Abstract
Poly(p-xylylene tetrasulfide) was synthesized from 1,4-bis(chloromethyl)-benzene and sodium tetrasulfide using interfacial polycondensation technique to examine the effect of methyl tributyl ammonium chloride, tetrabutyl ammonium bromide, and benzyl triethyl ammonium chloride as phase transfer catalysts. The structure of the synthesized polysulfide was confirmed via elemental analysis, attenuated total reflectance Fourier transform infrared spectroscopy, Raman spectroscopy, and X-ray diffraction techniques. Moreover, the thermal behavior of this polymer was characterized using differential scanning calorimetry and thermo gravimetric analysis methods. Beyond, Friedman method was applied to analyze the degradation kinetics of the prepared polymer. The synthesized polymer had a molecular weight of about 2584 g/mol.
1. Introduction
Polysulfide polymers exhibit excellent resistance to a wide range of chemicals and have excellent physical and chemical properties Citation[1–2]. This class of materials shows great resistance against gases, organic solvents, and ozone effects Citation[3–5]. Incorporation of a sulfide linkage into a polymer backbone causes an increase in solvent resistance and other properties of polymer Citation[6–10]. On the other hand, aromatic polydisulfide polymers have superior resistance against environmental degradation, low water-vapor transmission, excellent resistance against acids and bases, and good adhesion to metals Citation[11–13]. Aromatic polydisulfide polymers are commercially synthesized using an organic dihalide with sodium polysulfide Citation[4].
Interfacial polycondensation in a liquid–liquid system is one of the most widely used polycondensation techniques Citation[14]. The essence of interfacial polycondensation in liquid–liquid systems lies in the fact that the monomers dissolve in immiscible liquids. The reaction proceeds at the interface or near it. Heterogeneous systems are classified based on the mutual correlation between the molecular weight of the polymer and the conversion yield. In this system one can obtain a high molecular weight polymer at low conversions of the reacting groups Citation[15]. Interfacial catalysis or phase transfer catalyst (PTC) is a powerful tool to carry out the reactions with environmentally friendly reagents and solvents Citation[16–18]. This technique allows the reagents present in different phases to react with water-insoluble hydrophobic species in the organic phase, in the presence of a catalyst which transfers anions from the aqueous phase into the organic one.
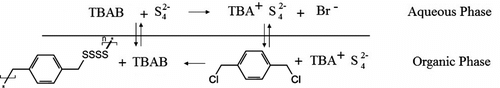
Recently, polysulfide polymers were synthesized by these researchers using methylene dichloride and ethylene dichloride via polycondensation techniques in the presence of PTCs Citation[19,20]. This study reports the synthesis of an aromatic polysulfide polymer namely poly(p-xylylene tetrasulfide) using tetrabutyl ammonium bromide (TBAB) as the phase transfer catalyst Equation (1). The polymer was characterized by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), Raman, elemental analysis (CHN), X-ray diffraction (XRD), thermo gravimetric analysis (TGA), and differential scanning calorimetry (DSC) analysis methods.
2 Experimental
2.1 Materials
1,4-bis(chloromethyl)-benzene, sodium hydroxide, sulfur, methyl tributyl ammonium chloride (MTBACl), TBAB, and benzyl triethyl ammonium chloride (BTEACl) were all purchased from Merck Co. and used as received.
2.2 Synthesis of monomer
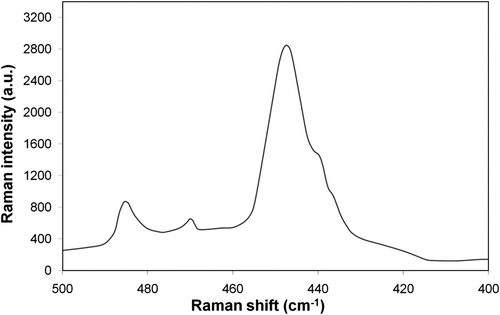
Anhydrous NaOH was mixed in the stoichiometric conditions required for producing tetrasulfide with elemental sulfur. Upon cooling the system down to the room temperature, a reddish brown solution of sodium tetrasulfide was produced which turned to yellow–orange color upon grinding. For chemical structure characterization of the prepared sodium terasulfide solution, Raman spectroscopy was employed as shown in Figure . Observed values of significant peak location in Raman spectra of Na2S4 were about 410.5, 446.5, and 484.7 cm−1 (see Figure ). The values reported for Raman peak locations are in close accordance with Janz’s results Citation[21].
2.3 Polymer synthesis
About 250 ml portion of a fresh solution of sodium tetrasulfide (2 M) was prepared through the above-mentioned procedure and then added to a 500 ml, four-necked round-bottomed flask equipped with a stirrer, a dropping funnel, a condenser, and a thermometer. The flask was heated up to 60 °C with constant stirring at 500 rpm, and then TBAB as a PTC was added to the reflux. During this process, the temperature of the solution was increased to 70 °C in 15 min. Next, 80 ml of 1, 4-bis(chloromethyl)-benzene solution was gently added through a dropping funnel during one hour. The reaction mixture was maintained at 70 °C while it was stirring vigorously for another hour. The resultant dispersed polymer was washed out by hot water, followed by decantation. After separation, some white powder was obtained as the final polymer.
2.4 Characterizations
FT-IR spectra of the polymer were recorded on a Bruker, Equinox 55 spectrometer. The Raman spectrum of the polymer was obtained using a Nicolet 950. Elemental analysis (C and H) was performed on a Perkin Elmer 2400 Series II CHNS/O elemental analyzer. TGA was performed using a PL-STA-1500 thermal analysis unit equipped with differential thermal analysis. The experiments were carried out in a nitrogen atmosphere at a heating rate of 10 °C/min. The glass transition temperature and the melting point of the polymer were calculated through DSC analysis using NETZSCH DSC 200 F3 instrument in N2 atmosphere (flow of 50 ml/min) at a heating rate of 5 °C/min. Powder XRD measurements were carried out using a JEOL GSM 20 A diffractometer equipped with CuKα as the radiation source with a scan rate of 4 °/min. Molecular weight of the polymer was determined by 1H NMR technique using 400 MHz Varian NMR, with dimethyl sulfoxide (DMSO) as the solvent.
3 Results and discussion
Poly(p-xylylene tetrasulfide) was prepared by the reaction of 1,4-bis(chloromethyl)-benzene and sodium tetrasulfide using TBAB as the phase transfer catalyst (Scheme ). The percentages of carbon and hydrogen of the observed polymer [C, H (40.96%, 3.31%)] are in accordance with the calculated values [C, H (41.38, 3.45)].
3.1 Characterization of poly(p-xylylene tetrasulfide)
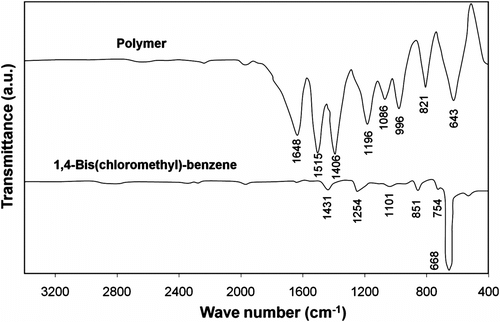
FT-IR spectra of prepared polymer and 1,4-bis(chloromethyl)-benzene as monomer are shown in Figure . The formation of polymeric product was confirmed by the disappearance of strong C–Cl stretching absorption at 668 cm−1. Also, the absorption bands corresponding to C–S and S–CH2 groups appeared at around 643 and 1200 cm−1, respectively. Absorption peaks at 1515, 1406, and 1648 cm−1 proved the presence of phenyl group in the structure of the prepared polymer. The strong bands at 820 and 996 cm−1 are indicators of the presence of mono-substituted aromatic ring.
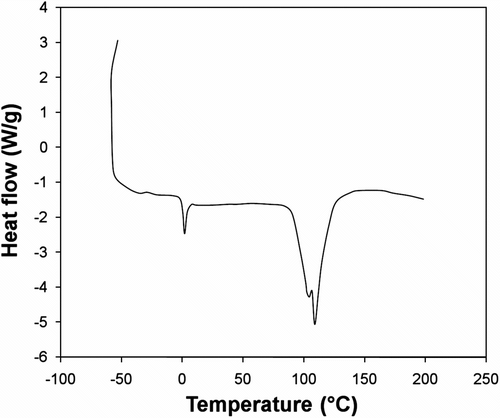
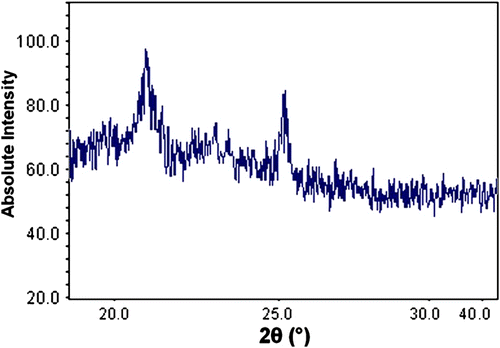
The thermal properties such as decomposition temperature, glass transition temperature (T g) and crystalline melting temperature (T m) of the prepared polymer were examined using TGA and DSC analysis. The glass-transition of the prepared polymer, obtained from DSC plot of polymer shown in Figure , was done under room temperature and showed the value of about – 0.8023 °C. The polymer also manifested a melting peak at around 110.05 °C (ΔH = 58.50 kJ/g). This is correlated to the fact that XRD pattern of the polymer showed a high degree of crystallinity (Figure ). This may be attributed to the incorporation of sulfur linkages in the backbone of the polysulfide polymer which facilitates the crystallization procedure Citation[19].
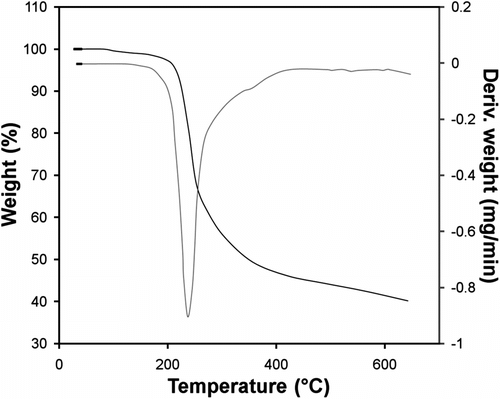
Thermogravimetric analysis-Differential thermal analysis (TGA-DTA) has been extensively used for thermal degradation studies of the polymer as shown in Figure . In the present study, the synthesized polymer exhibited a one-step exothermic degradation at 236.56 °C.
The degradation kinetics measurements were performed using Friedman method and the activation energy and the order of reactions were calculated using below equations Citation[21]:
Where dα/dt, Z, α, n, E, R, and T are the weight-loss rate, the frequency factor, the weight loss of the polymer undergoing degradation, the decomposition reaction order, the activation energy, the gas constant (8.3136 J/mol K), and the absolute temperature (K), respectively. The value of the activation energy can be calculated by plotting ln (dα/dt) against 1/T. Moreover, the value of n can be calculated by plotting ln (1–α) vs. 1/T and employing the logarithmic form of equation 2 and Z can be calculated as follows:
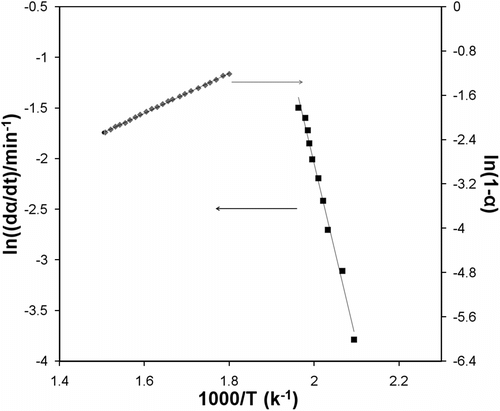
Figure illustrates the modeling results of experimental data using the Friedman method described above. Two sets of curves are presented in the figure; one set of parameters is the plot of ln (dα/dt) against 1/T, which gives the activation energy (E = 141.82 kJ/mol), and the other one is ln (1–α) against 1/T, which gives the order of reactions (n = 1.58).
3.2 The effect of PTC
In present work, the influence of catalyst on the reaction rate was studied and the results are summarized in Table . Adding TBAB as PTC caused an increase in polymerization rate in the polymer production; while adding MTBACl and BTEACl prevented the polymerization. As shown in equation 4, a stable type of benzylic carbocation will form by the reaction of the monomer with Na2S4 Equation (1)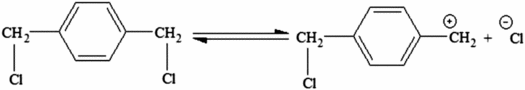
Table 1. The influence of PTC’s type on the amounts of synthesized polymer.
Therefore, a chloride ion will be formed using chlorine-contained catalysts according to equation (5). This ion that is formed acts as the common ion for the first equilibrium, which prevents 1,4-bis(chloromethyl) benzene monomer separation and consequently, the polymerization will not take place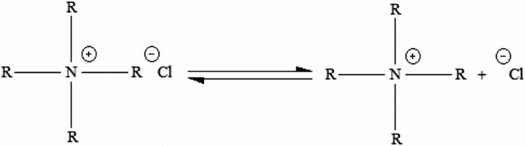
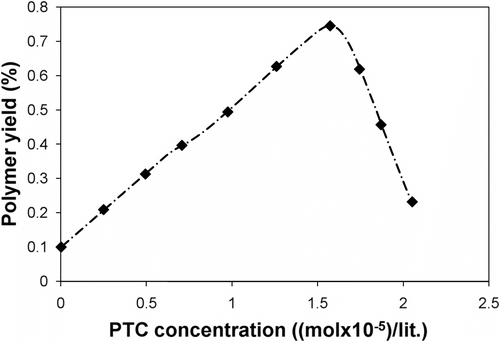
The effect of the PTC concentration on the polymerization process was also studied, and the results are shown in Figure . Further increases beyond 1.758 × 10−5 mol in the concentration of the PTC led to a decrease in polymer yield. This behavior was probably due to this fact that when more PTC is added, the catalyst is salted out of the aqueous and organic phases and turns into a separated phase. In concentrations above 1.758 × 10−5 mol, the salt formation increases. This increase in salt formation is probably because of ion exchange between the ions-agglomerated PTC particles that form solid-phase salt particles. As a result, the yield of the product is decreased.
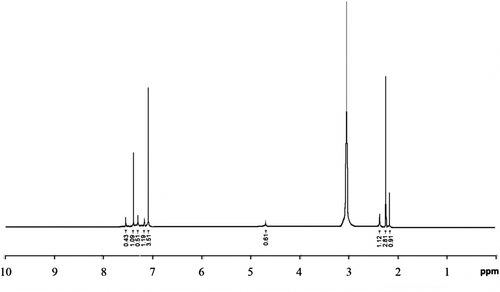
Moreover, the effect of PTC on the molecular weight of the prepared polymer was studied using 1H NMR technique as shown in Figure Citation[22]. The relative peak area ratio is C8H8Cl2:C8H8S4 = 1:11 which corresponds to a molecular weight of 2584.48 g/mol (–C8H8Cl2: 4.8, 7.6 ppm, –C8H8S4–: 2.18, 2.27, 2.41, 7.12, 7.16, 7.21, 7.39, and 7.45 ppm).
3.3 Resistance to solvent
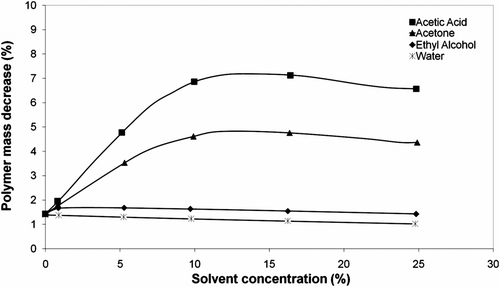
The polysulfide polymers exhibit superior solvent resistance when compared to other synthetic rubbers and this behavior is due to their backbone structure. The solvent resistance of the polymer depends largely on the sulfur content in the backbone. For surveying the swelling behavior of the prepared polymer, the organic polysulfide polymer was tested in different solvents such as ethyl alcohol, acetic acid, acetone, and water. Polymer solubility in each solvent was tested at four different concentrations at 25 °C to determine the solvent resistance properties of the specimens as shown in Figure . Distilled water was used as concentration reducer for preparing the different concentrations of solvents. According to the results, there was a high degree of the mass decrease of the polymer in acetone and acetic acid with an increase in the concentration of solvents. However, the obtained mass decrease of the polymer in water and ethyl alcohol was not noticeable.
4 Conclusion
P-xylylene tetrasulfide was synthesized from 1,4-bis(chloromethyl)-benzene and sodium tetrasulfide by interfacial polycondensation technique in the presence of tetrabutyl ammonium bromide as PTC. PTC was employed to increase the conversion rate of the polymer synthesis reaction. The structure of the targeted synthesized polymer was confirmed by FT-IR, Raman, CHN, and XRD spectroscopy methods. Thermogravimetric results indicate that the synthesized polymer was stable up to 200 °C. The synthesized polymer was insoluble in most common organic solvents.
References
- Kalaee , MR , Famili , MHN , Mortezaei , M and Zeeb , M . 2010 . Polymerization of ethylene dichloride and sodium tetrasulfide: synthesis and kinetic studies . Journal of Sulfur Chemistry , 31 : 247 – 253 .
- Mahon , A , Terence , J and Robert , J . 1998 . Thermal and photodegradation of polysulfide pre-polymers: products and pathways . Polymer Degradation and Stability , 62 : 15 – 24 .
- Catsiff , EH , Gillis , MN and Gobran , RH . 1971 . Poly(ethylene sulfide). III. Oxidative degradation . Journal of Polymer Science, Part A: Polymer Chemistry , 9 : 1271 – 1314 .
- Kishore , K and Ganesh , K . 1995 . Polymers containing disulfide, tetrasulfide, diselenide and ditelluride linkages in the main chain . Advances in Polymer Science , 121 : 81 – 121 .
- Kishore , K and Ganesh , K . 1993 . Synthesis, characterization, and thermal degradation studies on group VIA derived weak-link polymers . Macromolecules , 26 : 4700 – 4705 .
- Ganesh , K and Kishore , K . 1996 . Primary pyrolysis products of thiokol liquid polysulfide . Macromolecules , 29 : 26 – 29 .
- Sheydaei , M , Kalaee , MR , Allahbakhsh , A , Samar , M , Aghili , A , Dadghar , M and Mosavi , GS . 2012 . Characterization of synthesized poly(aryldisulfide) through interfacial polymerization using phase transfer catalyst . Journal of Sulfur Chemistry , 33 : 303 – 311 .
- Montaudo , G , Puglisi , C , Leeuw , JW , Hartgers , W , Kishore , K and Ganesh , K . 1996 . Thermal degradation processes in poly(xylylene sulfides) investigated by comparative direct pyrolysis MS and flash pyrolysis GC/MS experiments . Macromolecules , 29 : 6466 – 6474 .
- Lee TCP. Properties and applications of elastomeric polysulfides. Rapra; 1992.
- Sundarrajan , S , Ganesh , K and Srinivasan , KSV . 2003 . Synthesis, characterization, and reactivity ratio studies on new sulfide copolymers containing ethylbenzene units . Polymer , 44 : 61 – 71 .
- Meng , YZ , Tjong , SC and Hay , AS . 2001 . Synthesis of cocyclic(arylene disulfide) oligomers and their adhesion properties as heating-melt adhesive . Polymer , 42 : 5215 – 5224 .
- Yong Ding Y, Hay AS. Ring-opening polymerization study of cyclic (aromatic disulfide) oligomers derived from 4,4’-isopropylidene bisthiophenol. Polymer. 1997;38:2239–2244.
- Liang , ZA , Meng , YZ , Li , L , Du , XS and Hay , AS . 2005 . Aromatic disulfide polymers back to macrocyclic disulfide oligomers via cyclo-depolymerization reaction. Polymer , 46 ( 24 ) : 11117 – 11124 .
- Yamamoto , M , Uchimura , N , Adachi , K and Tsukahara , Y . 2010 . Multibranched polycarbonates synthesized via interfacial polycondensation using uniform size hemi-telechelic polystyrene macromonomers having a dihydroxyphenyl end-group . Designed Monomers and Polymers , 13 : 445 – 458 .
- Wittbecker , EL and Morgan , PW . 1959 . Interfacial polycondensation. I . Journal of Polymer Science, Part A: Polymer Chemistry , 40 : 289 – 297 .
- Tagle , LH , Diaz , FR , Nunez , M and Canario , F . 2003 . Polymerization by phase transfer catalysis. 27. Synthesis of polyesters containing silicon or germanium. Influence of the base concentration . International Journal of Polymer Material , 52 : 287 – 294 .
- Priyarega , S , Muthusamy , A , Kaniappan , K and Murugavel , SC . 2003 . Synthesis and characterization of photosensitive polyesters by phase-transfer-catalyzed polycondensation . Designed Monomers and Polymers , 6 : 187 – 196 .
- Alavi Nikje , MM and Hajifatheali , H . 2011 . Nanoclay-mediated epoxidation of HTPB using in-situ-generated dimethyl dioxirane . Designed Monomers and Polymers , 14 : 155 – 165 .
- Kalaee , MR , Famili , MHN , Mahdavi , H and Naderi , A . 2010 . Synthesis, characterization and properties of poly(methylenetetrasulfide) using interfacial catalysis . Polymer Science Series B. Polymer Chemistry , 52 : 286 – 291 .
- Kalaee , MR , Famili , MHN and Mahdavi , H . 2009 . Synthesis and characterization of polysulfide rubber using phase transfer catalyst . Macromolecular Symposia , 277 : 81 – 86 .
- Janz , GJ , Downey , JR , Roduner , E , Wasilczyk , GJ , Coutts , JW and Eluard , A . 1976 . Raman studies of sulfur-containing anions in inorganic polysulfides. Sodium polysulfides . Inorganic Chemistry , 15 : 1759 – 1763 .
- Kim , EA , Seyfferth , AL , Fendorf , S and Luthy , RGW . 2010 . Immobilization of Hg(II) in water with polysulfide-rubber (PSR) polymer-coated activated carbon . Water Research , 45 : 453 – 460 .