model. This is in sharp contrast with the frequent occurrence of some of these prohibited substructures in empirical food webs.
The problem of diet contiguity in the niche model has raised prior criticism (Cattin et al. Citation2004) and several variants of the niche model have been developed specifically to address this issue. The generalised niche model is an example for this, as it allows to create model food webs with a tunable bias towards diet contiguity. It may, therefore, seem surprising that the generalised niche model prohibits almost as many substructures as the original niche model. The reason is that the generalised niche model lifts the intervality constraint only for downward links where the prey has a lower niche index than the predator.
The relatively small advantage of the generalised niche model over the original one indicates that in order to accurately predict the full spectrum of ordered three-node substructures in natural food webs, removing the intervality constraint alone might not be sufficient. It seems to be necessary to also break the strict connection between feeding on prey with a higher niche position and cannibalism that is built into both the original and the generalised niche model. Considering that cannibalism likely occurs between differently sized individuals of the same species, there is no actual mechanism that makes such a connection between these ecologically quite different processes necessary. In fact, the high frequencies of occurrence of substructures with upward feeding links but no cannibalism suggest that there is no such connection. In more recent variants of the niche model like the probabilistic niche model (Williams & Purves Citation2011), no diet contiguity at either end of the feeding range is assumed. This model therefore does not prohibit any substructures, but it still assumes a positive correlation between cannibalism and upward feeding links. Whether or not this is justified could be revealed by a quantitative comparison of the predicted and the empirically observed spectrum of three-node substructures.
The quantitative evaluation of the spectrum of (connected) three-node substructures in empirical food webs revealed that approximately 30% of all substructures are prohibited in both the original and the generalised niche model. Between different habitat types, this fraction of prohibited substructures varied considerably and ranged from less than 9% of the three-node substructures found in lake food webs up to 45% of those found in terrestrial food webs. These differences might indicate that in terrestrial ecosystems body mass is not as important for generating feeding hierarchies as it is in lake ecosystems. In Brose et al. (Citation2006) similar results have been found and it was hypothesised that this is due to physical and morphological constraints on trophic interactions. For example, if a lack of hard surfaces (as in pelagic systems) requires predators to consume their prey in one piece, gape limitation determines the maximal prey size. However, this should affect river and stream food webs in a similar way as lake food webs, which is in contrast to our finding that more than 33% of the connected three-node substructures in river or stream food webs are prohibited by the model algorithms. At this point, we, therefore, cannot rule out the possibility that methodological differences in the assembly of the food webs are responsible for the different fractions of prohibited substructures in food webs from different habitats. The lake food webs in our database were on average the smallest networks. This might be due to lower taxonomic resolution or less complete sampling, which makes direct comparison of the quantitative spectra of food webs from different habitats difficult.
The extent of the disagreement between predictions by the two niche model variants and relative frequency of substructures in empirical food webs still might in part be due to inaccurate data. In a number of food webs, several species had identical body masses, which in reality should not be the case. This lead to some ambiguity in the order of species we used to calculate the spectrum of substructures. In our data-set of empirical food webs, 31.2% of the species had a non-unique body mass in their respective food webs. While this seems like a lot, we calculated that only 2.3% of all three-node substructures contained species that could not be ordered unambiguously. For the results presented in this manuscript, we, therefore, decided to use the empirical data ‘as is’, i.e., the order of species with identical body mass was set randomly. We also tried to order the species in a conservative way with respect to prohibited substructures. Whenever a unique feeding hierarchy existed between species with identical body mass (e.g. species i feeds on species j, but not vice versa) we assumed that i had a higher index (niche position) than j. Because the majority of the species with non-unique body mass are basal species between which such a hierarchy cannot exist, this decreased the fraction of three-node substructures with two or three species with identical body mass only marginally to 2.1%. A further literature search for more accurate body-mass data could further attenuate this issue, but limited taxonomic resolution of the food webs, especially at the basal level, will also limit the success of such an effort.
Our results might also be affected by another aspect of limited resolution in the empirical data. Nodes in food webs often represent average individuals of a population (Digel et al. Citation2014). This means that a node summarises the feeding relations of individuals with different body masses, e.g. due to different developmental status. Feeding links between (on average) small predator and large prey species could thus actually occur only between the largest individuals of the predator species and the smallest individuals of the prey species. If the size ranges of the two species overlap, this would reverse the direction of the feeding link relative to the niche axis and could turn a prohibited substructure into an allowed one. Given the prevalence of ontogenetic diet shifts in natural populations (Werner & Gilliam Citation1984), resolving the size or stage dependency of feeding interactions in both models and empirical data thus seems advisable (see (Rudolf & Lafferty Citation2011), for an example of a modelling study that resolves the stage dependency of feeding links).
Finally, our results have implications for the rich body of literature that studies the population dynamics of small modules of (usually two to four) interacting populations (McCann et al. Citation1998; Hastings & Powell Citation1991; Polis & Holt Citation1992; Gjata et al. Citation2012). Due to the allometric relationship between body mass and metabolic rates (Brown et al. Citation2004), the dynamics of interacting populations depends on their relative niche positions (i.e., predator–prey body-mass ratios, Yodzis and Innes Citation1992) and stable configurations that allow for the persistence of all species are usually found if predators are larger than their prey (Otto et al. Citation2007; Kartascheff et al. Citation2010; Heckmann et al. Citation2012). Most of the three-node substructures that are prohibited by the niche model include links between a small predator and a large prey, and our results suggest that these substructures are more common in natural food webs than previously assumed. For the three-species food chain (substructures k = 000, m = 6, 9, 17, 24, 34, and 36), it has been shown that almost all instances that are found in a number of empirical food webs have predator–prey body-mass ratios that allow for coexistence of all three species in an isolated food chain (Otto et al. Citation2007). It might be interesting to see whether this also applies for other three-node substructures, or if additional mechanisms that promote species coexistence (such as coupling to the surrounding food web, or cannibalistic links) are required.
Acknowledgements
We are grateful to Ulrich Brose and Christoph Digel for providing the experimental data on food-web structures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albert R, Barabási AL. 2002. Statistical mechanics of complex networks. Rev Mod Phys. 74:47–97.
- Binzer A, Brose U, Curtsdotter A, Eklöf A, Rall BC, Riede JO, de Castro F. 2011. The susceptibility of species to extinctions in model communities. Basic Appl Ecol. 12:590–599.
- Brose U, Jonsson T, Berlow EL, Warren PH, Banasek-Richter C, Bersier LF, Blanchard JL, Brey T, Carpenter SR, Blandenier M-FC, et al. 2006. Consumer-resource body-size relationships in natural food webs. Ecology. 87:2411–417.
- Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology. 85:1771–1789.
- Camacho J, Stouffer DB, Amaral, LAN. 2007. Quantitative analysis of the local structure of food webs. J Theor Biol. 246:260–268.
- Capitán JA, Arenas A, Guimerà R. 2013. Degree of intervality of food webs: from body- size data to models. J Theor Biol. 334:35–44.
- Cattin MF, Bersier LF, Banasek-Richter C, Baltensperger R, Gabriel JP. 2004. Phylogenetic constraints and adaptation explain food-web structure. Nature. 427:835–839.
- Claessen D, de Roos AM, Persson, L. 2004. Population dynamic theory of size-dependent cannibalism. Proc Biol Sci. 271:333–340.
- Cohen JE, Newman CM. 1985. A stochastic theory of community food webs: I. Models and aggregated data. Proc Biol Sci. 224:421–448.
- Cohen JE, Stephens DW. 1978. Food Webs and Niche Space. Princeton (NJ): Princeton University Press.
- Digel C, Curtsdotter A, Riede JO, Klarner B, Brose U. 2014. Unravelling the complex structure of forest soil food webs: higher omnivory and more trophic levels. Oikos. 123:1157–1172.
- Drossel B, McKane AJ. 2005. Modelling food webs. In: Bornholdt S, Schuster HG, editors. Handbook of graphs and networks: from the genome to the internet. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; p. 218–247.
- Dunne JA, Williams RJ, Martinez ND. 2002a. Food-web structure and network theory: the role of connectance and size. Proc Natl Acad Sci USA. 99:12917–12922.
- Dunne JA, Williams RJ, Martinez ND. 2002b. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol Lett. 5:558–567.
- Elton CS. 1927. Animal ecology. New York (NY): Macmillan Co.
- Fox LR. 1975. Cannibalism in natural populations. Ann Rev Ecol Syst. 6:87–106.
- Gjata N, Scotti M, Jordán F. 2012. The strength of simulated indirect interaction modules in a real food web. Ecol Complex. 11:160–164.
- Hastings A, Powell T. 1991. Chaos in a three species food chain. Ecology. 72:896–903.
- Heckmann L, Drossel B, Brose U, Guill C. 2012. Interactive effects of body-size structure and adaptive foraging on food-web stability. Ecol Lett. 15:243–250.
- Jeong H, Tombor B, Albert R, Oltvai ZN, Barabási AL. 2000. The large-scale organization of metabolic networks. Nature. 407:651–654.
- Kartascheff B, Heckmann L, Drossel B, Guill C. 2010. Why allometric scaling enhances stability in food web models. Theor Ecol. 3:195–208.
- Kéfi S, Berlow EL, Wieters EA, Navarrete SA, Petchey OL, Wood SA, Boit A, Joppa LN, Lafferty KD, Williams RJ, et al. 2012. More than a meal... integrating non-feeding interactions into food webs. Ecol Lett. 15:291–300.
- McCann K, Hastings A, Huxel GR. 1998. Weak trophic interactions and the balance of nature. Nature. 395:794–798.
- Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. 2002. Network motifs: simple building blocks of complex networks. Science. 298:824–827.
- Newman MEJ. 2003. The structure and function of complex networks. SIAM Rev. 45:167–256.
- Otto SB, Rall BC, Brose U. 2007. Allometric degree distributions facilitate food-web stability. Nature. 450:1226–1229.
- Paulau P, Feenders C, Blasius B. 2015. Extended structural analysis of ordered networks. (Unpublished manuscript, available upon request from P. Paulau.)
- Persson L, de Roos AM, Claessen D, Bystrom P, Lovgren J, Sjogren S, Svanback R, Wahlström E, Westman E. 2003. Gigantic cannibals driving a whole-lake trophic cascade. Proc Natl Acad Sci USA. 100:4035–4039.
- Polis GA. 1981. The evolution and dynamics of intraspecific predation. Ann Rev Ecol Syst. 12:225–251.
- Polis GA, Holt RD. 1992. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol Evol. 7:151–154.
- Riede JO, Rall BC, Banasek-Richter C, Navarrete SA, Wieters EA, Emmerson MC, Brose U. 2010. Scaling of food-web properties with diversity and complexity across ecosystems. Adv Ecol Res. 42:139–170.
- Rudolf VHW, Lafferty KD. 2011. Stage structure alters how complexity affects stability of ecological networks. Ecol Lett. 14:75–79.
- Stouffer DB, Camacho J, Guimera R, Ng CA, Amaral LAN. 2005. Quantitative patterns in the structure of model and empirical food webs. Ecology. 86:1301–1311.
- Stouffer DB, Camacho J, Amaral LAN. 2006. A robust measure of food web intervality. Proc Natl Acad Sci USA. 103:19015–19020.
- Stouffer DB, Camacho J, Jiang W, Amaral LAN. 2007. Evidence for the existence of a robust pattern of prey selection in food webs. Proc Biol Sci. 274:1931–1940.
- Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. 2011. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput Biol. 7:e1001066.
- Werner EE, Gilliam JF. 1984. The ontogenetic niche and species interactions in size- structured populations. Ann Rev Ecol Syst. 15:393–425.
- Williams RJ, Martinez ND. 2000. Simple rules yield complex food webs. Nature. 404:180–183.
- Williams RJ, Martinez ND. 2008. Success and its limits among structural models of complex food webs. J Anim Ecol. 77:512–519.
- Williams RJ, Purves DW. 2011. The probabilistic niche model reveals substantial variation in the niche structure of empirical food webs. Ecology. 92:1849–1857.
- Yodzis P, Innes S. 1992. Body size and consumer resource dynamics. Am Naturalist. 139:1151–1175.
