Abstract
Dendrimers are a new kind of polymers. They have gained widespread attentions due to their remarkable properties in recent years. In the present work, a facile and efficient synthetic approach to prepare poly(ester amine) dendrimers is presented. Three different generations of poly(ester amine) dendrimers are synthesized starting from using pentaerythritol tetraacrylate as a core, followed by Michael addition and acrylate esterification reactions. This synthetic protocol is highly efficient, high yielding, and environmentally benign. To the best of our knowledge, this is the first time that these novel poly(ester amine) dendrimers are synthesized by using Michael addition reaction as the key step. The chemical structures of these poly(ester amine) dendrimers were measured with Fourier transform infrared (FT-IR) spectroscopy, NMR, elemental analysis, gas permeation chromatography, and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF). In addition, the hydroxyl value and solubilities of the poly(ester amine) dendrimers were also examined. These synthesized poly(ester amine) dendrimers, which have well-defined structures with very narrow polydispersities, can be used in many fields such as chemical engineering, medicine, and nanoengineering.
Introduction
In recent years, dendrimers have gained widespread attentions due to their remarkable properties such as low viscosity, good solubility, and a large number of terminal functional groups relative to their linear analogs Citation[1–5]. These polymers have potential applications in the fields of medicine, additives, coatings, composites, catalysis, etc. Citation[6–10].
Several different methods have been developed to synthesize dendrimers. The divergent methodology is the most commonly used method of synthesis dendrimers. In a divergent strategy, polymeric segments are grown from a suitable core molecule and subsequent branching reactions produced a desired higher generation dendrimer. Tomalia et al. Citation[11] have synthesized poly(amido amine) (PAMAM) dendrimers by this approach. The convergent methodology is another commonly used method to synthesis dendrimers. In a convergent strategy, different living polymeric segments are synthesized firstly, and then link them to a suitable core. Using this approach, Hadjichristidis et al. Citation[12] have achieved dendrimer-like star block copolymers of styrene (St) and isoprene. However, both the divergent and convergent strategies also have some disadvantages. The difficulty to synthesize most of dendrimers consists of the fact that it involves multiple steps of protection/deprotection and complicated purification. In order to overcome this drawback, some efficient reactions, such as Cu-catalyzed azide-alkyne click chemistry and thiol-ene reaction, have been used for preparing dendrimers. For instance, Killops et al. Citation[13] have synthesized several generations of poly (thio-ether) dendrimers using thiol-ene addition reactions for construction of the dendritic backbone. Although there are many preparation methods, these novel poly(ester amine) dendrimers synthesized by Michael addition and acrylate esterification have not yet been reported.
Herein, we present a facile and efficient method to synthesize a series of dendrimers by using Michael addition and acrylate esterification. The reaction steps we chose are highly efficient, high yielding, and environmentally benign. The synthesized dendrimers can be easily modified for application in various areas by functionalizing the hydroxyl end groups.
Experimental
Materials
Pentaerythritol tetraacrylate (PETEA) was purchased from Aldrich, USA. Acrylyl chloride was provided by TCI-EP (Tokyo Kasei, Japan). Diethanolamine (DEA), triethylamine, and dibutylamine were purchased from Lingfeng Chemical Reagent Co. Ltd. (Shanghai, China). Methanol, acetone, and dichloromethane were obtained from Sinopharm Chemical Reagent Co. Ltd, China. All other common chemical reagents were all analytical grade and used as received.
Synthesis of first-generation poly(ester amine) dendrimer (G1-OH)
PETEA (7.05 g, 0.02 mol), DEA (8.41 g, 0.08 mol), and methanol (50 ml) were charged into a 250 ml three-neck flask equipped with a magnetic stirrer, nitrogen inlet, and a cooler. The mixture was stirred for 36 h at 35 °C under nitrogen atmosphere (Scheme ). After cooling to room temperature, the white precipitate was filtrated, washed with methanol, and dried under vacuum for 24 h. The first-generation poly(ester amine) dendrimer was obtained, named G1-OH (Yield: 12.01 g, 78%).
Synthesis of first-generation acrylate-terminated oligomer (G1-ene)
G1-OH (1.54 g, 2 mmol), triethylamine (1.62 g, 16 mmol), and 30 ml acetone were mixed in a 100 ml three-neck flask equipped with a magnetic stirrer, a dripping funnel, and a drying tube. The flask was cooled in a water/ice-bath, acryloyl chloride (1.62 g, 16 mmol) diluted in 10 ml acetone was added into the mixture. Then, the mixture was stirred at room temperature for 24 h. The solid was filtered off from the reaction mixture and the acetone was concentrated by rotary evaporation to give the crude product as pale yellow oil. The obtained crude product was dissolved in CH2Cl2 and washed twice with a 10% Na2CO3 solution. The organic layer was separated, dried over MgSO4, filtered, and then concentrated at reduced pressure to give colorless oil, named G1-ene (Yield: 2.34 g, 74%). The different generations of poly(ester amine) dendrimers were synthesized by repeating the previous two steps.
Measurements
1H NMR spectrums were obtained by a Bruker 300 MHz spectrometer (Karlsruhe, Germany) with DMSO-d6 as solvent. Infrared absorption spectra (IR) were measured on a Nicolet Magna IR650 (Madison, WI). The molecular weight and polydispersity indexes of the oligomers were estimated by Water 515-2410 gel permeation chromatography (GPC, Water, USA). Linear polystyrene standards were used for calibration and dimethylformamide (DMF) as a solvent. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) experiment was performed on a Hewlett Packard G2025 a MALDI-TOF system using dihydroxy benzoic acid as matrix. Hydroxyl value was determined according to ASTM D-4274 method. Elemental analysis was performed with a Leeman CE440 instrument. The solubility of the samples was determined by the observation of the soluble process of the samples in different solvents at room temperature.
Results and discussion
FT-IR and 1H NMR spectra
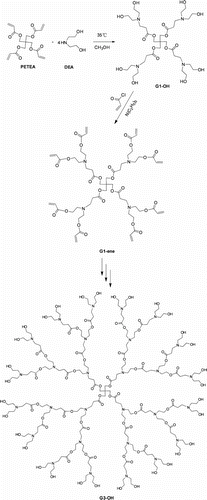
The first generation poly(ester amine) dendrimer (G1-OH) was synthesized by the Michael addition reaction of PETEA and DE in 78% yield. A subsequent esterification of G1-OH with acryloyl chloride, furnished the ene-functional poly(ester amine) dendrimer G1-ene. After repetition of the previous two steps, the third generation poly(ester amine) dendrimer, G3-OH, is obtained. The Michael addition and acrylate esterification reactions we chose are highly efficient, high yielding, and environmentally benign. Furthermore, the purification operations are extremely simple, even for the G1-ene and G2-ene. To the best of our knowledge, this is the first time that these novel poly(ester amine) dendrimers are synthesized by using Michael addition and acrylate esterification. Figure shows the proton peaks of G1-OH, G2-OH, and G3-OH, respectively. From the 1H NMR spectrum of G1-OH in Figure , proton signals at δH (300MHZ; DMSO-d6
; Me4Si) 4.16–4.32 (8H, S, –C–CH2–O–), 3.16–3.57 (22H, br, –CH2–OH, –CH2–N<), and 2.42–2.75 (16H, S, –OOC–CH2, –N–CH2–) were observed. The other poly(ester amine) dendrimers (G2-OH and G3-OH) show analogous 1H NMR spectra as that for G1-OH because they have similar structures. After acrylate esterification, three groups of characteristic peaks at 5.85–6.56 ppm which belong to the three different protons of the terminal double bond groups are clearly visualized in Figure . This indicates that the acrylyl chloride has been reacted with G1-OH and G2-OH, respectively. Figure shows the Fourier transform infrared (FT-IR)spectra of poly(ester amine) dendrimers. A strong and broad peak around 3326 cm−1 confirmed high concentration of hydroxyl groups in poly(ester amine) dendrimers molecules. The peaks at 1722 cm−1 (
C=O in ester bond), 1128 cm−1 (C–O symmetric stretching), and 1046 cm−1 (asymmetric stretching) were the characteristic absorption bonds of poly(ester amine) dendrimers. After acrylate esterification reactions, three absorption peaks at around 1661, 1408, and 810 cm−1 can be observed Citation[14]. It is the characteristic peaks of the double bonds in G1-ene and G2-ene, as shown in Figure . It can also be concluded that the acrylate esterification reactions have taken place as expected.
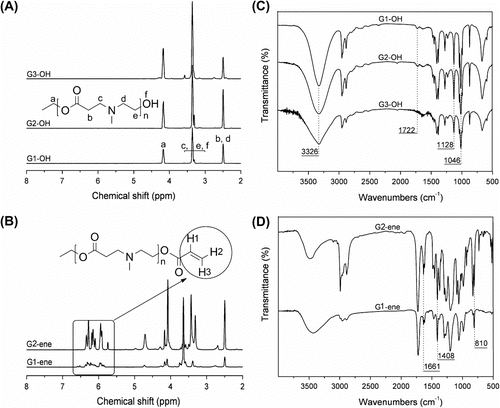
MALDI-TOF MS spectra
Figure shows the MALDI-TOF MS analyses of the G1-OH (a) and G2-OH (b). In all cases strong signals of molecular ions are observed at the expected molecular weights of ideal structures. For example, the [M + K]+ ion peak for G1-OH was present at 810 and in excellent agreement with the calculated value. Even in the case of G2-OH, the peak signal at m/z = 2085 was close to the calculated mass value of 2086.
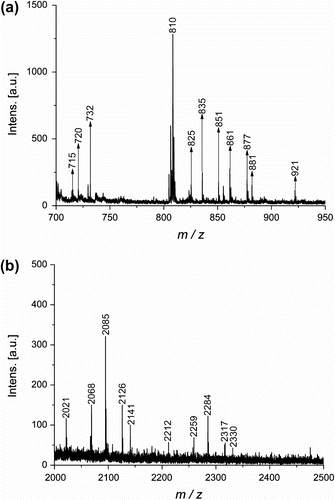
GPC
The polydispersity of the poly(ester amine) dendrimers were analyzed by GPC in DMF, using a linear polystyrene (PS) standard. As no appropriate dendrimer standards are available for calibration, the results of molecular weights are much lower than theoretical values. However, the GPC measurement is still useful in providing polydispersity of the poly(ester amine) dendrimers Citation[15,16]. As can be seen in Figure , all the poly(ester amine) dendrimers are monodisperse, the predicted and the observed values are in reasonable agreements. It can be predicted that the synthesized poly(ester amine) dendrimers have well-defined structures.
Element analyses and hydroxyl values
The hydroxyl values (mmol/g) and element analyses of poly(ester amine) dendrimers are summarized in Table . It is evident that the hydroxyl values of G1-OH, G2-OH, and G3-OH are close to their theoretically calculated ones. The hydroxyl value of G1-OH and G3-OH is 10.4 and 6.9 mmol/g, respectively. Although poly(ester amine) dendrimers with larger generation numbers have more hydroxyl groups and larger molecular weights, the hydroxyl values in fact decrease along with generation numbers increased. This can be attributed to the fact that the increasing extents of molecular weights were greater than those of hydroxyl numbers Citation[17]. The results of element analyses of N, C, and H in poly(ester amine) dendrimers are shown in Table , which shows that the measured results are closed to their theoretically calculated ones. The element contents of N, C, and H in G2-OH were 8.11, 52.04, and 8.13%, respectively. It can be concluded that the real structures of poly(ester amine) dendrimers are similar to the ideal ones drawn in Scheme .
Table 1. Characteristics of the G1-OH, G2-OH, and G3-OH.
Solubility
The solubilities of the G1-OH, G1-ene, G2-OH, G2-ene, and G3-OH were examined at room temperature. These results are summarized in Table . It can be seen that the G1-OH, G2-OH, and G3-OH are well soluble in water, DMAC, DMF, and DMSO, and partially soluble in methanol, acetone, THF, and 1,4-dioxane. Though G1-OH, G2-OH, and G3-OH were well dissolved in water, G1-ene and G2-ene were insoluble in water. This means that the solubility of poly(ester amine) dendrimers (G1-OH, G2-OH, and G3-OH) changed by functionalizing the hydroxyl end groups of poly(ester amine) dendrimers.
Table 2. Solubilities of the G1-OH, G1-ene, G2-OH, G2-ene, and G3-OH.
Conclusion
In conclusion, we have synthesized three different generations of poly(ester amine) dendrimers from pentaerythritol tetraacrylate by Michael addition and acrylate esterification reactions. The reaction steps we chose are highly efficient, high yielding, and environmentally benign. The different generations of poly(ester amine) dendrimers can be easily synthesized by repeating the previous two steps. These synthesized poly(ester amine) dendrimers, which have well-defined structures with very narrow polydispersities, can be easily modified and have potential applications in many fields such as chemical engineering, medicine, and nanoengineering.
Acknowledgments
This work was supported by National High Technology Research and Development Program of China (Grant No. 2008AA03A323) and National Natural Science Foundation of China (Grant No. 21002012).
References
- Ponnapati , R , Felipe , MJ and Advincula , R . 2011 . Macromolecules , 44 : 7530
- Hourani , R and Kakkar , A . 2010 . Macromolecular Rapid Communications. , 31 : 947
- Comanita , B and Roovers , J . 1999 . Designed Monomers and Polymers , 2 : 111
- Ornelas , C , Ruiz , J and Astruc , D . 2009 . Organometallics , 28 : 2716
- Lee , H , Choi , JS and Larson , RG . 2011 . Macromolecules , 44 : 8681
- Lin , QM , Jiang , GH and Tong , KK . 2010 . Designed Monomers and Polymers , 13 : 301
- Rajakumar , P , Visalakshi , K , Ganesan , S , Maruthamuthu , P and Suthanthiraraj , SA . 2012 . Journal of Materials Science , 47 : 1811
- Sarkar , A , Carver , PI , Zhang , T , Merrington , A , Bruza , KJ , Rousseau , JL , Keinath , SE and Dvornic , PR . 2010 . Journal of Membrane Science , 349 : 421
- Deng , LB , Wang , L , Yu , HJ , Dong , XC and Huo , J . 2007 . Designed Monomers and Polymers , 10 : 131
- Natarajan , B and Jayaraman , N . 2011 . Journal of Organometallic Chemistry , 696 : 722
- Tomalia , DA , Baker , H , Dewald , J , Hall , M , Kallos , G , Martin , S , Roeck , J , Ryder , J and Smith , P . 1985 . Polymer Journal , 17 : 117
- Chalari , I and Hadjichristidis , N . 2002 . Journal of Polymer Science Part A: Polymer Chemistry , 40 : 1519
- Killops , KL , Campos , LM and Hawker , CJ . 2008 . Journal of the American Chemical Society , 130 : 5062
- Kim , HD and Kim , TW . 1998 . Journal of Applied Polymer Science , 67 : 2153
- Fu , Q , Cheng , LL , Zhang , Y and Shi , WF . 2008 . Polymer , 49 : 4981
- Vukovic , J , Jovanovic , S , Lechner , MD and Vodnik , V . 2009 . Journal of Applied Polymer Science , 112 : 2925
- Murillo , EA , Vallejo , PP , Sierra , L and Lopez , BL . 2009 . Journal of Applied Polymer Science , 112 : 200