Abstract
Nanocomposites of high performance polyimide and modified multiwalled carbon nanotube were prepared via in situ polymerization technique. Neat fluorinated polyimide was synthesized from the reaction between 4,4′-(hexafluoroisopropylidene) diphthalic anhydride and 2-(3,5-diaminophenyl)-benzimidazole. Polyimide/modified carbon nanotube nanocomposites enclosing 0.5, 1, 3, and 5% of functionalized carbon nanotube were successfully prepared by an in situ polymerization reaction through thermal imidization. The Fourier transform infrared spectroscopy, X-ray diffraction, field emission type scanning electron microscope, transmission electron microscopy, and thermogravimetric analysis techniques were used to evaluate the properties of the novel nanocomposites. According to thermogravimetric analysis results, the addition of the modified multiwalled carbon nanotube improved thermal stability of the obtained hybrid materials. The morphological images show that the carbon nanotubes were dispersed homogeneously in polymer matrix. The mechanical properties of nanocomposites were substantially improved by the incorporation of functionalized carbon nanotube into the polymer matrix.
1. Introduction
Over the past two decades, nanocomposites (NCs) have attracted considerable academic and industrial interest Citation[1]. They are an important class of new generation materials, which typically exhibit properties superior to those of their individual components Citation[2]. To optimize the performance properties of these materials, the dispersion of inorganic domain in the organic matrix at nanolevel is desirable Citation[3]. Polymer-based NCs are a subject of considerable research due to their ability to combine the advantages of both polymers and nanoscale fillers Citation[4]. Whenever nanofillers are added to a polymer matrix, dispersion is of critical importance, while well dispersed nanophase may be advantageous, low dispersion can have negative results. Therefore, the investigation of the affect of nanoparticles on the properties of a polymer matrix is necessary in order to be able to better foretell the final properties of the composite Citation[5,6].
Carbon nanotubes (CNTs) were first discovered by Oberlin et al. Citation[7] and aroused great interest since the rediscovery by Iijima Citation[8]. They have been considered as excellent reinforcing fillers in polymer NCs because of their outstanding properties like high aspect ratio, nanosize in diameter, very low density, and, more importantly, excellent physical properties such as extremely high mechanical strength, high electrical, and thermal conductivity Citation[9]. Introducing of CNTs to polymer matrices modifies mechanical, electrical, thermal, and morphological properties of the produced NCs Citation[10–13]. The challenges for developing high-performance CNTs/polymer NCs are homogeneous dispersion of CNTs in the polymeric matrix and strong interfacial interactions so as to affect efficient load transfer from the polymeric matrix to the CNTs Citation[14]. Without chemically bonding, load transfer between the CNTs and the matrix mainly comes from electrostatic and van der Waals interactions Citation[15]. Much efficiency of load transfer can be realized by chemical bonding of polymeric matrix with functionalized CNTs Citation[16,17]. It has been confirmed that refluxing CNTs with concentrated nitric acid create acidic sites on CNTs, such as carboxylic, carbonyl, and hydroxyl groups Citation[18]. These reactive groups on CNTs will greatly enhance the combination of CNTs with polymeric matrix, thus improving the mechanical strength of the NCs Citation[19]. Sidewall functionalization of CNTs also improves the dispersion of CNTs in a polymeric matrix Citation[20–24].
Among the heat resistant and high performance macromolecules, polyimides (PIs) have received considerable scientific and technological attention due to their outstanding performance such as dielectrical and mechanical properties, thermal and chemical stability Citation[25]. Because of their exceptional physical and mechanical properties, they have several potential applications for advanced technology such as light emitting diode, internal dielectric photoresist, membrane, photoconductor, and nonlinear optical materials Citation[26,27]. However, owing to their rigid backbones and strong interactions between chains, wholly aromatic PIs, do not always provide the optimum properties for many specialty applications owing to deficiencies in processability, solubility, and transparency as well as their relatively high dielectric constant Citation[28,29]. To overcome these problems, much research effort has been focused on the synthesis of soluble and processable PIs in fully imidized form without deterioration of their own excellent properties. Several approaches were reported to synthesize soluble PIs including introduction of flexible linkage and heteroaromatic rings into the polymer backbone Citation[30]. The benzimidazole rings with heteroaromatic structure have excellent stabilities derived from its molecular symmetry and aromaticity, so the incorporation of benzimidazole group into PI backbone would improve the solubility without deterioration of their own excellent properties Citation[31–33].
Due to their unique properties, extensive researches have been conducted concerning the development of PI-based NCs. In fact a unique set of property through a combination of PI and different inorganic nanoparticle by rational selection of the raw materials and the preparative approaches, so as to achieve various properties required in different applications with lower technical risk than developing a new kind of material Citation[34,35]. Aromatic PIs serve as excellent matrices for CNT-based NCs. It has been reported that doping of a polyimide matrix with less than 1 vol.% of CNTs resulted in NCs of significantly improved conductivity Citation[36–38].
Allowing for the importance of the NCs, the present work describes the in situ preparation of PI/MWNT NCs by conversion of 4,4′-(hexafluoroisopropylidene) diphthalic anhydride and 2-(3,5-diaminophenyl)-benzimidazole in the presence of acid-modified multiwall carbon nanotubes (MWNTs-COOH). The resulting hybrid materials were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), transmission electron microscopy (TEM), field emission type scanning electron microscopy (FE-SEM), thermogravimetric analysis (TGA), and mechanical properties techniques.
2 Experimental
2.1 Materials
Solvents and chemicals were obtained from the Merck Chemical Co. (Germany) and Aldrich Chemical Co. (Milwaukee, WI, USA). The 4,4-(Hexafluoroisopropylidene) pyromellitic dianhydride (6FDA) was purified by vacuum sublimation and stored under vacuum. N,N-Dimethylacetamide (DMAc) was dried over BaO, then distilled in vacuum. Hydrazine monohydrate, 3,5-Dinitrobenzoyl chloride, 1,2-phenylenediamine, phosphorus pentoxide (P2O5), and methanesulfonic acid were obtained from commercial sources and used as received. CNTs used in this work were MWNTs-COOH which was supplied by the Nanosabz chemical Co. of Iran. They were dried at 100 oC in an oven for at least 10 h prior to use. The purity of MWNTs-COOH was more than 95% and -COOH content nearly reached 3.86%.
2.2 Synthesis of benzimidazol diamine
According to literature Citation[39], for the preparation of 2-(3,5-diaminophenyl)-benzimidazole (1), at first corresponding dinitro was synthesized from the reaction of 1,2-phenylenediamine and 3,5-dinitrobenzoyl chloride using methanesulfonic acid and P2O5 as condensation medium. Then, diamine 1 was fabricated by reduction of the dinitro precursor using palladium on activated carbon (Pd/C) and hydrazine monohydrate in refluxed ethanol (yield: 77%) melting point: 242–243 oC; FT-IR (KBr, cm−1): 3409 (s, br), 3063 (w), 1607 (s), 1577 (s), 1484 (w), 1446 (m), 1274 (w), 841 (m), and 743 (s).
The existence of the characteristic peaks for NH2 functions and the absence of the original peaks arising from the NO2 groups in the corresponding dinitro provided the successful formation of the 2-(3,5-diaminophenyl)-benzimidazole (DABI) Absorption of amine NH2 and NH benzimidazole bonds appeared around 3394 and 3323 cm−1 and the peak at 1627 cm−1 confirms the presence of NH deformation. Two absorption bands at 1577 and 1484 cm−1, were characteristic peaks for aromatic rings. The benzimidazole groups showed bands at 1484 cm−1, 1590 cm−1 and a shoulder at 1630 cm−1.
In the 1H NMR spectra of DABI, appearances of the N–H protons of benzimidazole group at 12.62 ppm as broad singlet peaks, indicate presence of this group. The absorption of aromatic protons appeared in the range of 5.96–7.50 ppm. The proton of the amine groups appeared as broad singlet peaks at 4.91 ppm.
2.3 Synthesis of reference PAAs and PI
Into a 100 ml three-neck round-bottom flask equipped with a mechanical stirrer, nitrogen inlet, and drying tube containing calcium chloride were placed with diamine 1 (1.00 g, 4.46 mmol) and 7 ml of DMAc. The solution was stirred until the diamine completely dissolved. Then 6FDA with the same molar ratio of diamine was added into the solution six times within 1 h. The viscosity increased quickly over 2 h. The mixture was stirred under nitrogen at room temperature for another 12 h. The resulting yellow poly(amic acid) (PAA) solution was clear and viscous. The solution was subsequently used to prepare thin films for characterization.
FT-IR (KBr, cm−1) of PAA: 2750–3400 (m, br), 1665 (m), 1600 (w), 1552 (s), 1490 (w), 1397 (m), 1238 (m), 1100 (w), 1068 (br), 1032 (s), 1013 (w), 876 (w), 758 (m), 654 (w), 621 (m), 5740 (w), and 431 (m).
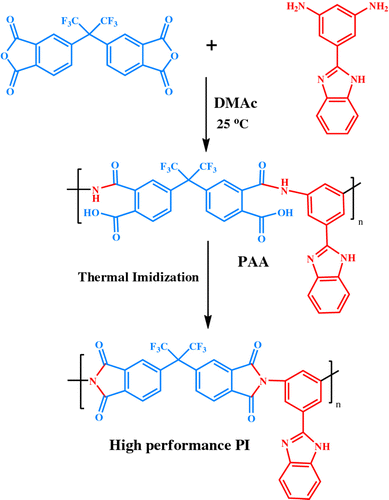
Neat PI film was fabricated by casting the PAA solution onto a glass plate. After the film was dried at room temperature for 3 h, it was heated at 80, 100, 150, and 200 oC for 1 h each and at 250 oC for 2 h, to obtain yellow colored transparent films.
FT-IR (KBr, cm−1): 3343 (m), 1778 (m), 1720 (s), 1607 (m), 1530 (w), 1457 (m), 1440 (m), 1376 (s), 1320 (s), 1276 (w), 1238 (m), 1124 (w), 1097 (s), 1071 (w), 1010 (m), 830 (s), 754 (m), 722 (s), 647 (m), 620 (m), and 565 (w).
2.4 Synthesis of PI/MWNTs-COOH NC films
A mixture of DMAc (5 mL) and an appropriate amount of MWNTs-COOH was sonicated with an ultrasonic horn at room temperature under nitrogen atmosphere. After 1 h, sonication was stopped and diamine 1 (0.25 g, 1.12 mmol) was added, which dissolved within 15 min under magnetic stirring. Finally, 6FDA (0.5 g, 1.12 mmol) was added. The whole polymerization reaction was carried out at ambient temperature under magnetic stirring until a stable high viscous solution was obtained (ca. 4 h). The overall concentration of MWNT and poly(amic acid) (PAA) in DMAc was 10 wt.%. The viscous solution prepared was cast into a Petri dish and dried overnight at 40 oC under vacuum. Subsequently, the obtained films were cured at 80, 100, 150, and 200 oC for 1 h each and at 250 oC for 2 h in a nitrogen circulated tube oven to obtain solvent-free films. Various compositions (0.5%, 1%, 3%, and 5 wt.%.) of MWNT were prepared by mixing various amounts of MWNT to the PI solution.
2.5 Methods for characterization
A Jasco-680 FT-IR spectroscope (Japan) was employed to examine the chemical bonds on the polymer and NCs. Spectra of solids were obtained with KBr pellets. Vibration bands were reported as wavenumber (cm−1). The band intensities were assigned as weak (w), medium (m), shoulder (sh), strong (s), and broad (br). TGA was performed with a STA503 win TA at a heating rate of 10 °C/min from 25 to 800 °C under nitrogen. The XRD pattern was acquired by using a Philips Xpert MPD X-ray diffractometer. The diffractograms were measured for 2θ, in the range of 10°–100°, using Cu Kα incident beam (λ = 1.51418 Å). The dispersion morphology of the nanoparticles on PI matrix was observed using FE-SEM (HITACHI (S-4160). TEM images were obtained using a Philips CM 120 microscope with an accelerating voltage of 100 kV. Tensile properties of the NC films were measured according to DIN procedure 53,455 having a crosshead speed of 5 mmmin−1 using Zwick 1446-60.
3 Results and discussion
3.1 Fabrication PI/MWNTs-COOH NC films
In this study, acid functionalized MWNTs were used for preparation of hybrid materials. These reactive groups on CNTs will greatly enhance the combination of CNTs with PI matrix, thus improving the thermal and mechanical strength of the resulted NCs. For preparation of NCs, fluorinated PI with benzimidazole side group was chosen as a matrix and it was synthesized by polymerization reaction of 6FDA and diamine with 2-(3,5-dinitrophenyl)-benzimidazole in dry DMAc as shown in Scheme . There are some suggestions for interaction of modified CNTs with PI such as the formation of H-bond between a carbonyl, benzimidazole as well as fluor group with carboxylic acid functional groups in the modified CNTs.
3.2 Characterizations
3.2.1 FT-IR
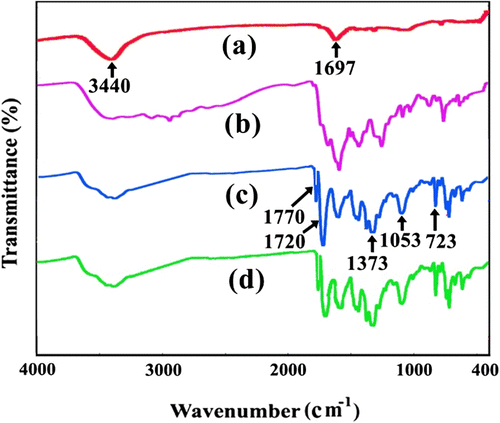
The FT-IR spectra of functionalized MWNTs, PAA, neat PI, and PI/MWNTs-COOH NC5% are shown in Figure . The FT-IR spectra of functionalized MWNTs showed peaks with low intensity at 3440, 1697, and 1182 cm−1, corresponding to OH, CO, and C–C–O stretching, respectively (Figure ). The FT-IR spectrum of PI showed distinct features that clearly indicate imide ring formation during the thermal cyclization step. The characteristic absorption bands of amic acid and carboxyl groups in the 2600–3500 and 1650 cm−1 regions disappear and those of the imide ring appear near 1770 cm−1 (asym. C=O stretching) and 1720 cm−1 (sym. C=O stretching), 1373 cm−1 (C–N stretching) 1053 cm−1 and 723 cm−1 imide (ring deformation) (Figures ). The FT-IR spectra of NCs prepared by MWNT and PI with 5% MWCNT were also included. The incorporation of MWNT in PI caused the slight changes in the intensities of absorption bands of NCs. The main interaction can be hydrogen bonding between carboxylic carbonyl group of CNT and NH of benzimidazole. Moreover, aromatic pi–pi stacking interactions can also be considered. From Figure , two characteristic peaks near 1614 and 1718 cm−1 due to the stretching vibration of –CO group can be seen. It can also be clearly seen that the peaks for C=O groups were gradually shifted from 1718 cm−1 in pure PI to 1700 cm−1 in composites with the content of functionalized MWNTs. The results suggest that the composites are not the simple mixtures of polymer and MWNTs but imply the existence of a strong interaction between them. The presence of the carboxylic groups on the nanotube surface is likely to give the interfacial interaction between the polymer matrix and the nanotubes in polymer composites.
3.2.2 X-ray diffraction
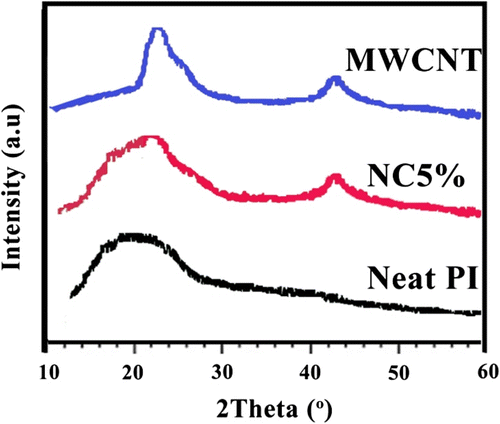
XRD patterns of functionalized MWNTs, PI, and PI/MWNT NC5% are presented in Figure . The X-ray patterns of the MWNT displayed the presence of two peaks at 2θ = 25.8o and 42.7o corresponding to the (0 0 2) and (1 0 0) reflections of the carbon atoms, respectively, which is in good agreement with the literature values Citation[40]. Pure PI showed broad diffraction peaks at 2θ = 18–23o. The X-ray pattern of the NC5% showed mixed peaks appearing in the MWNT and pure PI, respectively.
3.2.3 Field emission-scanning electron microscopy
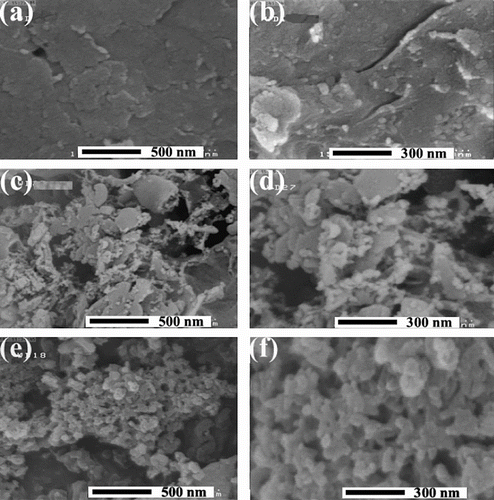
FE-SEM study was carried out in order to investigate the surface morphology of the tensile fractured surface and distribution of CNTs in the PI matrix. The morphological images of the pure PI, NC3%, and NC5% were studied by FE-SEM with different magnifications (Figure ). Pure PI shows massive, aggregated morphology, and in some instances, there are some bulky flakes. Bright dots and thread-like structures in the NCs micrographs are attributed to the MWCNTs. This homogeneous dispersion may be due to the fact that induced hydrogen bonding and van der Waals force between polymer and CNTs during in situ formation of NC films. For NC3%, the FE-SEM images show that MWNTs-COOH are homogeneously dispersed in the polymer matrix. In addition, the results demonstrate that the structure of the prepared NC3% is more compact and uniform than that of NC5%. In Figures , maximum numbers of CNTs are dispersed individually with some little agglomerates.
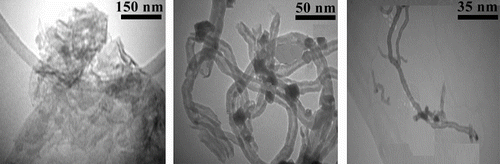
3.2.4 Transmission electron microscopy
Homogeneous dispersion of CNTs in polymer matrix is one of the most important factors in CNT-based polymer NCs because any inhomogeneities lead eventually to structural defects in the composite materials. To study the dispersion of CNTs in polymer matrix, morphological inspection is very much important. Figure shows the representative TEM micrographs of PI/ MWNTs-COOH NC3% with different magnifications. The micrographs confirmed that the MWNTs-COOH particles were well dispersed in the PI matrix. According to these images, the carbon nanotubes are randomly oriented throughout the PI matrix and interconnecting network is formed. The obtained results indicate that the effect of acid functionality in the CNT plays an important role in dispersion of the CNTs in polymer matrix. The relatively strong interactions between PI matrix and MWNTs-COOH are responsible for good dispersion of CNTs in polymer network.
3.3 Thermogravimetric analysis of the composites
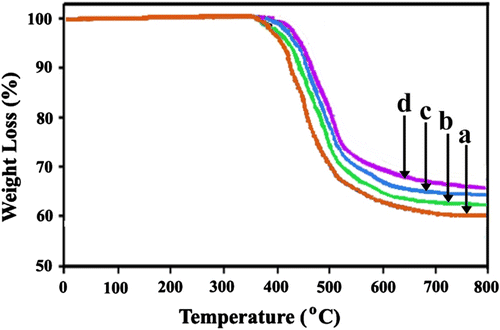
The thermal properties of PI and hybrid materials were studied by means of TGA at heating rate of 10 °C/min under a nitrogen atmosphere. The TGA scanned results of four samples under N2 environment are shown in Figure . The corresponding thermoanalysis data, including the temperatures at which 5% (T 5) and 10% (T 10) degradation occur and char yield at 800 °C are summarized in Table . The thermal decomposition behavior of all samples mainly takes place in one step process. It can be observed that the thermal degradation curves of samples with MWNTs-COOH move towards higher temperature, and the composites show more char residues than neat PI. For instance, the initial temperature is 406 oC and char residue is 59 wt.% for neat PI; however, the initial temperature could be increased to 428 oC and char residue is 68 wt.% when the loading of MWNTs-COOH is 5 wt.% in the composites. The improvement in the thermal stability of samples with MWNTs-COOH can be explained by the numerous interconnected CNTs acting as a barrier that prevents the transfer of heat and promotes the formation of stable char layers, which efficiently protect the underlying polymeric materials from thermal degradation. Increase in the char yield is almost equal to amount of added CNT so the effect on char yield mainly comes from the initially added CNT or it can be considered as initially added char.
Table 1. Thermal properties of the neat PI and different NCs.
3.4 Mechanical properties
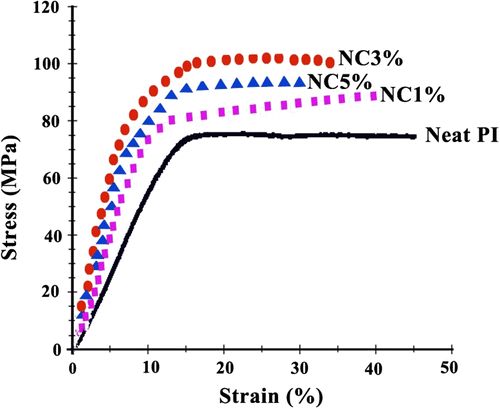
The amount of MWNTs-COOH added to PI significantly affects the observed mechanical properties. The mechanical tensile properties of PI hybrid films with various MWNTs-COOH contents are shown in Figure . The strength and modulus values were found to be enhanced with respect to those of PI for filler contents up to a critical content, with inferior values above that content. It appears that there is a critical amount of filler beyond which the filler’s reinforcing capacity diminishes. The tensile strength of the neat PI matrix is 74.60 MPa along with 1, 3, and 5 wt.% MWNT filled PI matrix are 90.32, 105.50, and 98.75 MPa, respectively. The maximum stress at break was found to increase initially with increase in MWNTs-COOH content, and at 3 wt.% MWNTs-COOH showed a maximum value of 105.50 MPa relative to the 90.32 MPa of the neat PI. This decrease in the ultimate tensile strength is mainly owing to the agglomeration of filler particles over critical MWNTs-COOH content Citation[39]. The general tendency for improvement in the stress level is increased by the addition of CNTs which play the role of reinforcement. Another reason for the enhancements in the tensile modulus of NCs is the strong interaction between the PI matrix and MWNTs-COOH via formation of hydrogen and chemical bonding. From these results, it is deduced that the reinforcing effect of CNTs is very marked. As the CNTs content in the polymer increases, the stress level gradually increases but at the same time the strain of the NCs decreased. Similar results have previously been obtained with other polymer NCs Citation[22,24,41].
4 Conclusions
In the present work, the effects of acid functionalization of MWNTs on the dispersion and thermal of PI/MWNTc-COOH NCs were investigated for different MWNT contents. High performance fluorinated PI with imidazole pendant group was synthesized from the reaction between 4,4′-(hexafluoroisopropylidene) diphthalic anhydride and 2-(3,5-diaminophenyl)-benzimidazole and then NCs of the synthesized polymer and modified MWNTs were successfully prepared by an in situ polymerization reaction through thermal imidization. The FT-IR, XRD, FE-SEM, and TEM were used to evaluate the properties of the novel NCs. FE-SEM and TEM observation showed that a homogeneous dispersion of functionalized MWNTs throughout the PI matrix and a strong interfacial adhesion between functionalized MWNTs and the matrix were achieved in PI composites, which strengthened the mechanical properties. So, the functional groups on the MWNTs surface played an important role in accelerating both the dispersion of MWNTs and the interfacial adhesion in the composites. According to TGA results, the thermal stability of the NCs improved under a N2 environment by about 10 oC at 5 wt.% MWNT loading. The strong interaction between the functionalized fillers and the PI matrix greatly enhances the dispersion as well as the interfacial adhesion, thus improving the overall mechanical performance of the resulting NCs.
Acknowledgments
The work described in this paper was supported by a grant from the Islamic Azad University-Gachsaran branch. The authors gratefully acknowledge the Research Vice Chancellor of Islamic Azad University of Gachsaran and his coworkers.
References
- Schaefer , DW and Justice , RS . 2007 . How nano are nanocomposites? . Macromolecules , 40 : 8501 – 17 .
- Chiu , FC and Kao , GF . 2012 . Polyamide 46/multi-walled carbon nanotube nanocomposites with enhanced thermal, electrical, and mechanical properties . Composites Part A , 43 : 208 – 18 .
- Zulfiqar , S , Lieberwirth , I , Ahmad , Z and Sarwar Ilyas , M . 2008 . New aramid-based nanocomposites: synthesis and characterization . Polymer Engineering & Science , 48 : 1624 – 33 .
- Liang , BI , Huang , Y , Zhang , L , Wang , Y , Ma , Y , Guo , T and Chen , Y . 2009 . Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites . Advanced Functional Materials , 19 : 2297 – 302 .
- Kuljanin , J , Comor , MI , Djokovic , V and Nedeljkovic , JM . 2006 . Synthesis and characterization of nanocomposite of polyvinyl alcohol and lead sulfide nanoparticles . Materials Chemistry and Physics , 95 : 67 – 71 .
- Parvinzadeh , M , Moradian , S , Rashidi , A and Yazdanshenas , ME . 2010 . Surface characterization of polyethylene terephthalate/silica nanocomposites . Applied Surface Science , 256 : 2792 – 802 .
- Oberlin , A , Endo , M and Koyama , T . 1976 . Filamentous growth of carbon through benzene decomposition . Journal of Crystal Growth , 32 : 335 – 49 .
- Iijima , S . 1991 . Helical microtubules of graphitic carbon . Nature , 354 : 56 – 8 .
- Saito , R , Dresselhause , G and Dresselhause , MS . 1998 . Physical properties of carbon nanotubes , London : Imperial College Press .
- Chang, TE, Jensen LR, Kisliuk A, Pipes RB, Pyrz R, Sokolov AP. Microscopic mechanism of reinforcement in single-wall carbon nanotube/polypropylene nanocomposite. Polymer. 2005;46:439–46.
- Xu , Y , Ray , G and Abdel-Magid , B . 2006 . Thermal behavior of single-walled carbon nanotube polymer–matrix composites . Composites Part A , 37 : 114 – 21 .
- Yuen SM, Ma CCM, Wu HH, Kuan HC, Chen WJ, Liao SH, Hsu CW, Wu HL. Preparation and thermal, electrical, and morphological properties of multiwalled carbon nanotube and epoxy composites. Journal of Applied Polymer Science. 2007;103:1272–9.
- Rahmat , M and Hubert , P . 2011 . Carbon nanotube–polymer interactions in nanocomposites: a review . Composites Science and Technology , 72 : 72 – 84 .
- Zhang , WD , Shen , L , Phang , IY and Liu , T . 2004 . Carbon nanotubes reinforced nylon-6 composite prepared by simple melt-compounding . Macromolecules , 37 : 256 – 9 .
- Liao , K and Li , S . 2001 . Characteristics of carbon nanotube-polystyrene interface . Applied Physics Letters , 79 : 4225 – 7 .
- Ebbsen , TW , Hiura , H and Bischer , ME . 1996 . Decoration of carbon nanotubes . Advanced Materials , 8 : 155 – 7 .
- Zhu , J , Kim , JD , Peng , H , Margrave , JL , Khabashesku , VN and Barrera , EV . 2003 . Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization . Nano Letters , 3 : 1107 – 13 .
- Can Zaman , A , Ustundag , CB , Kaya , F and Kaya , C . 2012 . OH and COOH functionalized single walled carbon nanotubes-reinforced alumina ceramic nanocomposites . Ceramics International , 38 : 1287 – 93 .
- Hirsch , A . 2002 . Functionalization of single-walled carbon nanotubes . Angewandte Chemie International Edition , 41 : 1853 – 9 .
- Mickelson , ET , Chiang , IW , Zimmerman , JL , Lozano , PJBJ , Liu , J , Smalley , RE , Hauge , RH and Margrave , JL . 1999 . Salvation of fluorinated singlewall carbon nanotubes in alcohol solvents . Journal of Physical Chemistry B , 103 : 4318 – 22 .
- Shieh , YT , Liu , GL , Wu , HH and Lee , CC . 2007 . Effects of polarity and pH on the solubility of acid-treated carbon nanotubes in different media . Carbon , 45 : 1880 – 90 .
- Barick , AK and Tripathy , DK . 2011 . Preparation, characterization and properties of acid functionalized multi-walled carbon nanotube reinforced thermoplastic polyurethane nanocomposites . Materials Science and Engineering B , 176 : 1435 – 47 .
- Huang , JM , Tsai , MF , Yang , SJ and Chiu , WM . 2011 . Preparation and thermal properties of multiwalled carbon nanotube/polybenzoxazine nanocomposites . Journal of Applied Polymer Science , 122 : 1898 – 904 .
- Li , C , Zhao , Q , Deng , H , Chen , C , Wang , K , Zhang , Q , Chen , F and Fu , Q . 2011 . Preparation, structure and properties of thermoplastic olefin nanocomposites containing functionalized carbon nanotubes . Polymer International , 60 : 1629 – 37 .
- Yu , YY , Chien , WC and Tsai , TW . 2010 . High transparent soluble polyimide/silica hybrid optical thin films . Polymer Testing , 29 : 33 – 40 .
- Yang , CP , Hsiao , SH and Hsu , MF . 2002 . Organosoluble and light-colored fluorinated polyimides from 4,4’-bis(4-amino-2-trifluoromethylphenoxy)biphenyl and aromatic dianhydrides . Journal of Polymer Science Part A: Polymer Chemistry , 40 : 524 – 34 .
- Chen , K , Kazuaki , XC , Endo , YN , Higa , M and Okamoto , K . 2009 . Synthesis and properties of novel sulfonated polyimides bearing sulfophenyl pendant groups for fuel cell application . Polymer , 50 : 510 – 6 .
- Eichstadt , AE , Ward , TC , Bagwell , MD , Farr , IV , Dunson , DL and McGrath , JE . 2002 . Synthesis and characterization of amorphous partially aliphatic polyimide copolymers based on bisphenol-A dianhydride . Macromolecules , 35 : 7561 – 8 .
- Wang , YW and Chen , WC . 2011 . New photosensitive colorless polyimide-silica hybrid optical materials: synthesis, properties and patterning . Materials Chemistry and Physics , 126 : 24 – 30 .
- Huang , XS , Huang , YG and Qing , FL . 2009 . Novel poly(aryl ether)s containing benzimidazole pendants derived from 1,4-bis(2-benzimidazolyl)-2,5-difluorobenzene . Journal of Materials Science , 44 : 3566 – 73 .
- Toiserkani , H , Saidi , K and Sheibani , H . 2009 . Synthesis and characterization of new soluble and thermally stable poly(amide-imide)s containing pendent benzimidazole moieties . Journal of Applied Polymer Science , 114 : 185 – 92 .
- Ayala , V , Maya , EM , Garcia , JM , De La Campa , JG , Lozano , AE and De Abajo , J . 2005 . Synthesis, characterization, and water sorption properties of new aromatic polyamides containing benzimidazole and ethylene oxide moieties . Journal of Polymer Science Part A: Polymer Chemistry , 43 : 112 – 21 .
- Wu , SY , Yuen , SM , Ma , CCM and Huang , YL . 2009 . Synthesis and properties of aromatic polyimide, poly(benzoxazole imide), and poly(benzoxazole amide imide) . Journal of Applied Polymer Science , 113 : 2301 – 12 .
- Pan , LY , Zhan , MS and Wang , K . 2011 . High-temperature-resistant polyimide/montmorillonite nanocomposite foams by solid blending . Polymer Engineering & Science , 51 : 1397 – 403 .
- Yu , YY , Chien , WC , Lin , JM and Yu , HH . 2011 . High transprent polyimide/titania multi layer anti-refelective hybrid films . Thin Solid Films , 519 : 4731 – 6 .
- Srivastava , R , Banerjee , S , Jehnichen , D , Voit , B and Bohme , F . 2009 . In situ preparation of polyimide composites based on functionalized carbon nanotubes . Macromolecular Materials and Engineering , 294 : 96 – 102 .
- Smith , JG , Connell , JW , Delozier , DM , Lillehei , PT , Watson , KA , Lin , Y , Zhou , B and Sun , YP . 2004 . Space durable polymer/carbon nanotube films for electrostatic charge mitigation . Polymer , 45 : 825 – 36 .
- Hu , N , Zhou , H , Dang , G , Rao , X , Chen , C and Zhang , W . 2007 . Efficient dispersion of multi-walled carbon nanotubes by in situ polymerization . Polymer International , 56 : 655 – 9 .
- Alvarez-Gallego , Y , Ruffmann , B , Silva , V , Silva , H , Lozano , AE , De La Campa , JG , Nunes , SP and de Abajo , J . 2008 . Sulfonated polynaphthalimides with benzimidazole pendant groups . Polymer , 49 : 3875 – 83 .
- Pirlot , C , Willems , I , Fonseca , A , Nagy , JB and Delhalle , J . 2002 . Preparation and characterization of carbon nanotube/polyacrylonitrile composites . Advanced Engineering Materials , 4 : 109 – 14 .
- Loos , MR , Abetz , V and Schulte , K . 2011 . Fast and highly efficient one-pot synthesis of polyoxadiazole/carbon nanotube nanocomposites in mild acid . Polymer International , 60 : 517 – 28 .