Abstract
Polybenzoxazine-based flexible composites have been prepared by blending benzoxazine with linear low density polyethylene (LLDPE) in presence of a compatibilizer (maleic anhydride grafted linear low density polyethylene [LLDPE-g-MA]) using a melt blending method. It has been observed that the thermal stability of the composites increased with increasing amount of polybenzoxazine in the composition. Mechanical properties of the composites have been investigated by using tensile and flexural tests. Presence of 5 wt% compatibilizer in the composition showed very good mechanical properties. Pure polybenzoxazine possessed high tensile strength and less elongation at break whereas LLDPE showed low tensile strength and significantly more elongation at break. Composites consist of LLDPE and polybenzoxazine with additional LLDPE-g-MA, exhibited higher tensile strength than pure LLDPE and more elongation at break than pure polybenzoxazine. Prepared composites possess very good mechanical flexibility, which makes them good candidates to prepare of complex structures and coating.
1. Introduction
Recently, polybenzoxazine-based materials have gained immense interest in the field of polymer research because polybenzoxazine exhibits several interesting properties, such as (1) near zero volumetric change upon curing, (2) very low water absorption, (3) for some polybenzoxazines T g is much higher than cure temperature, (4) high char yield, (5) no strong acid catalysts are required for curing, (6) release of no byproduct during curing, and (7) good thermal and flame retarding properties Citation[1–4]. Shrinkage does not occur during curing because of ring opening polymerization, which is a very striking feature of benzoxazine-based polymers Citation[5]. Though, polybenzoxazine offers a variety of advantages, pure polybenzoxazine-based polymers also suffer a number of disadvantages such as poor mechanical strength (brittleness) and difficulty in processing Citation[6,7]. These factors limit the industrial applications of polybenzoxazine-based polymers. To improve the processibility and mechanical properties, several researchers have attempted various strategies, such as (1) preparation of modified monomers with additional functionality Citation[8–15], (2) synthesis of novel polymeric precursors by incorporating benzoxazine units either as side chain Citation[16–20] or as end chain Citation[21–27] or in main chain of polymer Citation[28–36], and (3) blending with a high-performance polymer or filler and fiber Citation[6]. However, most of the preparation methods are complex in nature, require costly starting materials, and limited to laboratory scale only. Therefore, there is a need to develop polybenzoxazine-based composites, which can be prepared in large scale by using a simple method and cheap starting materials and the composites will possess mechanical flexible property so that they can be used to make complex structures or coating.
In this paper, we report the preparation of the composites, composed of polybenzoxazine and linear low density polyethylene (LLDPE) and a compatibilizer (maleic anhydride grafted LLDPE [LLDPE-g-MA]). LLDPE was chosen as one of the components of the composites because LLDPE possesses excellent low-temperature flexibility, extraordinary processibility, better environmental stress cracking resistance, mechanical flexibility, more elongation at break, and puncture resistance Citation[37]. Polybenzoxazine–LLDPE composites, with various compositions, were prepared by using a simple melt blending method. LLDPE-g-MA was used as a compatibilizer to enhance the interfacial interaction between LLDPE and polybenzoxazine. The prepared composites were characterized by using X-ray diffraction (XRD), thermo gravimetric analysis (TGA), differential scanning calorimetric (DSC) analysis, and Fourier transform infrared (FTIR) spectroscopy. Tensile and flexural properties of the composites were carried out by using a universal testing machine.
2 Experimental
2.1 Materials
The chemicals used were aniline, paraformaldehyde, bisphenol-A (99%, S D Fine-Chem Limited, India), and CHCl3 (99.7%, Qualigens Fine Chemicals, India). Linear low density polyethylene (LLDPE, R35A042), having a density of 0.935 gm/cm3 and melt flow index (MFI) of 4.2 gm/10 min, was obtained from GAIL (India) Ltd and LLDPE-g-maleic anhydride (LLDPE-g-MA) (OPTIM E-126, with 0.73% maleic anhydride content and MFI 2.16 gm/10 min) from Pluss Polymers Pvt. Ltd, India. All chemicals were used as received.
2.2 Synthesis of benzoxazine monomer
Benzoxazine monomer (bis (3-phenyl-3, 4-dihydro-2H-1, 3-benzoxazinyl) isopropane) was synthesized using a solventless method by reacting bisphenol-A, aniline, and paraformaldehyde Citation[38]. In a typical synthesis, bisphenol-A (0.02 mol, 4.48 gm), aniline (0.04 mol, 3.68 ml), and paraformaldehyde (0.08 mol, 2.4 gm) were mixed in a round bottom flask and heated slowly at 90 °C in an oil bath for 90 min. After cooling, benzoxazine monomer was extracted from reaction mixture by dissolving in CHCl3 followed by filtration. Pure benzoxazine monomer was finally obtained by evaporating CHCl3. Benzoxazine monomer was then dried in a vacuum oven for 24 h at 55 °C to remove traces of chloroform. Now onwards, benzoxazine monomer will be referred as ‘BA’ and polybenzoxazine as ‘PB.’
2.3 Preparation of composite
Composites, having different compositions, were prepared by blending benzoxazine with LLDPE and LLDPE-g-MA with various amounts (Table ). The blending of LLDPE, LLDPE-g-MA, and BA was carried out in a custom made cylindrical mixing chamber (65 mm diameter × 65 mm height) using a two-sided blend stirrer. The temperature of the mixture during mixing was maintained at 180 °C and the stirring speed was 80 rpm. LLDPE and LLDPE-g-MA were first melted for 10 min then benzoxazine was added to this melt and mixed for 20 min. This hot mass was then transferred into a pot and heated at 200 °C for 30 min in an oven. The hot semi-viscous mixture thus obtained was immediately poured into a closed mold under hydraulic pressure through a 5 mm gate. The material was then allowed to cool at room temperature inside the mold cavity. After cooling, the mold was opened to get the final product. Now onwards, we will refer the composites composed of LLDPE, LLDPE-g-MA, and polybenzoxazine as ‘L-LgM-PB.’
Table 1. Compositions of the composites.
2.4 Sample characterization
Room temperature XRD spectra of the LLDPE, polybenzoxazine, and their composites were recorded by using a wide angle powder X-ray diffractometer (Mini Flex II, Rigaku, Japan) with Cu Kα (λ = 0.15405 nm) radiation. TGA and DSC analysis were carried out for the neat polymers and composites by using DTG-60 and DSC-60 (Shimadzu, Japan), respectively. Thermal analysis was performed at a constant heating rate 10 °C/min in air atmosphere. Tensile measurements were performed according to ASTM D638 standard using a universal testing machine (INSTRON 3366, USA). Type I dog-bone specimens with the overall dimensions of 165 mm × 19 mm × 3.2 mm were prepared for tensile testing. Room temperature measurements were carried out at a constant crosshead speed of 5 mm/min. The flexural properties of the neat polymers and blend were determined in accordance with ASTM D790 using a universal testing machine (INSTRON 3366, USA) with 10 kN load cell. Specimens were tested in a three point loading with 50 mm support span at crosshead speed of 5 mm/min at room temperature. The surface morphology of the composites was studied using a scanning electron microscope (SEM) (JSM-7500F, JEOL, Japan).
3 Results and discussion
3.1 XRD analysis
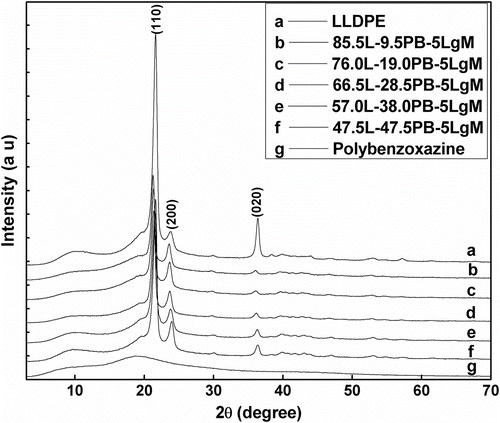
Room temperature wide angle powder XRD spectra of pure LLDPE showed diffraction peaks at 2θ = 21.6, 23.8, and 36.3° corresponding to (1 1 0), (2 0 0), and (0 2 0) diffraction planes of the orthorhombic crystal planes of polyethylene Citation[39] along with a broad amorphous peak in between 2θ = 8° and 12°. Pure cured polybenzoxazine exhibited a broad peak from 2θ = 11.7° to 37.5° indicating its amorphous nature. XRD spectra of the prepared composites showed the presence of characteristic peaks of LLDPE along with amorphous broad peak of polybenzoxazine (Figure ). The crystalline nature of LLPDE was remained preserved in the cured blend samples. However, with increasing amount of polybenzoxazine in the composites broadening of the peaks of LLDPE was observed. This indicates that blending of polybenzoxazine with LLDPE enhances the amorphous nature. The change of percentage of crystallinity of the composites with changing composition is summarized in Table .
Table 2. Amount of crystallinity present in the prepared composites.
3.2 Thermal analysis
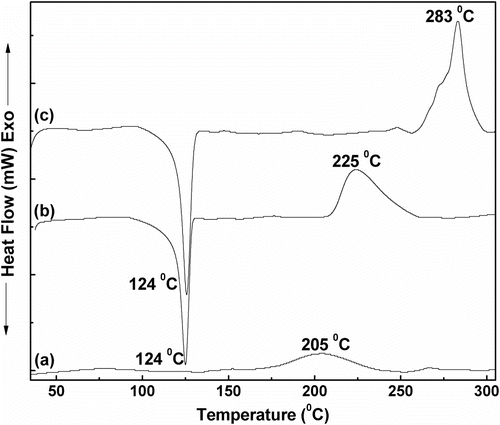
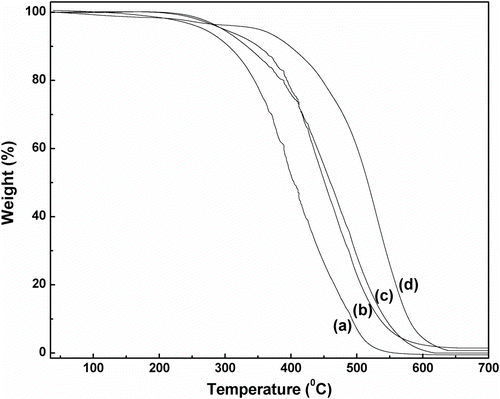
In the DSC thermogram of benzoxazine monomer, an exothermic peak at ∼205°C was observed (Figure ), which corresponds to the ring opening polymerization of benzoxazine Citation[40]. In case of pure LLDPE, (1) an endothermic peak at ∼124 °C, corresponding to its melting temperature Citation[39] and (2) an exothermic peak at ∼225 °C, which indicates its thermal decomposition Citation[41], were observed (Figure ). In the DSC thermograms of LLDPE–polybenzoxazine composites, the endothermic peak at ∼124 °C was present, but the exothermic ring opening polymerization peak of benzoxazine was absent (Figure ). This indicates that benzoxazine monomers were fully polymerized to polybenzoxazine during composite preparation as the melt blending was performed at 200 °C. An exothermic peak at ∼283 °C, corresponding to the thermal decomposition, was present in the DSC thermograms of the composites. It was observed that decomposition temperature of the composites shifted to higher temperatures with increasing amount of polybenzoxazine. This indicates that presence of polybenzoxazine enhances the thermal stability of the composites. Same observation was found in the TGA analysis of the composites. Thermal decomposition of the composites was found to be started after ∼250 °C. The temperatures at which 5 and 10% weight loss occurred are listed in Table . Char yield of the all the composites was found to be zero at ∼600 °C in air. Full decomposition temperatures of the composites were also found to be increased with increasing amount of polybenzoxazine in the composition (Figure ).
Table 3. Temperatures required for 5 and 10% weight loss due to thermal decomposition of the composites in air.
3.3 FTIR analysis
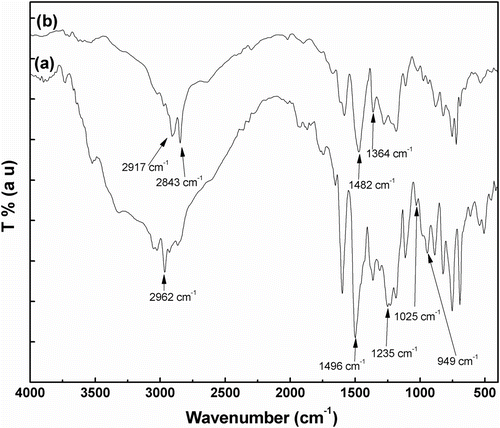
FTIR spectra of benzoxazine and 47.5L-47.5PB-5LgM blend are shown in Figure and (b), respectively. In case of benzoxazine monomer, a peak at 949 cm−1 for C–H out of plane deformation of 1,2,4-tri-substituted benzene ring was observed. Other peaks observed at 1496, 1235, and 1025 cm−1 are due to tri-substituted benzene ring mode in the oxazine ring structure Citation[7], C–O–C asymmetric and symmetric stretching mode, respectively. The methyl group vibration was found at 2962 cm−1 Citation[42,43]. In the FTIR spectra of composites, (Figure ) the characteristic peaks of LLDPE and polybenzoxazine were present. A strong peak around 2917 and 2843 cm−1, associated with the C–H stretching vibration, along with peaks at 1482 cm−1 due to –CH2 rocking vibration and at 1364 cm−1 for –CH3 symmetric vibration for LLDPE Citation[39] were observed. It was also observed that the peak at 949 cm−1, corresponds to the tri-substituted benzene ring of benzoxazine, was disappeared in the blend samples. This indicates the complete formation of polybenzoxazine from benzoxazine occurred during blend preparation Citation[7].
3.4 Mechanical properties
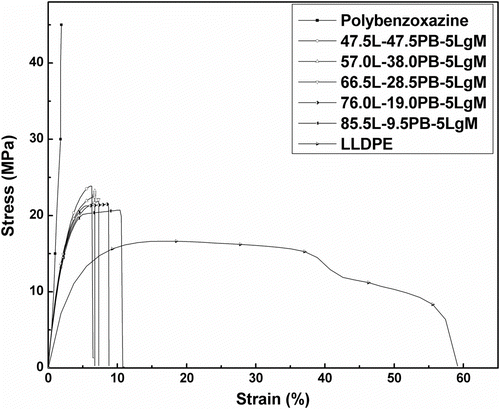
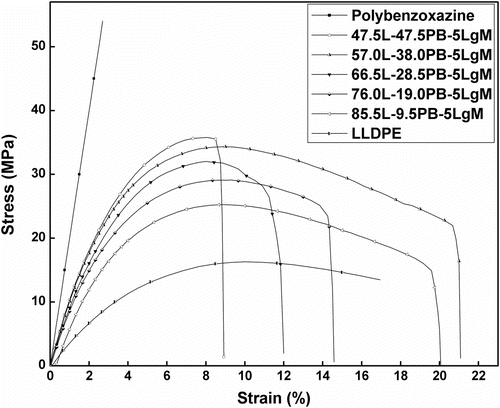
In this study, composites were prepared by blending LLDPE with polybenzoxazine in presence of LLDPE-g-MA compatibilizer. To determine the optimum amount of compatibilizer, required to achieve good mechanical property, samples were prepared by blending various amount of LLDPE-g-MA (0–8 wt%) with LLDPE and PB and tensile and flexural tests were performed. It was observed that tensile strength and flexural strength of the composites increased with increasing amount of LLDPE-g-MA (up to 5 wt%) and then started to decrease (Figure ). Pure polybenzoxazine possessed high tensile strength (47.05 MPa) and less elongation at break (2.2%), whereas LLDPE showed low tensile strength (16.75 MPa) and significantly more elongation at break (57%). Composites, consist of LLDPE and PB with 5 wt% LLDPE-g-MA (47.5L-47.5PB-5LgM), exhibited higher tensile strength (23.81 MPa) than pure LLDPE and more elongation at break (6.11%) than pure polybenzoxazine. The increase of tensile strength and flexural strength with increasing amount of LLDPE-g-MA might be due to the fact that, LLDPE-g-MA molecules are placed at the interface of LLDPE and polybenzoxazine during melt blending processes. The polar functional groups of LLDPE-g-MA interact with polar functional groups of polybenzoxazine while the LLDPE backbone of LLDPE-g-MA molecules compatibilizer with the LLDPE matrix Citation[44]. This interaction enhances the interfacial adhesion and helps to reduce the interfacial tension between the two distinct phases (LLDPE and polybenzoxazine). At 5 wt%, the concentration of LLDPE-g-MA in the blend may reaches to the critical concentration value, where compatibilizer molecules already occupy most of the interfacial area. Beyond 5 wt% excess LLDPE-g-MA molecules form a third phase Citation[44]. So, decrease in tensile strength and flexural strength of the composites was observed beyond 5 wt% LLDPE-g-MA. SEM micrographs of the composites also showed the enhancement of homogeneity of LLDPE and polybenzoxazine phases with increasing amount of LLDPE-g-MA (Figure ). Table summarizes the tensile and flexural properties of the composites having various amount of LLDPE-g-MA. As the composites containing 5 wt% LLDPE-g-MA exhibited highest tensile and flexural strength, so this concentration of LLDPE-g-MA was chosen to prepare composites having various amount of LLDPE and polybenzoxazine to assess the effect of blending of LLDPE with polybenzoxazine.
Figure 5 Change of tensile and flexural strength of the composites with the variation of LLDPE-g-MA in the composition.
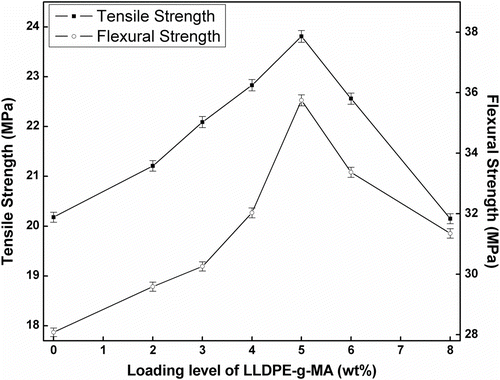
Figure 6 SEM micrograph of (a) 50.0L-50.0PB-0LgM blend, (b) 49.0L-49.0PB-2LgM blend, (c) 48.5L-48.5PB-3LgM blend, and (d) 47.5L-47.5PB-5LgM blend.
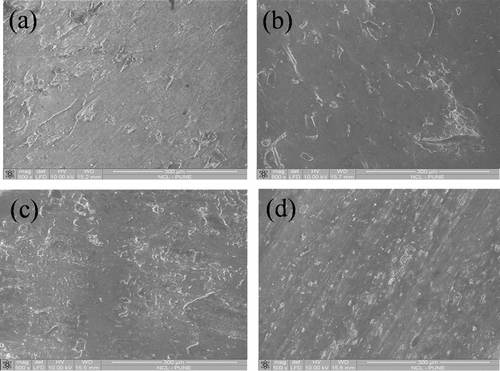
Table 4. Tensile and flexural properties of the composites having various amount of LLDPE-g-MA.
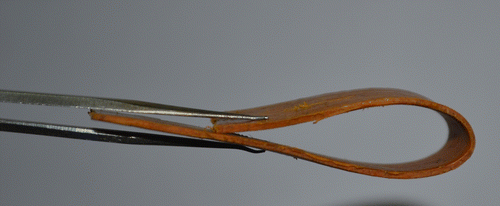
Tensile tests of the composites showed that blending of LLDPE with polybenzoxazine resulted in decrease of tensile strength and modulus with increasing amount of LLDPE. But significant amount of increase of the elongation at break was observed due to lending of LLDPE with polybenzoxazine compare to pure polybenzoxazine (Figure ). Flexural strength and modulus were also decreased with increasing amount of LLDPE in the composition of composites (Figure ). However, toughness of the samples decreases very less with increasing amount of LLDPE in the composite. These facts indicate that blending of LLDPE with polybenzoxazine in presence of 5 wt% LLDPE-g-MA compatibilizer increases the mechanical flexibility of the composites. The tensile and flexural properties of the composites are listed in Table . Figure demonstrates the flexibility of the composites, composed of 47.5 wt% LLDPE, 47.5 wt% polybenzoxazine, and 5 wt% LLDPE-g-MA.
Table 5. Change of mechanical properties of the composites with the variation of LLDPE and polybenzoxazine in the composite.
4 Conclusions
Polybenzoxazine–LLDPE-based composites were prepared by blending LLDPE with polybenzoxazine in presence of a compatibilizer (LLDPE-g-MA) by employing a simple melt blending method. Thermal stability of the composites was found to be increased with increasing polybenzoxazine content in the blend. However, as the melting point of the LLDPE is 124 °C, these composites should be used below this temperature. Tensile and flexural testing of the composites revealed that 5 wt% is the optimum amount of compatibilizer that can be used in blend preparation. Mechanical flexibility of the composites was found to be increased with increasing amount of LLDPE in the composition.
In summary, in this paper, a melt blending method has been reported for preparation of mechanically flexible polybenzoxazine-based blend. This method is very simple and cheap. LLDPE was used to blend with polybenzoxazine. So, these composites can easily be prepared in large scale. As the prepared composites possess good mechanical flexibility along with good thermal stability, these composites have the potential to be used in preparation of complex structures.
References
- Ghosh , NN , Kiskan , B and Yagci , Y . 2007 . Polybenzoxazines - new high performance thermosetting resins: synthesis and properties . Progress in Polymer Science , 32 : 1344 – 1391 .
- Yagci , Y , Kiskan , B and Ghosh , NN . 2009 . Recent advancement on polybenzoxazine - a newly developed high performance thermoset . Journal of Polymer Science Polimer Chemistry , 47 : 5565 – 5576 .
- Kiskan , B , Dogan , F , Durmaz , YY and Yagci , Y . 2008 . Synthesis, characterization and thermally-activated curing of azobenzene-containing benzoxazines . Designed Monomers and Polymers , 11 : 473 – 482 .
- Ghetiya , RM , Kundariya , DS , Parsania , PH and Patel , VA . 2008 . Synthesis and characterization of cardo bisbenzoxazines and their thermal polymerization . Polymer-Plastics Technology , 47 : 836 – 841 .
- Jang , J and Seo , D . 1998 . Performance improvement of rubber-modified polybenzoxazine . Journal of Applied Polymer Science , 67 : 1 – 10 .
- Kiskan , B , Ghosh , NN and Yagci , Y . 2011 . Polybenzoxazine-based composites as high-performance materials . Polymer International , 60 : 167 – 177 .
- Ishida H. Overview and historical background of polybenzoxazine research. In: Ishida H, Agag T, editors. Handbook of benzoxazine resins. Amsterdam: Elsevier; 2011. p. 3–81.
- Takeichi , T , Nakamura , K , Agag , T and Muto , H . 2004 . Synthesis of cresol-based benzoxazine monomers containing allyl groups and the properties of the polymers therefrom . Designed Monomers and Polymers , 7 : 727 – 740 .
- Kim , HJ , Brunovska , Z and Ishida , H . 1999 . Synthesis and thermal characterization of polybenzoxazines based on acetylene-functional monomers . Polymer , 40 : 6565 – 6573 .
- Agag , T and Takeichi , T . 2003 . Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high performance thermosets . Macromolecules , 36 : 6010 – 6017 .
- Agag , T and Takeichi , T . 2001 . Novel benzoxazine monomers containing p-phenyl propargyl ether: polymerization of monomers and properties of polybenzoxazines . Macromolecules , 34 : 7257 – 7263 .
- Brunovska , Z and Ishida , H . 1999 . Thermal study on the copolymers of phthalonitrile and phenylnitrile-functional benzoxazines . Journal of Applied Polymer Science , 73 : 2937 – 2949 .
- Ishida , H and Ohba , S . 2005 . Synthesis and characterization of maleimide and norbornene functionalized benzoxazines . Polymer , 46 : 5588 – 5595 .
- Kiskan , B and Yagci , Y . 2007 . Thermally curable benzoxazine monomer with a photodimerizable coumarin group . Journal Polymer Science Polymer Chemistry , 45 : 1670 – 1676 .
- Allen , DJ and Ishida , H . 2009 . Effect of phenol substitution on the network structure and properties of linear aliphatic diamine-based benzoxazines . Polymer , 50 : 613 – 626 .
- Cao , H , Yan , D , Sun , X , Xu , R and Yu , D . 2009 . Synthesis and characterization of a novel 2-oxazoline-benzoxazine compound with incorporated polyhedral oligomeric silsesquioxane . Designed Monomers and Polymers , 12 : 565 – 578 .
- Gacal , B , Cianga , L , Agag , T , Takeichi , T and Yagci , Y . 2007 . Synthesis and characterization of maleimide (co)polymers with pendant benzoxazine groups by photoinduced radical polymerization and their thermal curing . Journal Polymer Science Polymer Chemistry , 45 : 2774 – 2786 .
- Kiskan , B , Demiray , G and Yagci , Y . 2008 . Thermally curable polyvinylchloride via click chemistry . Journal Polymer Science Polymer Chemistry , 46 : 3512 – 3518 .
- Ergin , M , Kiskan , B , Gacal , B and Yagci , Y . 2007 . Thermally curable polystyrene via click chemistry . Macromolecules , 40 : 4724 – 4727 .
- Kukut , M , Kiskan , B and Yagci , Y . 2009 . Self-curable benzoxazine functional polybutadienes synthesized by click chemistry . Designed Monomers and Polymers , 12 : 167 – 176 .
- Kiskan , B , Colak , D , Muftuoglu , AE , Cianga , I and Yagci , Y . 2005 . Synthesis and characterization of thermally curable benzoxazine-functionalized polystyrene macromonomers . Macromolecular Rapid Communications , 26 : 819 – 824 .
- Kiskan , B and Yagci , Y . 2005 . Synthesis and characterization of naphthoxazine functional poly(ϵ-caprolactone) . Polymer , 46 : 11690 – 11697 .
- Yildirim , A , Kiskan , B , Demirel , AL and Yagci , Y . 2006 . Synthesis, characterization and properties of naphthoxazine-functional poly(propyleneoxide)s . European Polymer Journal , 42 : 3006 – 3014 .
- Tasdelen , MA , Kiskan , B and Yagci , Y . 2006 . Photoinitiated free radical polymerization using benzoxazines as hydrogen donors . Macromolecular Rapid Communications , 27 : 1539 – 1544 .
- Ishida , H and Lee , YH . 2001 . Study of hydrogen bonding and thermal properties of polybenzoxazine and poly-(ϵ-caprolactone) blends . Journal of Polymer Science Polymer Physics , 39 : 736 – 749 .
- Zheng , SX , Lu , H and Guo , QP . 2004 . Thermosetting blends of polybenzoxazine and poly(ϵ-caprolactone): phase behavior and intermolecular specific interactions . Macromolecular Chemistry and Physics , 205 : 1547 – 1558 .
- Nakamura , M and Ishida , H . 2009 . Synthesis and properties of new crosslinkable telechelics with benzoxazine moiety at the chain end . Polymer , 50 : 2688 – 2695 .
- Liu , J . 1995 . Synthesis, characterization, reaction mechanism and kinetics of 3,4-dihydro-2H-1,3- benzoxazine and its polymers , Cleveland , OH : Case Western University .
- Chernykh , A , Liu , JP and Ishida , H . 2006 . Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain . Polymer , 47 : 7664 – 7669 .
- Takeichi , T , Kano , T and Agag , T . 2005 . Synthesis and thermal cure of high molecular weight polybenzoxazine precursors and the properties of the thermosets . Polymer , 46 : 12172 – 12180 .
- Kiskan , B , Yagci , Y and Ishida , H . 2008 . Synthesis, characterization, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain . Journal of Polymer Science Polymer Chemistry , 46 : 414 – 420 .
- Kiskan , B , Aydogan , B and Yagci , Y . 2009 . Synthesis, characterization, and thermally activated curing of oligosiloxanes containing benzoxazine moieties in the main chain . Journal of Polymer Science Polymer Chemistry , 47 : 804 – 811 .
- Velez-Herrera , P , Doyama , K , Abe , H and Ishida , H . 2008 . Synthesis and characterization of highly fluorinated polymer with the benzoxazine moiety in the main chain . Macromolecules , 41 : 9704 – 9714 .
- Chou , CI and Liu , YL . 2008 . High performance thermosets from a curable Diels-Alder polymer possessing benzoxazine groups in the main chain . Journal of Polymer Science Polymer Chemistry , 46 : 6509 – 6517 .
- Nagai , A , Kamei , Y , Wang , XS , Omura , M , Sudo , A , Nishida , H , Kawamoto , E and Endo , T . 2008 . Synthesis and crosslinking behavior of a novel linear polymer bearing 1,2,3-triazol and benzoxazine groups in the main chain by a step-growth click-coupling reaction . Journal of Polymer Science Polymer Chemistry , 46 : 2316 – 2325 .
- Chernykh , A , Agag , T and Ishida , H . 2009 . Synthesis of linear polymers containing benzoxazine moieties in the main chain with high molecular design versatility via click reaction . Polymer , 50 : 382 – 390 .
- Bhadrakumari , S and Predeep , P . 2008 . PTCR characteristics in YBa2Cu3O7- x -linear low density polyethylene (LLDPE) composite materials . Journal of Superconductivity and Novel Magnetism , 21 : 313 – 316 .
- Brunovska , Z , Liu , JP and Ishida , H . 1999 . 1,3,5-Triphenylhexahydro-1,3,5-triazine-active intermediate and precursor in the novel synthesis of benzoxazine monomers and oligomers . Macromolecular Chemistry and Physics , 200 : 1745 – 1752 .
- Borah , JS and Chaki , TK . 2011 . Dynamic mechanical, thermal, physico-mechanical and morphological properties of LLDPE/EMA blends . Journal of Polymer Research , 18 : 569 – 578 .
- Sarangi , PP , Naik , B , Vadera , SR , Patra , MK , Prakash , C and Ghosh , NN . 2010 . Preparation of polybenzoxazine-Ni-Zn-ferrite magnetic nanocomposite and its magnetic property . Materials and Technology , 25 : 271 – 275 .
- Celal , B and Flotek , P . 2011 . DSC A good R&D and QC tool for the Rotomolder . RotoWorld , 7 : 24 – 26 .
- Tekeichi , T , Guo , Y and Agag , T . 2000 . Synthesis and characterization of poly(urethanebenzoxazine) films as novel type of polyurethane/phenolic resin composites . Journal of Polymer Science Polymer Chemistry , 38 : 4165 – 4176 .
- Rajput , AB and Ghosh , NN . 2011 . Preparation and characterization of novel polybenzoxazine-polyester resin blends . International Journal of Polymeric Materials , 60 : 27 – 39 .
- Borah , JS and Chaki , TK . 2011 . Dynamic rheological, morphology and mechanical properties of compatibilized LLDPE/EMA blends . Journal of Polymer Research , 18 : 907 – 916 .