Abstract
Synthesis of dextran-graft-poly(hydroxyethyl methacrylate) gels as a new fluoride biosorbent was considered in this work. For this propose, the Taguchi experimental design method was used for optimizing the synthetic conditions of the gels to reach high level of fluoride absorbency. The effects of three main parameters including concentrations of monomer (hydroxyethyl methacrylate), crosslinking agent (ethylene glycol dimethacrylate), and initiator (ammonium persulfate) on the final properties of the prepared gels were investigated. The proposed mechanism for grafting and chemically crosslinking reactions was proved with equilibrium water absorption, Fourier-transformed infrared, scanning electron microscopy, and thermogravimetric analysis. Under the optimized synthetic conditions, the maximum fluoride adsorption capacity in distilled water was found to be 8312 (mg fluoride/kg dried gel). As well, the loading of prepared gels with silver nanoparticles has been considered to achieve antibacterial activity. The silver nanoparticles loaded gels showed strong antibacterial activity against Gram-negative Escherichia coli bacterium. Therefore, these new gels can be considered as a potential candidate to develop an efficient antibacterial fluoride biosorbent for water treatment applications.
1. Introduction
Nowadays, fluoride contamination in ground water has been recognized as one of the serious problems Citation[1]. The World Health Organization (WHO) classified fluoride as one of the contaminants for human consumption, only comparable with arsenic and nitrate Citation[2,3]. The permissible limit of fluoride in drinking water specified by the WHO is 1.5 mg/l Citation[2]. Excess intake of fluoride leads to various diseases such as osteoporosis, arthritis, brittle bones, cancer, infertility, brain damage, Alzheimer syndrome, and thyroid disorder Citation[4]. The incidence of dental and bone fluorosis symptom was found to be related to the consumption of water containing high fluoride concentrations Citation[2,5]. Increasing amount of wastewater containing fluoride is being released from various engineering process, such as semiconductor manufacturing, electroplating, glass and ceramic production, coal power plants, and rubber and fertilizer manufacturing. Therefore, the necessity to remove the fluoride from water in an efficient way is great.
Various treatment methods have been reported for defluoridation of water: ion adsorption Citation[6,7], ion exchange resins Citation[8–10], precipitation–coagulation Citation[11,12], and membrane processes such as reverse osmosis Citation[13,14], nanofiltration Citation[15,16], electrodialysis Citation[17], and Donnan dialysis Citation[18,19]. Each of the methods has been found to be limited, since the membrane processes often have high operational costs Citation[14,16] and the chemical precipitation may generate secondary wastes Citation[12]. Among of these methods, adsorption technique has been widely studied in recent years due to its cost-effectiveness, simplicity of design and operation Citation[7,20,21]. However, there is still a great demand for identification of environmentally friendly, simple, and low-cost technologies for the removal of fluoride from drinking water.
Recently, considerable interest has been generated in the development of polymeric adsorbents as a tool for removing fluoride from polluted systems Citation[22–24]. On the other hand, utilization of natural resources in developing ion adsorbents (biosorbent) is increasing, because of their cost effective, eco-friendly, and renewable nature. Polysaccharides are a rich resource of structurally and functionally different chemical moieties and possess additional advantage of undergoing simple chemical modifications. Being biodegradable and hydrophilic, these polymers are important materials in the areas of separation, enrichment, and water management Citation[25]. Further modification of these polymers through grafting and crosslinking reactions affords tailor-made products with desirable and targeted features Citation[25,26].
Among various polysaccharides, chitin and chitosan derivatives have been gained wide attention as effective biosorbents due to their low cost and high contents of amine and hydroxyl functional groups which show significant adsorption potential for the removal of various aquatic pollutants Citation[26–31]. Dextran is another polysaccharide that possesses a complex glucan structure of nearly 5–10% (1,3) α-linked branched units, on average; along with its major linear backbone composed of (1,6) α-D-glycoside residues derived from bacterial sources having three hydroxyl functional groups per each glucose moiety. Dextran is hydrophilic, biocompatible, water-soluble, and inert biopolymer, which does not affect viable cells in biological systems Citation[32]. However, a comprehensive survey of literature shows that there is no report regarding utilization of dextran for preparation of fluoride biosorbent.
The aim of this study is utilization of dextran as a environmentally friendly and renewable resource material to prepared biosorbent with fluoride absorbency. In this relate, dextran-graft-poly(hydroxyethyl methacrylate) gels were prepared for the first time and their feasibility for remove of fluoride from aqueous solution was evaluated. The synthetic conditions for preparation of gels, i.e. concentrations of monomer, crosslinking agent, and initiator to achieve maximum fluoride absorbency were optimized through the Taguchi experimental design method. Equilibrium water absorption, fourier-transformed infrared (FTIR), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA) were used to characterize the prepared gels. As well as, the combination of silver nanoparticles with prepared gel was considered as a promising route to design fluoride adsorbent with antibacterial activity for water treatment applications.
2 Experimental
2.1 Materials
The polysaccharide dextran 70 (Mn = 45,040 g/mol, PDI = 1.47) was purchased from Pharmacosmos A/S (Holbaek, Denmark). All chemicals including hydroxyethyl methacrylate (HEMA), ethylene glycol dimethacrylate (EGDMA), ammonium persulfate (APS), potassium fluoride (KF), silver nitrate (AgNO3), tri-sodium citrate, parahydroxy benzoic acid (PHBA), and methanol were of analytical grades supplied by Merck Chemical Co. (Darmstadt, Germany). Double distilled water and acetonitril were used for preparation of gels.
2.2 Experimental design
A Taguchi methodology was adopted using Qualitek-4 software (version 6.3, USA) to investigate the effects of selected formulation variables comprising concentration of monomer (HEMA), crosslinking agent (EGDMA), and initiator (APS), on the gel characteristics. These parameters were varied at three levels as shown in Table . The applied ranges of the variables were determined based on the literature Citation[33] and our preliminary experiments.
Table 1. Experimental control factors and their levels.
Standard tables known as orthogonal arrays (OA) are used for the design of the experiments in the Taguchi method. An OA three factors with a three level are shown in Table . This OA is particularly designed with the symbol of L9. The columns correspond to the factors specified in this study and each column contains three levels for the factors assigned to the column (a total of 9 conditions). Calculations and statistical analysis of the results by analysis of variance were carried out to determine which factors had statistically significant effect on the response parameters.
Table 2. Experimental layout of an L9 standard orthogonal array according to Taguchi design.
2.3 Preparation of dextran-graft-poly(HEMA) gels
The crosslinking and graft copolymerization reactions were carried out using APS as a water soluble initiator, EGDMA as a crosslinker, and distilled water as the reaction medium. A general procedure was conducted as follows. Dextran (1.00 g) was dissolved in 30 ml degassed distilled water in a three-neck reactor equipped with mechanical stirrer (Motogen Company, 400 rpm). The reactor was placed in a water bath preset at 75 °C. After complete dissolution of the polysaccharide to form a homogeneous solution, the certain amount of monomer (0.235–0.470 mol/l) and EGDMA (0.03 –0.09 mol/l, previously dissolved in 5 ml acetonitril) were simultaneously added the reactor and the reaction mixture was stirred for 15 min. Then, a definite amount of APS solution (0.006–0.018 mol/l) was added to the reactor. The gelation was observed after about 40 min. Finally, the obtained gel was poured into 100 ml of ethanol for 2 h and then cut into small pieces. Then the ethanol was decanted and 100 ml fresh ethanol was added. The gel pieces were kept for 24 h in order to complete dehydration. The dried gel particles were filtered and placed in a vacuum oven at 45 °C for 6 h. After grinding, the powdered gels were stored away from moisture, heat, and light.
2.4 Loading of silver nanoparticles into dextran-graft-poly(HEMA) network
The silver nanoparticles were loaded into the dextran-graft-poly(HEMA) according to a reported approach Citation[34]. A dextran-graft-poly(HEMA) gel (sample S3) was put in AgNO3 solution (15 mg AgNO3 in 40 ml distilled water) for 12 h, then was taken out and put in tri-sodium citrate solution (20 mg dissolved in 25 ml water) for next 12 h to reduce Ag+ ions into silver nanoparticles. Then, the produced silver nanoparticles loaded gels were dried in a vacuum oven at 45 °C for 6 h.
2.5 Characterization
The FTIR spectroscopy was accomplished quantitatively on the gels to identify its structure. To this end, a definite weight of the dried gels thoroughly mixed with KBr using a mortar and pestle, and pressed into disks. Spectral scanning was done using an Equinox 55 spectrophotometer (Bruker, Germany) in the range between 400 and 4000 cm−1 at 4 cm−1 resolution and 16 scans at room temperature. The HPLC system consisted of an anion exchange column (PRP-X100, Hamilton) with length of 100 mm, diameter of 4.1 mm, and pore size of 10 μm, an isocratic pump, and an UV detector (Agilent 1200) was used to determined concentration of fluoride. Samples morphology was evaluated using SEM (Vega Tescan, Czech Republic). Samples were mounted on metal stubs and sputter coated with gold for 4 min prior to examination. TGA was carried out for TGA of dextran and dextran-graft-poly(HEMA) gel using a TGA-PL instrument (Polymer Laboratories, UK) under dynamic nitrogen atmosphere (50 ml/min) at a heating rate of 10 °C/min and temperature interval between ambient temperature to complete destruction at 600 °C.
Equilibrium water absorption (EWA) was determined for all of samples. The completely dried, powdered, and accurately (0.2 ± 0.001 g) weighted samples with average particle sizes between 40 and 60 mesh (250–400 mm) were completely immersion in distilled water for three days at ambient conditions. Sol fractions of the batches were previously extracted during post-fabrication work up; however, swelling medium was replaced each day and refurnished with distilled water. The surface water on the bulk of gels was blotted using filter paper then weighed accurately. EWA (g/g) was calculated using the following equation Citation[31]:
where W 0 and W 1 are the weights of dried (initial) and swollen gels, respectively. The values reported are an average of three measurements.
For fluoride adsorption capacity (FAC) measurements, exactly 0.30 g of each gel was placed into the flask containing 100 ml aqueous solution of potassium fluoride with a concentration of 100 ppm at pH = 2. The mixture was shaken by a rotary shaker for 24 h at room temperature to reach the equilibrium adsorption. Then, 20 microliters aliquot of the solution was injected with an auto injector to the anion exchange column and eluted with a mobile phase consisting of PHBA and methanol (97.5/2.5, v/v) with pH = 8.7 (adjust with NaOH) at a flow rate of 1.0 ml/min. The fluoride was detected using the UV detector at 310 nm. Mean values of total content of fluoride were calculated from three replicates. To determine the equilibrium fluoride concentration in the solutions, over the range 0.5–100 ppm, a five-point calibration curve was constructed, with triplicate injections at each concentration and plotting the mean peak height obtained for each concentration against the respective fluoride concentration. Fluoride concentration was subjected to linear least-square regression analysis and was found to be linear over the range tested with a regression coefficient of 0.998. The FAC (mg/kg) values were calculated by subtracting the equilibrium concentration of fluoride from the initial one. The values reported are an average of three measurements.
To investigate antibacterial activity of both prepared gel and silver nanoparticles loaded gels against Escherichia coli, the method ISO 6887 (microbiology of food and animal feeding stuffs – preparation of test sample, initial suspension, and decimal dilutions for microbiological examination) and the method ISO 8443 (microbiology of food and animal feeding stuffs – colony count technique at 30 °C) were applied.
3 Results and discussion
3.1 Synthesis and characterization of dextran-graft-poly(HEMA) gels
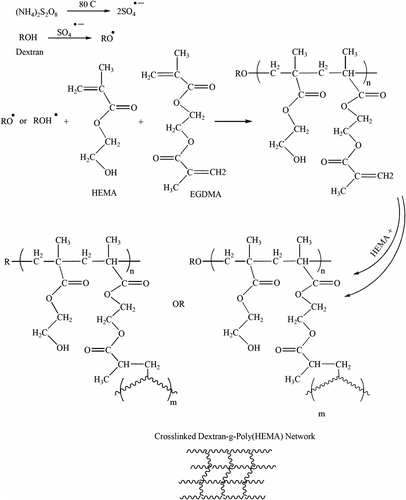
Crosslinking and graft copolymerization of HEMA onto the dextran backbone were carried out in the aqueous medium using APS and EGDMA as free-radical initiator and crosslinking agent. A simple mechanism for the grafting and chemically crosslinking reactions are outlined in Scheme .
The persulfate initiator is decomposed under heating to generate sulfate anion radical. The radical abstracts hydrogen from the hydroxyl group of the dextran to form alkoxy radicals on the dextran backbone. These active centers initiate the polymerization of HEMA leading to grafting of poly(HEMA) on the dextran backbone (Scheme ). Since a crosslinking agent, EGDMA, is present in the system, the copolymer comprises a crosslinked network structure. It should be pointed out that the sulfate ion radical may also initiate HEMA homopolymerization. However, the probable crosslinked hydrophilic homopoly(HEMA) does not cause appreciable undesired effects on the absorbency properties of the final gels. According to preliminary measurements, the sol (soluble) content of the gel networks was as little as 2.5%. This fact practically supports the idea that most homopoly(HEMA)s are involved in the gel network. Therefore, the percentage of HEMA moieties in the prepared gel networks is very similar to that of the initial feed.
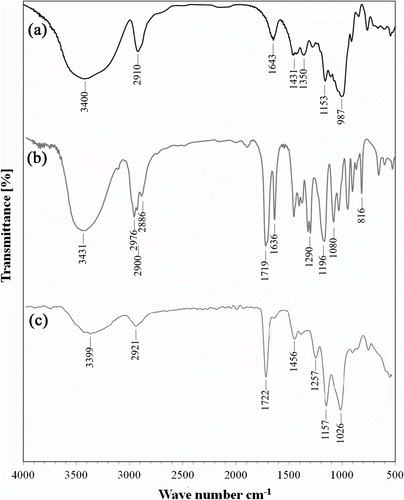
The grafting was confirmed by comparing the FTIR spectra of the polysaccharide substrate with that of the grafted products. The FTIR spectra of pure dextran, pure HEMA monomer, and sample S3 are shown in Figure . For pure dextran (Figure ), the strong peak at 3400 cm−1 was devoted to the stretching vibration of O–H bond. The stretching vibrations of aliphatic C–H groups were observed at 2910 and the corresponding bending vibrations of C–H groups were detected at 1431 and 1350 cm−1. The strong peak appears at 987 cm−1 were due to stretching vibration of C–O–C bonds of polysaccharide structure. Meanwhile, the peak at 1575 cm−1 was due to stretching vibration of C–N bonds. HEMA monomer exhibits some characteristic peaks at 3431, 1719, 1169, and 1080 cm−1 related to stretching vibrations of O–H, C=O, and C–O groups, respectively (Figure ). As well, the peaks were observed around 2956 and 1636 cm−1 can be attributed to the stretching vibration of=C–H and C=C bonds of HEMA, respectively. As well the peak belongs to bending vibration C=C group appeared at 815 cm−1.
Among mentioned peaks, corresponding peaks of double bonds can be used to monitor the polymerization process and the degree of conversion. As it is obvious in Figure , the peaks at 2956, 1636, and 815 cm−1, belonged to double bounds of HEMA monomer, disappeared at FTIR spectra of sample S3. Obviously, all other peaks mentioned for pure dextran and HEMA monomer can be recognized in FTIR spectra of sample S3.
3.2 Thermogravimetric analysis
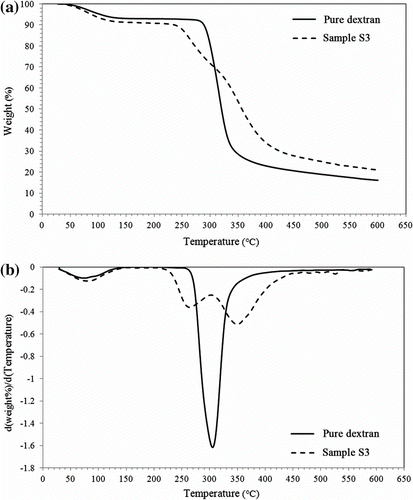
In order to further investigate the grafting of poly(HEMA) onto the dextran backbone, the TGA of pure dextran was compared with that of sample S3 (Figure ). TGA and DTG curves for sample S3 are completely different from that of pure dextran. According to Figure , some changes occurred during polymerization reaction, which improved the thermal stability of the prepared gels in comparison to that of pure dextran. Decomposition process in N2 occurs in three stages for sample S3 and in two stages for pure dextran. In the first stage, ranged from room temperature to 170 °C and the weight loss for pure dextran and sample S3 were about 7.5 and 9.4%, respectively. This weight loss may be due to desorption of moisture as hydrogen-bonded water molecules to the glucose moieties. As well as, this stage perhaps could be ascribed to the elimination of the residual organic solvents used in drying of gels.
The degradation process in the second stage can be attributed to the rupture of the main chains of dextran and also crosslinking bonds. The onset of thermal degradation is lower for sample S3 (i.e. 230 vs. 281 °C), which may be attributed to the different bonds strength that existence on copolymer network.
A significant increase in thermal stability of sample was observed in comparison to the pure dextran especially, considering the remained ash (T = 600 °C, 21.8 vs. 16.2% for sample S3 and pure dextran, respectively) which is certainly due to its crosslinking and consequent improvement in thermal stability. Furthermore, the thermal degradation of the synthesized gels is sufficiently high for different applications of the gels.
3.3 Optimization of grafting conditions for maximum fluoride absorbency
The Taguchi experimental design method was used for optimizing of the synthetic conditions of the dextran-graft-poly(HEMA) gels, i.e. concentrations of monomer (HEMA), crosslinking agent (EGDMA), and initiator (APS), to reach high level of fluoride absorbency. After selection of factors and their levels, an OA appropriate for three factors with three levels for each factor was applied (L9 OA). After synthesis of defined gels, the FAC values were determined at neutral (pH = 7.4) and acidic (pH = 2) conditions. As expected, the prepared gels showed no fluoride absorbency at neutral, but their fluoride absorbability significantly increased at acidic condition (Table ). The electrostatic attraction between fluoride ions and hydroxyl groups in the backbone of dextran and HEMA moieties, protonated at such pH values, was proposed as the main sorption mechanism Citation[29,35].
Table 3. Experimental results for water absorption and fluoride adsorption studies.1
Finding the optimum conditions and contribution of each factor was performed by ANOVA analysis. It should be emphasized that the interaction between the variables were neglected. In the case of optimum results proposed by Qualitek-4 software, and according to ANOVA analysis, optimum conditions are as follows: 0.235 mol/l HEMA, 0.09 mol/l EGDMA, and 0.018 mol/l APS. According to Table , the proposed optimum conditions resulted in FAC of 8312 mg/kg (refers to sample S3). In order to achieve high level of confidence and reproducibility for results, FAC of sample S3 was measured three additional times. Results were found to be 8081, 8352, and 8504 mg/kg for consecutive trials. The slight differences observed can be attributed to the materials and apparatus used for the synthesis and FAC determination. As well, it can be seen from the ANOVA results that the most effective factor is HEMA concentration. The contribution of HEMA is 55.21% and those of the EGDMA and APS are 12.03, and 32.76%, respectively (Table ).
Table 4. The results of analysis of variance.
EWA values of the prepared gels were also determined and reported in Table . As expected, sample S3 with higher EWA value (1061 g/g), showed higher capacity for fluoride removal. In fact, dextran-based gels with higher EWA values, like other biosorbents, expanded in aqueous solutions in more extent. This surface expansion improved the adsorb ability of fluoride.
A brief comparison of the fluoride absorbency of the prepared gel from water under various experimental conditions with that of some recently reported biosorbents is given in Table . According to Table , the FCA value of dextran-based gel is comparable with that value of other reported biosorbents. Therefore, prepared gels can be used as a potential candidate for defluoration of wastewater.
Table 5. Adsorption capacity and other parameters for the removal of fluoride by different biosorbents.
3.4 Scanning electron microscopy
Water absorption of gels and its retention rate depends on gel porosity and mean pore size. Hence, one of the most important properties, which should be considered, is gel microstructure morphologies. This porous microstructure brings about an increased surface area and capillary effect Citation[36,37]. The microstructure morphologies of prepared gels were observed using SEM. The SEM images of pure dextran and sample S3 are shown in Figures and , respectively. Considerable morphological changes in the gels have been observed compared with pure dextran. These provide strong evidence that dextran backbone has been functionalized with poly (HEMA). According to Figure , pure dextran is like microparticles that does not show any porous structure on the surface. But the microstructure of sample S3 was highly porous (Figure ). It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers. Another interesting point of SEM images is the relationship between porosity and fluoride absorbency of samples in distilled water. The gel sample has a highly nanoporous and cellular structure. As one can see in Figure , the size of porosity for this gel is not monodisperse, but is in range of nanometer scales (50–500 nm). As well as, this result shows that the more porous and smaller size porous structures can give higher water absorbency.
Figure 4 SEM images of sample S3 (a) and sample S3 loaded with silver nanoparticles (b). EDXA spectra of sample S3 (c) and sample S3 loaded with silver nanoparticles (d).
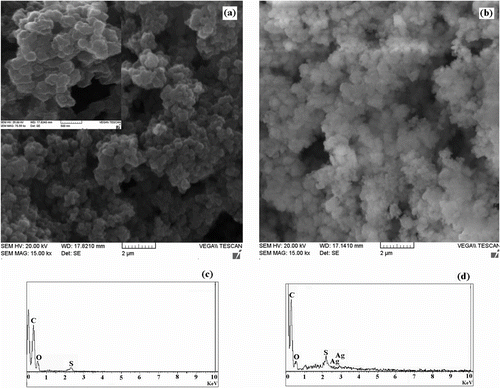
Figure shows the SEM micrographs of sample S3 after loading of silver nanoparticles. There were apparent differences between the beads with many cavities before loading and no such cavities are observed after loading. EDXA spectrum of sample S3 (Figure ) shows the presence of elements in it. An emergence of Ag peak in the EDXA spectra of sample S3 loaded with nanoparticles (Figure ) confirmed the Ag existence on the surface.
3.5 Antibacterial studies
Thus, herein, we explored the feasibility of precisely producing silver nanoparticles in gel networks and demonstrated their antibacterial activity. In particular, polymers with silver nanoparticles have been identified as materials to overcome infections, dates back as early as ancient times. Silver, both as a metal and in ionic form, exhibits strong antibacterial activity against a broad range of micro-organisms, and its use as an antibacterial agent is well known Citation[38]. It has been reported that the mode of antibacterial action of silver nanoparticles is similar to that of silver ion. However, the effective biocidal concentration of silver nanoparticles is at a nanomolar level in contrast to a micromolar level of silver ions Citation[38]. Silver is capable of causing a bacteriostatic (growth inhibition) or a bactericidal (antibacterial) impact. Research indicates that silver is also effective in purification systems for disinfecting water or air, still there is a great interest to generate antibacterial material, because of their superior biomedical relevance.
For this purpose, sample S3 was loaded with silver nanoparticles and the in vitro antibacterial screening for it has been carried out against E. coli as a common micro-organism in water. The results suggest that the silver nanoparticles loaded gels showed high antibacterial activity with an inhibition zoom of 2 cm, while sample S3 as a control one did not show any zoom of inhibition. Therefore, these new gels can be considered as a candidate to develop an efficient antibacterial fluoride biosorbent for water treatment applications.
4 Conclusions
Dextran is a environmentally friendly, renewable resource-based and low-cost material used for removing fluoride from water. Dextran-graft-poly(HEMA) gels was prepared for the first time, which offered satisfactory results in terms of fluoride removal. The synthetic conditions of dextran-based gels were optimized using the Taguchi method performing nine experimental runs. A maximum value of 8312 (mg fluoride/kg dried gel) was achieved under the optimum conditions that were found to be HEMA, 0.235 mol/l; EGDMA, 0.09 mol/l; and APS, 0.018 mol/l. In addition, incorporation of silver nanoparticle, resulted in gels, which demonstrated antibacterial activity against bacteria such as E. coli. These new gels can be used as potential candidate for simultaneous defluoridation and killing pathogenic bacterial of wastewater during water treatment processes.
Acknowledgment
The authors express their sincere gratitude to Sharif University of Technology for providing financial support to this research.
References
- Amini , M , Mueller , K , Abbaspour , KC , Rosenberg , T , Afyuni , M , Moller , KN , Sarr , M and Johnson , CA . 2008 . Environmental Science and Technology , 42 : 3662 – 3668 .
- Guidelines for drinking-water quality. 4th ed. Geneva: World Health Organization (WHO); 2011.
- Bhatnagar , A , Kumar , E and Sillanp , M . 2006 . Chemical Engineering Journal , 13 : 203 – 217 .
- Harrison , PTC . 2005 . Journal of Fluorine Chemistry , 126 : 1448 – 1456 .
- Islam , M and Patel , R . 2011 . Chemical Engineering Journal , 169 : 68 – 77 .
- Wu , X , Zhang , Y , Dou , X and Yang , M . 2007 . Chemosphere , 69 : 1758 – 1764 .
- Onyango , MS , Kojima , Y , Aoyi , O , Bernardo , EC and Matsuda , H . 2004 . Journal of Colloid and Interface Science , 279 : 341 – 350 .
- Sundaram , CS and Meenakshi , S . 2009 . Journal of Colloid and Interface Science , 333 : 58 – 62 .
- Viswanathan , N and Meenakshi , S . 2008 . Journal of Fluorine Chemistry , 129 : 645 – 653 .
- Chubar , NI , Samanidou , VF , Kouts , VS , Gallios , GG , Kanibolotsky , VA , Strelko , VV and Zhuravlev , IZ . 2005 . Journal of Colloid and Interface Science , 291 : 67 – 74 .
- Zhao , H-Z , Yang , W , Zhu , J and Ni , J-R . 2009 . Chemosphere , 74 : 1391 – 1395 .
- Emamjomeh , MM and Sivakumar , M . 2006 . Journal of Hazardous Materials , B131 : 118 – 125 .
- Sehn , P . 2008 . Desalination , 223 : 73 – 84 .
- Ndiaye , PI , Moulin , P , Domingwez , L , Millet , JC and Charbit , F . 2005 . Desalination , 173 : 25 – 32 .
- Diawara , CK . 2008 . Separation and Purification Reviews , 37 : 303 – 325 .
- Hu , K and James , MD . 2006 . Journal of Membrane Science , 279 : 529 – 538 .
- Kabay , N , Arar , O , Samatya , S , Yuksel , U and Yuksel , M . 2008 . Journal of Hazardous Materials , 153
- Durmaz , F , Kara , H , Cengeloglu , Y and Ersoz , MM . 2005 . Desalination , 177 : 51 – 57 .
- Ruiz T, Persin F, Hichour M, Sandeaux J, Journal of Membrane Science. 2003, 212:113.
- Liu , Q , Guo , H and Shan , Y . 2010 . Journal of Fluorine Chemistry , 131 : 635 – 641 .
- Ayoob , S and Gupta , AK . 2006 . Critical Reviews in Environmental Science and Technology , 36 : 433 – 487 .
- Thakur , N , Kumar , SA , Wagh , DN , Das , S , Pandey , AK , Kumar , SD and Reddy , AVR . 2012 . Journal of Hazardous Materials , 201–202 : 193 – 201 .
- Cui , H , Li , Q , Qian , Y , Tang , R , An , H and Zhai , J . 2011 . Water Research , 45 : 5736 – 5744 .
- Fang , L , Ghimire , KN , Kuriyama , M , Inoue , K and Makino , K . 2003 . Journal of Chemical Technology and Biotechnology , 78 : 1038 – 1047 .
- Miretzky , P and Cirelli , AF . 2011 . Journal of Fluorine Chemistry , 132 : 231 – 240 .
- Viswanathan , N and Meenakshi , S . 2008 . Journal of Fluorine Chemistry , 129 : 503 – 509 .
- Sundaram , CS , Viswanathan , N and Meenakshi , S . 2009 . Journal of Hazardous Materials , 172 : 147 – 151 .
- Ma , W , Ya , F-Q , Han , M and Wang , R . 2007 . Journal of Hazardous Materials , 143 : 296 – 302 .
- Davila-Rodriguez , JL , Escobar-Barrios , VA , Shirai , K and Rangel-Mendez , JR . 2009 . Journal of Fluorine Chemistry , 130 : 718 – 726 .
- Bansiwal , A , Thakre , D , Labhshetwar , N , Meshram , S and Rayalu , S . 2009 . Colloids and Surfaces B: Biointerfaces , 74 : 216 – 224 .
- Viswanathan , N , Sundaram , CS and Meenakshi , S . 2009 . Journal of Hazardous Materials , 161 : 423 – 430 .
- Heinze , T , Liebert , T , Heublein , B and Hornig , S . 2006 . Advances in Polymer Science , 205 : 199 – 291 .
- Pourjavadi , A , Soleyman , R and Barajee , GR . 2008 . Starch Strake , 60 : 467 – 475 .
- Thomas , V , Yallapu , MM , Sreedhar , B and Bajpai , SK . 2007 . Journal of Colloid and Interface Science , 315 : 389 – 395 .
- Niu , CH , Volesky , B and Cleiman , D . 2007 . Water Research , 41 : 2473 – 2478 .
- Doo-Won , L , Jong , YK and Sohk-Won , K . 2000 . Journal of Applied Polymer Science , 78 : 2525 – 2532 .
- Pourjavadi , A , Ayyari , M and Amini-Fazl , MS . 2008 . European Polymer Journal , 44 : 1209 – 1216 .
- Marambio-Jones , C and Hoek , EMV . 2010 . Journal of Nanoparticle Research , 12 : 1531 – 1551 .