Abstract
In the present study, a new class of biodegradable graft copolymers of polystyrene (PS) with aliphatic polyester (APE) side chains were synthesized through combination of nitroxide-mediated free radical polymerization (NMRP) and ring-opening polymerization (ROP) with copper (I)-catalyzed ‘click’ chemistry. First, the click component, azido-functional PS (PS-N3) was prepared by copolymerization of styrene and chloromethyl styrene by NMRP followed by the conversion of chlorine groups of the resulting copolymer to azido groups by using NaN3 in N,N-dimethylformamide. Propargyl functional APEs were prepared independently by ROP of lactic acid and glycolic acid using tin octoate in the presence of propargyl alcohol at 130 °C. Finally, PS-N3 was coupled with the resulting polymers by click chemistry to yield graft copolymers (PS-g-poly (L-lactic-co-glycolic acid) [PLLA] and PS-g-PLLGA, respectively). The intermediates and final graft copolymers were characterized by proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectral and gel permeation chromatography analyses. The hydrolytic degradation behavior of the obtained graft copolymers in phosphate buffer saline solution were investigated by the weight loss and molecular weight measurements. It is shown that both copolymers undergo slow hydrolytic degradation, PS-g-PGLLA being relatively faster due to the presence of hydrophilic glycolic acid groups in the structure.
1. Introduction
Biodegradable polymers have gained much attention due to their feasibility to biomedical applications such as sutures, bone screws, tissue engineering scaffolds, and drug delivery systems Citation[1,2]. Physical properties, hydrolytic degradation profiles, and biocompatibility of aliphatic polyesters and co-polyesters make them incomparable materials within the biomedical products Citation[3]. For biological applications, mostly poly (L-lactic acid) (PLLA) and poly (L-lactic-co-glycolic acid) (PLLGA) are preferred due to their resorbability and their non-hazardous properties are approved by Food and Drug Administration (FDA) Citation[4,5]. Despite their superior properties, PLLA or PLLGA by themselves are not perfect materials for certain applications such as those involving drug delivery devices and implant systems. Hydrophobicity and lack of any functional groups at the backbone of the polymer chain for possible modifications limit their utilization Citation[6,7]. Chemical compositions of biodegradable polyesters affect degradation mechanism through hydrolytic and/or microbial routes Citation[8–10]. Both degradation processes occur by the cleavage of the backbone ester linkages. Hydrophilicity, surface chemistry, morphology, and crosslink density are major factors that influences degradation rate Citation[11]. The glycolic acid content contributes to a higher degradation rate due to hydrophilic nature of glycolic acid, while the lactic acid content of the polymer slows down the rate Citation[12–14]. The degradation rate can be controlled by the incorporation of new segments to biodegradable aliphatic polyesters Citation[9]. Typically, such modifications were facilitated through functional groups attached at the chain ends Citation[11–15]. Polyesters are commonly prepared by ring-opening polymerization (ROP) of lactones and cyclic diesters such as lactide and glycolide by alcohols in the presence of tin(II) octoate (Sn(Oct)2) catalyst Citation[16–20]. This methodology affords possibility to incorporate functional groups into polyesters provided that in addition to the hydroxyl groups, the initiator contains desired functionality. Previously, we have prepared thermal- Citation[21–27], photo- Citation[28,29], and electro-functional Citation[30] polymers of biocompatible polymers by taking advantage of the functional initiator approach Citation[31].
It has now well established that click chemistry Citation[32] based on Cu(I)-catalyzed Huisgen 1, 3-dipolar cycloaddition reaction of azides with terminal alkynes provides a powerful tool forming building blocks, in high yields and with no by-products.Citation[33,34] Many examples of click reactions involving macromolecules were reported. In this laboratory, we have focused on the use of such click reactions for the synthesis of various macromolecular architectures Citation[34–41] and functionalization of polymers Citation[42–47], microspheres Citation[48], clays Citation[49,50], and silsesquioxanes Citation[51] with thermal-, photo-, and electro-active groups.
In this study, we report the use of ‘click’ reactions for preparing graft copolymers of polystyrene with two different biocompatible side chains, namely PLLA or PLLGA. We used nitroxide mediated radical polymerization (NMRP) method to obtain polystyrene with chloromethyl functions. As a next step, chlorine groups were converted to azide groups by the reaction with sodium azide in order to take part in click reaction with alkyne-functionalized polymers. For this reason, acetylene-functionalized PLLA and PLLGA were synthesized by using propargyl alcohol as a functional initiator in ROP of the corresponding monomers and grafted onto azide-functionalized PS via ‘click’ reaction. Success of the functionalization processes at different stages was followed by using Fourier transform infrared (FT-IR) and proton nuclear magnetic resonance (1H NMR) spectroscopies. The hydrolytic degradation behavior of the obtained graft copolymers in phosphate buffer saline solution (PBS) were investigated by the mass loss and molecular weight loss measurements.
2 Experimental
2.1 Materials
Sodium azide (NaN3, 99%, Aldrich), copper(I) bromide (CuBr, 98%, Acros), N,N-Dimethyl formamide (DMF) and dichloromethane(CH2Cl2, 99%, J. T. Baker), methanol (MeOH, Acros Organics, 99%), tetrahydrofuran (THF, Acros Organics, 99,9%), propargyl alcohol (C3H4O, Aldrich, 99%), and 2,2-dipyridyl (Acros Organics, 99%) were used as received. L-Lactide(LA) (Polysciences) and glycolide (GA) (Polysciences, 99,9%) were crystallized from dry benzene. Tin octoate (Sn(Oct)2, Sigma Corp.) was used as received. Styrene (S) (C8H8, Aldrich, 99%) and 4-vinyl benzyl chloride (C9H9Cl, Acros, 90%) were passed through a basic alumina column to remove the inhibitor.
2.2 Synthesis of poly(styrene-co-chloromethylstyrene), P(S-co-CMS) (1a) and polystyrene-azide PS-N3 (1b)
Poly(styrene-co-chloromethylstyrene) P(S-co-CMS) prepared via nitroxide-mediated radical polymerization (NMRP) of styrene (S) and chloromethylstyrene (CMS) at 125 °C for 18 h Citation[52]. The obtained polymer precipitated from methanol and dried under vacuum. Chlorine (Cl) units of the P(S-co-CMS) polymer successfully converted to azide groups. P(S-co-CMS) (1.00 g, 1.24 × 10−4 mol) dissolved in DMF and reacted with NaN3 (8.85 × 10−3 g, 1.36 × 10−4 mol) 1.1 times excess in mol of Cl units of P(S-co-CMS) polymer at room temperature for 24 h. The resulting polymer was filtered and precipitated from methanol and finally dried under vacuum.
1H NMR (CDCl3) δ (TMS, ppm) for P(S-co-CMS): 7.04–6.54 (aromatic protons), 4.5 (CH 2–Cl), 1.82–1.42 (aliphatic protons)
1H NMR (CDCl3) δ (TMS, ppm) for PS-N3: 7.04–6.54 (aromatic protons), 4.25 (CH 2–N3), 1.82–1.42 (aliphatic protons)
2.3 Synthesis of acetylene-terminated PLLA (2a) and PLLGA (2b)
Acetylene-terminated PLLA synthesis is conducted by ROP of monomer. L-lactide (2.00 g, 13.80 mmol) and propargyl alcohol (15.50 mg, 0.27 mmol) reacted in the presence of Sn(Oct)2 (catalytic amount) at 125 °C under N2 for 24 h. The resulting polymer was diluted by dichloromethane and precipitated from cold methanol and finally dried under vacuum.
1H NMR (CDCl3) δ (TMS, ppm): 5.16–5.13 (CH–CH3), 4.32 (CH
2-O), 2.50 (CCH), 1.58 (CH–CH
3).
The same procedure using L-lactide and glycolide with 3:1 mol ratios, respectively, is followed for the synthesis of corresponding PLLGA.
1H NMR (CDCl3)δ (TMS, ppm): 5.16–5.13 (CH–CH3), 4.81 (CH
2–CO), 4.32 (CH
2–O), 2.50 (C
CH), 1.58 (CH–CH
3).
2.4 Synthesis of polystyrene-g-poly(lactic acid) (PS-g-PLLA) (3a) and PS-g-PLLGA (3b)
To a round-bottom flask equipped with a magnetic stirring bar, PS-N3 (1.00 g, 1.96 mmol), PLLA (1.94 g, 0.12 mmol), ligand (2, 2-dipyridyl, 0.24 mmol), and catalyst (CuBr, 0.12 mmol) were dissolved in 7 mL of dry THF and stirred at room temperature under N2. After the reaction, the mixture was diluted with THF and then passed through a column of neutral alumina to remove metal salt. The reaction mixture was concentrated and precipitated from cold methanol. The final product was washed with methanol and dried for 24 h in a vacuum oven at room temperature.
1H NMR (CDCl3) δ (TMS, ppm): 7.04–6.54 (aromatic protons), 5.16–5.13 (CH–CH3), 4.20 (CH 2–triazole ring), 3.48 (CH 2–O), 1.58 (CH–CH 3).
Same procedure is followed for the PLLGA.
1H NMR (CDCl3) δ (TMS, ppm): 7.04–6.54 (aromatic protons), 5.16–5.13 (CH–CH3), 4.81 (CH
2–CO), 4.20 (CH
2–triazole ring), 3.48 (CH
2–O), 1.58 (CH–CH
3).
2.5 Characterizations
1H NMR measurements were recorded in CDCl3 with Si(CH3)4 as internal standard, using a Bruker AC250 (250.133 MHz) instrument. FT-IR spectra were recorded on a Perkin-Elmer FT-IR Spectrum One-B spectrometer. Molecular weights were determined by gel permeation chromatography (GPC) instrument, Viscotek GPCmax Autosampler system consisting of a pump, three ViscoGEL GPC columns (G2000HHR, G3000HHR and G4000HHR), a Viscotek UV detector, and a Viscotek differential refractive index (RI) detector with a THF flow rate of 1.0 mL min−1 at 30 °C. Both detectors were calibrated with PS standards having narrow molecular weight distribution. Data were analyzed using Viscotek OmniSEC Omni-01 software.
2.6 Degradation studies
The in vitro degradation studies were performed at 37 °C in phosphate buffer saline (pH: 7.4, PBS). The phosphate buffer consisted of Na2HPO4 (1.15 g/L), KH2PO4 (0.20 g/L), KCl (0.20 g/L), and NaCl (8.00 g/L) in water. Graft copolymer films prepared by solvent casting were placed in vials, each containing 15 mL PBS. The vials were incubated at 37 °C for various periods of time during six weeks. The buffer solution was replaced every 48 h. The films were subsequently dried at 25 °C in vacuum until a constant weight was reached. The percentage change in mass for each film was calculated as follows:
Where m f is the final mass and m i is the initial mass.
3 Results and discussion
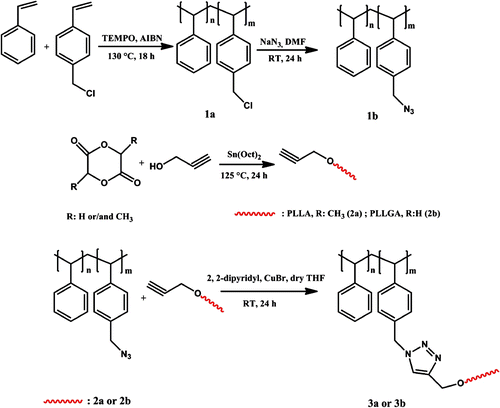
The synthetic strategy for obtaining desired graft copolymers involves combination of two controlled polymerization methods with Cu(I)-catalyzed azide-alkyne click chemistry.
The overall process is shown in Scheme .
3.1 Synthesis and characterization of polymers
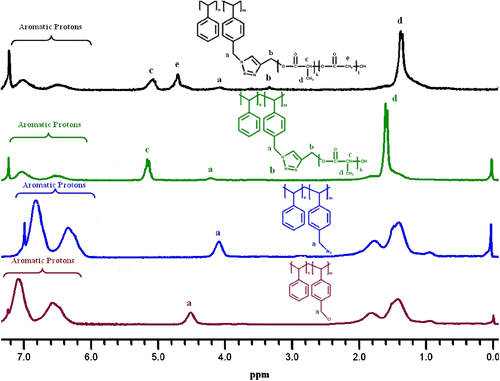
For the synthesis of parent azide-functionalized polymer, we first prepared P(S-co-CMS) via NMRP of S and CMS at 125 °C according to the described procedure Citation[19]. NMRP was deliberately selected as the controlled radical polymerization method for its suitability for the polymerization of S-based monomers and tolerance to halide functional groups. The composition of copolymer was determined using 1H NMR spectroscopy. The mole fractions of CMS and S were calculated from the ratio of the peak areas around 4.5 ppm, corresponding to two methylene protons of in the side chain of CMS to the total area between 6.3 and 7.4 ppm, which was attributed to the total aromatic protons (Figure ). P(S-co-CMS) with M n(GPC) 7.690 and 16 mol% chloromethyl groups was then quantitatively converted into polystyrene-azide (PS-N3) in the presence of NaN3/DMF at room temperature. From the 1H NMR spectrum of PS-N3 shown in Figure and it was observed that while the signal at 4.5 ppm corresponding to CH 2–Cl protons of the precursor P(S-co-CMS) completely disappeared, and a new signal appeared at 4.25 ppm due to CH2 linked to azide groups. The structure of PS-N3 was further supported by the observation from FT-IR spectrum of the azide stretching band at 2094 cm−1 (vide infra).
The second step in the synthesis of PS with biodegradable side chains was to obtain alkyne-functionalized polymers (PLLA or PLLGA) (Scheme ). To obtain the desired functionalities, combined with proper molecular weights and polydispersities, polymers were synthesized by ROP of L-lactide and glycolide, respectively, by using propargyl alcohol as a functional initiator, in the presence of tin octoate. In both cases, the GPC traces are unimodal and narrow, indicating that no important side reactions occurred. Moreover, as these polymers were intended to be used in the subsequent click reactions, efforts were directed toward obtaining low molecular weights, combined with convenient yields. Some characteristics are presented in Table .
Table 1. The yields of the reactions and molecular weight characteristics of the products at different modification stages.
Finally, the obtained PLLA and PLLGA containing acetylene groups were clicked to PS-N3 to yield PS-g-PLLA and PS-g-PLLGA, respectively, by copper-catalyzed cycloaddition reaction (see Scheme ). After the click reaction, new peaks appeared at around 1.58, 3.48, and 5.13 ppm for PS-g-PLLA copolymer, and 1.58, 3.48, 4.81, and 5.13 ppm for PS-g-PLLGA copolymer which correspond to PLLA and PLLGA segments of the graft copolymers, respectively.
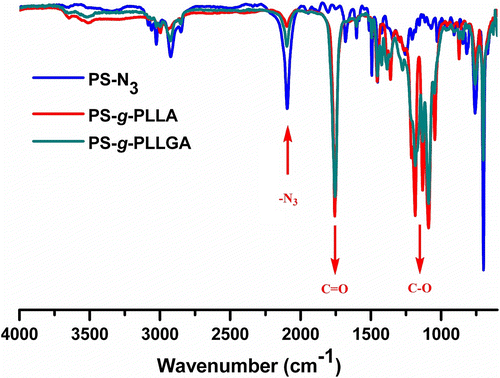
The graft copolymer formation was also followed by FT-IR spectroscopy. The FT-IR spectrum associated with graft copolymers showed the presence of both components (PS and PLLA or PLLGA) in the structure (Figure ). As anticipated, the azide stretching band of the precursor polymers at 2100 cm−1 decreased significantly. However, it did not disappear completely probably due to the steric hindrance Citation[53].
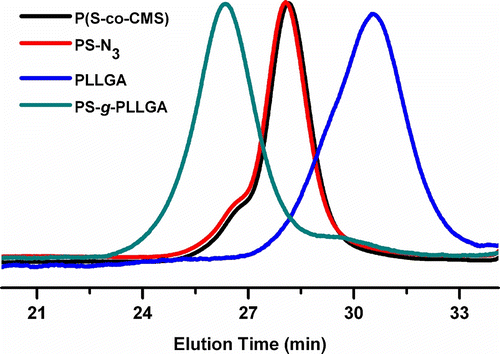
Successful grafting was also confirmed by GPC measurements. In Figure , the normalized GPC curves of the individual precursor polymers as well as of the resulting PS-g-PLLGA graft copolymer were shown. In the elution curves of graft copolymers, although slight shoulders of the chromatographic peak corresponding to precursor polymers are noted, clear shifts toward lower retention times were observed. PS-g-PLLA graft copolymer exhibited the same characteristics as that of PS-g-PLLGA. These observations confirm that the described click approach is useful for the desired graft copolymer formation.
The yields of polymerization, modification and click reactions, and the molecular weight characteristics of the resulting polymers are collected in Table . As can be seen, the measured molecular weights generally fit with those determined by 1H NMR analyses. Some discrepancies observed may be due to the different hydrodynamic volumes of the graft copolymers and strong interactions of the polar segments with the stationary phase of the GPC column. It is also noted that no side reactions such as coupling or chain scission occurred during the azidation process and the molecular weight of the precursor polymer was preserved.
3.2 Hydrolytic degradation of graft copolymers
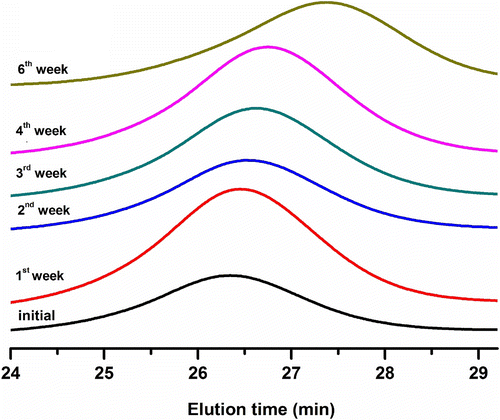
Preliminary studies were conducted to study the hydrolytic degradation behavior of the graft copolymers. The hydrolytic degradation behavior of the graft copolymers were carried out in phosphate buffer saline at pH 7.4 and 37 °C as described in the Experimental section. Typical GPC curves of PS-g-PLLGA before and during hydrolytic degradation are shown in Figure .
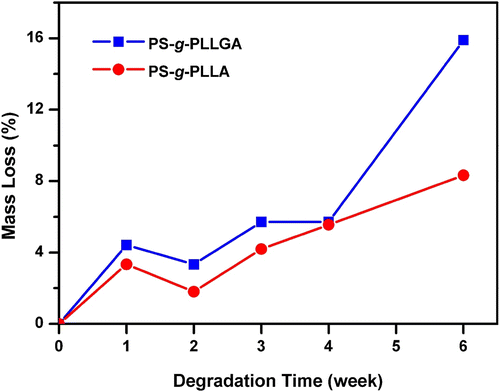
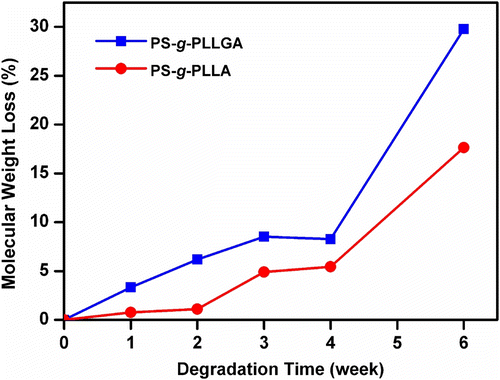
As can be seen, during hydrolysis the molecular weight peak of the graft copolymer is gradually shifted to a low molecular weight region, thereby showing the hydrolytic degradation capability of the new graft copolymers synthesized. The degradation behavior of the graft copolymers was also followed by mass losses of the polymers. Figures and compare the molecular weight and mass losses of PS-g-PLLA and PS-g-PLLGA, respectively, during the hydrolytic degradation.
In both cases, similar two-stage degradation patterns were observed. The initial slow degradation is leveled off at four weeks. The relatively faster rate observed after this point may be due to the autocatalytic degradation favored by the acid released.
Relatively faster rate of the degradation observed with the PS-g-PLLGA polymer is due to the presence of hydrophilic glycolide groups present in the structure. The degradations occurred through ester groups (lactic acid and glycolic acid repeating units). This was confirmed by the 1H NMR spectroscopy. Although the 1H NMR spectra of the chloroform extracted degradation products were complex for a definite estimation, they clearly indicated that the remaining product was mainly polystyrene.
4 Conclusions
In conclusion, we have demonstrated that biodegradable graft copolymers of polystyrene and aliphatic polyesters could easily be synthesized by combination of controlled polymerization methods (NMRP and ROP) and click chemistry. The structural characterization of the graft copolymers was made by spectral and molecular weight analyses. The resulting graft copolymers showed slow hydrolytic degradation profile. The graft copolymers with PLLGA side chains degrade faster relatively due to the hydrophilic nature of the glycolic acid repeating units. Due to their slow degradation, these copolymers are ideally suitable for long-term drug delivery usage. Further investigations on the applications of graft copolymers in the form of microspheres in drug delivery systems are now being studied and will be reported elsewhere.
Acknowledgements
The authors thank Istanbul Technical University, Research Fund, and TUBITAK-1001 (Turkish Scientific and Technological Research Council) research grants (SBAG-108S062) for the financial support.
References
- Edlund , U and Albertsson , AC . 2002 . Degradable polymer microspheres for controlled drug delivery . Advances in Polymer Science , 157 : 67 – 112 .
- Moravek , SJ and Storey , RF . 2009 . Copolymers of rac-lactide and ϵ-caprolactone: conventional copolymerization vs. macroinitiator copolymerization . Journal of Macromolecular Science, Pure Applied Chemistry , 46 : 339 – 345 .
- Lue , J-M , Wang , X , Marin-Muller , C , Wang , H , Lin , PH , Yao , Q and Chen , C . 2009 . Current advances in research and clinical applications of PLGA-based nanotechnology . Expert Review of Molecular Diagnostics , 9 : 325 – 341 .
- Gupta , AP and Kumar , V . 2010 . Synthesis and characterization of novel chain-linked biodegradable polymers . Designed Monomers and Polymers , 13 : 65 – 72 .
- Zhou , ZH , Liu , XP and Lin , LH . 2008 . Preparation and biocompatibility of poly(L-lactide-co-glycolide) scaffold materials for nerve conduits . Designed Monomers and Polymers , 11 : 447 – 456 .
- Carrot , G , Hilborn , JG , Trollsas , M and Hedrick , JL . 1999 . Two general methods for the synthesis of thiol-functional polycaprolactones . Macromolecules , 32 : 5264 – 5269 .
- Ouchi , T , Seike , H , Miyazaki , H , Tasaka , F and Ohya , Y . 2000 . Synthesis of a block copolymer of L-lactide and depsipeptide with pendant thiol groups . Designed Monomers and Polymers , 3 : 279 – 287 .
- Lu , FZ , Xiong , XY , Li , ZC , Du , FS , Zhang , BY and Li , FM . 2002 . A convenient method for the synthesis of amine-terminated poly(ethylene oxide) and poly(epsilon-caprolactone) . Bioconjugate Chemistry , 13 : 1159 – 1162 .
- Cometa , S , Bartolozzi , I , Corti , A , Chiellini , F , De Giglio , E and Chiellini , E . 2010 . Hydrolytic and microbial degradation of multi-block polyurethanes based on poly(ϵ-caprolactone)/poly(ethylene glycol) segments . Polymer Degradation and Stability , 95 : 2013 – 2021 .
- Corti , A , Muniyasamy , S , Vitali , M , Imam , SH and Chiellini , E . 2010 . Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: synergistic effects of sunlight exposure, thermal aging and fungal biodegradation . Polymer Degradation and Stability , 95 : 1106 – 1114 .
- Cordova , A , Iversen , T and Hult , K . 1999 . Lipase-catalyzed formation of end-functionalized poly(ϵ-caprolactone) by initiation and termination reactions . Polymer , 40 : 6709 – 6721 .
- Kayaman-Apohan , N , Karal-Yilmaz , O , Baysal , K and Baysal , BM . 2001 . Polymer , 42 : 4109
- Porjazoska , A , Kayaman-Apohan , N , Karal-Yilmaz , O , Cvetkovska , M , Baysal , K and Baysal , BM . 2002 . Synthesis and characterization of glycolide, L-lactide and PDMS based terpolymers as a support for cell cultures . Journal of Biomaterials Science Polymer Edition , 13 : 1119 – 1134 .
- Porjazoska , A , Karal-Yilmaz , O , Kayaman-Apohan , N , Cvetkovska , M and Baysal , BM . 2004 . Biocompatible polymer blends of poly(D, L-lactic acid-co-glycolic acid) and triblock PCL-PDMS-PCL copolymers: their characterizations and degradations , Zagreb : Croatian Chemical Society . p. 545–551
- Nampoothiri , KM , Nair , NR and John , RP . 2010 . An overview of the recent developments in polylactide (PLA) research . Bioresource Technology , 101 : 8493 – 8501 .
- Wiggins , JS , Hassan , MK , Mauritz , KA and Storey , RF . 2006 . Hydrolytic degradation of poly(D,L-Lactide) as a function of end group: carboxylic acid vs. hydroxyl . Polymer , 47 : 1960 – 1969 .
- Moravek , SJ , Messman , JM and Storey , RF . 2009 . Copolymers of rac-lactide and ϵ-caprolactone: conventional copolymerization vs. macroinitiator copolymerization . Journal of Polymer Science Part A: Polymer Chemistry , 47 : 797 – 803 .
- Detta , N , El-Fattah , AA , Chiellini , E , Walkenstrom , P and Gatenholm , P . 2008 . Biodegradable polymeric micro-nanofibres by electrospinning of polyester/polyether block copolymers . Journal of Applied Polymer Science , 110 : 253 – 261 .
- Cometa , S , Chiellini , F , Bartolozzi , I , Chiellini , E , De Giglio , E and Sabbatini , L . 2010 . Surface segregation assessment in poly(3-caprolactone)-poly(ethylene glycol) multi-block copolymer films . Macromolecular Bioscience , 10 : 317 – 327 .
- Tasdelen , MA . 2011 . Poly(epsilon-caprolactone)/clay nanocomposites via ‘click’ chemistry . European Polymer Journal , 47 : 937 – 941 .
- Colak , DG , Cianga , I , Yagci , Y , Cirpan , A and Karasz , FE . 2007 . Novel poly(phenylene vinylenes) with well-defined poly(epsilon-caprolactone) or polystyrene as lateral substituents: synthesis and characterization . Macromolecules , 40 : 5301 – 5310 .
- Kiskan , B and Yagci , Y . 2005 . Synthesis and characterization of naphthoxazine functional poly(ϵ-caprolactone) . Polymer , 46 : 11690 – 11697 .
- Yurteri , S , Cianga , I , Demirel , AL and Yagci , Y . 2005 . New polyphenylene-g-polystyrene and polyphenylene-g-polystyrene/poly(ϵ-caprolactone) copolymers by combined controlled polymerization and cross-coupling processes . Journal of Polymer Science Part A: Polymer Chemistry , 43 : 879 – 896 .
- Yurteri , S , Cianga , I , Degirmenci , M and Yagci , Y . 2004 . Synthesis and characterization of poly(p-phenylene)-graft-poly(ϵ-caprolactone) copolymers by combined ring-opening polymerization and cross-coupling processes . Polymer International , 53 : 1219 – 1225 .
- Nur , Y , Yurteri , S , Cianga , I , Yagci , Y and Hacaloglu , J . 2007 . Thermal degradation of poly(p-phenylene-graft-ϵ-caprolactone) copolymer . Polymer Degradation Stability , 92 : 838 – 848 .
- Muftuoglu , AE , Cianga , M , Colak , D and Yagci , Y . 2004 . Synthesis, characterization and electrochromic properties of a conducting copolymer of pyrrole functionalized polystyrene with pyrrole . Designed Monomers Polymers , 7 : 563 – 582 .
- Tasdelen , MA , Beyazit , S , Gunes , D , Bicak , N , Tatar , P , Demirel , AL and Yagci , Y . 2011 . Poly(p-phenylene methylene)-based block copolymers by mechanistic transformation . Journal of Polymer Science Part B: Polymer Chemistry , 49 : 4021 – 4026 .
- Degirmenci , M , Hizal , G and Yagci , Y . 2002 . Synthesis and characterization of macrophotoinitiators of poly(ϵ-caprolactone) and their use in block copolymerization . Macromolecules , 35 : 8265 – 8270 .
- Degirmenci , M , Izgin , O and Yagci , Y . 2004 . Synthesis and characterization of cyclohexene oxide functional poly (epsilon-caprolactone) macromonomers and their use in photoinitiated cationic homo- and copolymerization . Journal of Polymer Science Part A: Polymer Chemistry , 42 : 3365 – 3372 .
- Kerman , I , Toppare , L , Yilmaz , F and Yagci , Y . 2005 . Thiophene ended epsilon-caprolactone conducting copolymers and their electrochromic properties . Journal of Macromolecular Science, Pure and Applied Chemistry , A42 : 509 – 520 .
- Tasdelen , MA , Kahveci , MU and Yagci , Y . 2011 . Telechelic polymers by living and controlled/living polymerization methods . Progress in Polymer Science , 36 : 455 – 567 .
- Kolb , HC , Finn , MG and Sharpless , KB . 2001 . Click chemistry: diverse chemical function from a few good reactions . Angewandte Chemie International Edition , 40 : 2004 – 2021 .
- Rostovtsev , VV , Green , LG , Fokin , VV and Sharpless , KB . 2002 . A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective ‘ligation’ of azides and terminal alkynes . Angewandte Chemie International Edition , 41 : 2596 – 2599 .
- Tornoe , CW , Christensen , C and Meldal , M . 2002 . Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides . Journal of Organic Chemistry , 67 : 3057 – 3064 .
- Iskin , B , Yilmaz , G and Yagci , Y . 2011 . ABC type miktoarm star copolymers through combination of controlled polymerization techniques with thiol-ene and azide-alkyne click reactions . Journal of Polymer Science Part A: Polymer Chemistry , 49 : 2417 – 2422 .
- Iskin , B , Yilmaz , G and Yagci , Y . 2011 . Synthesis of ABC type miktoarm star copolymers by triple click chemistry . Polymer Chemistry , 2 : 2865 – 2871 .
- Tasdelen , MA , Yilmaz , G , Iskin , B and Yagci , Y . 2012 . Photoinduced free radical promoted copper(I)-catalyzed click chemistry for macromolecular syntheses . Macromolecules , 45 : 56 – 61 .
- Cengiz , H , Aydogan , B , Ates , S , Acikalin , E and Yagci , Y . 2011 . Intramolecular cross-linking of polymers by using difunctional acetylenes via click chemistry . Designed Monomers and Polymer , 14 : 69 – 78 .
- Yilmaz , G , Toiserkani , H , Demirkol , DO , Sakarya , S , Timur , S , Orun , L and Orun , L . 2011 . Polysulfone based amphiphilic graft copolymers by click chemistry as bioinert membranes . Materials Science and Engineering: C , 31 : 1091 – 1097 .
- Gacal , BN , Koz , B , Gacal , B , Kiskan , B , Erdogan , M and Yagci , Y . 2009 . Pyrene functional poly(vinyl alcohol) by ‘click’ chemistry . Journal of Polymer Science Part A: Polymer Chemistry , 47 : 1317 – 1326 .
- Kukut , M , Kiskan , B and Yagci , Y . 2009 . Self-curable benzoxazine functional polybutadienes synthesized by click chemistry . Designed Monomers and Polymers , 12 : 167 – 176 .
- Toiserkani , H , Yilmaz , G , Yagci , Y and Torun , L . 2010 . Functionalization of polysulfones by click chemistry . Macromolecular Chemistry and Physics , 211 : 2389 – 2395 .
- Durmaz , YY , Sangermano , M and Yagci , Y . 2010 . Surface modification of UV-cured epoxy resins by click chemistry . Journal of Polymer Science Part A: Polymer Chemistry , 48 : 2862 – 2868 .
- Okcu , SS , Durmaz , YY and Yagci , Y . 2010 . Synthesis and characterization of telechelic block co-polymers by combination of atom transfer radical polymerization and click chemistry processes . Designed Monomers and Polymers , 13 : 459 – 472 .
- Yilmaz , G , Toiserkani , H , Demirkol , DO , Sakarya , S , Timur , S , Yagci , Y and Torun , L . 2011 . Modification of polysulfones by click chemistry: amphiphilic graft copolymers and their protein adsorption and cell adhesion properties . Journal of Polymer Science Part A: Polymer Chemistry , 49 : 110 – 117 .
- Ates , S , Durmaz , YY , Torun , L and Yagci , Y . 2010 . Synthesis and characterization of polystyrene possessing triptycene units in the main chain by combination of ATRP and click chemistry processes . Journal of Macromolecular Science: Pure and Applied Chemistry , 47 : 809 – 815 .
- Tasdelen , MA and Yagci , Y . 2010 . Light-induced copper(I)-catalyzed click chemistry . Tetrahedron Letters , 51 : 6945 – 6947 .
- Karagoz , B , Durmaz , YY , Gacal , BN , Bicak , N and Yagci , Y . 2009 . Functionalization of poly(divinylbenzene) microspheres by combination of hydrobromination and click chemistry processes: a model study . Designed Monomers and Polymers , 12 : 511 – 522 .
- Tasdelen , MA , Van Camp , W , Goethals , E , Dubois , P , Du Prez , F and Yagci , Y . 2008 . Polytetrahydrofuran/clay nanocomposites by in situ polymerization and ‘click’ chemistry processes . Macromolecules , 41 : 6035 – 6040 .
- Oral , A , Tasdelen , MA , Demirel , AL and Yagci , Y . 2009 . Poly(methyl methacrylate)/clay nanocomposites by photoinitiated free radical polymerization using intercalated monomer . Polymer , 50 : 3905 – 3910 .
- Ak , M , Gacal , B , Kiskan , B , Yagci , Y and Toppare , L . 2008 . Enhancing electrochromic properties of polypyrrole by silsesquioxane nanocages . Polymer , 49 : 2202 – 2210 .
- Gacal , B , Durmaz , H , Tasdelen , MA , Hizal , G , Tunca , U , Yagci , Y and Demirel , AL . 2006 . Anthracene-maleimide-based Diels-Alder ‘click chemistry’ as a novel route to graft copolymers . Macromolecules , 39 : 5330 – 5336 .
- Li , J , Li , H , Wu , C , Ke , Y , Wang , D , Li , Q , Zhang , L and Hu , Y . 2009 . Morphologies, crystallinity and dynamic mechanical characterizations of polypropylene/polystyrene blends compatibilized with PP-g-PS copolymer: effect of the side chain length . European Polymer Journal , 45 : 2619 – 2628 .