Abstract
A new class of spherical and swellable microbeads was obtained by a newly proposed suspension polymerization protocol. Hydrophilic monomers in the form of crosslinking agent, glycerol dimethacrylate (GDMA), and glycerol 1,3-diglycerolate diacrylate (GDGDA) were copolymerized in an aqueous suspension medium. Poly(GDMA-co-GDGDA) microbeads were highly hydrophilic due to hydroxyl functionality from both crosslinking agents. In the proposed method, the organic phase including monomers and diluent (i.e. cyclohexanol) was dispersed in an aqueous medium by using poly(vinyl alcohol) as the stabilizer. Poly(GDMA-co-GDGDA) microbeads were obtained by changing GDMA/GDGDA ratio, monomer/diluent ratio, and stirring rate. The average size and size distribution properties, microbead yields, and the equilibrium swelling behavior of the gel beads were presented. Spherical and swellable bifunctional microbeads carrying hydroxyl–carboxyl or hydroxyl–amine groups were also obtained by including amine and carboxyl carrying functional monomers, 2-(dimethylamino)ethyl methacrylate, and methacrylic acid, respectively, in the suspension copolymerization proposed.
Introduction
Polymeric microbeads have been used as carriers in various medical and biological applications such as affinity chromatography, drug delivery, enzyme immobilization, cell immobilization, embolization, and immunochemistry Citation[1–6]. Various parameters including particle size and size distribution, porosity and pore structure, surface area, swellability, and specific functional residuals (or reactive sites) indicate the overall performance of polymer microbeads in these applications. The polymer beads with optimized parameters are widely used as functional support materials Citation[7–13]. Additionally, polymeric microbeads have attracted much attention because they can be produced easily in a wide variety of compositions and can be modified by inserting various ligands into the structure in order to make them specific sorbent Citation[14–20]. Polymer beads in the size range of 50nm–2 mm are obtained by various manufacturing processes including suspension, emulsion, and dispersion polymerization. Suspension polymerization is particularly used for the synthesis of spherical polymer beads in the size range of 10–1000 μm Citation[21].
The acrylate polymers with biocompatible properties are suitable matrices in most of the biomedical and biotechnological applications. Poly(hydroxyethylmethacrylate) (poly(HEMA)) beads are very versatile because of their hydrophilic nature, high chemical and mechanical stability, and resistance toward microbial and enzymatic attacks as well as with blood compatibility Citation[4,22,23]. Cross-linked poly(HEMA) beads produced by suspension polymerization were used as sorbents in various chromatographic applications. 2-Hydroxypropyl methacrylate (HPMA)-based microbeads, whose physical properties and molecular structures are very similar to those of HEMA, were previously prepared in mono and bifunctional forms by Tuncel et al. Citation[24]. Microbeads with controlled hydrophilicity and functionality were also obtained by adjusting the feed ratio of poly(ethylene glycol) methacrylate to the ethylene glycol dimethacrylate or divinylbenzene as an alternative matrix to poly(HEMA)-based particles Citation[25].
Saracoglu et al. Citation[26] reported that monodisperse cross-linked poly(glycerol dimethacrylate) (poly(GDMA))-based microgel particles carrying hydroxyl and carboxyl functionalities from nanometer to micrometer size range were synthesized by single-stage precipitation polymerization. The authors claimed that the produced poly(GDMA) microbeads could be used widely as a promising material for biotechnological and biomedical applications. In another study, the poly(2-(dimethyl-amino) ethyl methacrylate (DMAEM)-grafted-glycerol dimethacrylate (GDMA)) particles exhibiting thermosensitive properties were obtained by grafting of poly(DMAEM) brushes onto the surfce of poly(GDMA) particles by means of surface-initiated atom transfer radical polymerization [27]. The results showed that cell attachment and proliferation were facilitated due to the presence of positively charged amino groups on their surfaces. Glycerol 1,3-diglycerol diacrylate-grafted poly(3-hydroxyoctanoate) (GDD-g-PHO) copolymers were prepared by Kim et al. [28], and they reported that the GDD-g-PHO copolymers became more hydrophilic as the GDD grafting density in the copolymer increased, and biocompatibility was also enhanced by grafting of GDD groups.
In the present study, spherical and swellable hydrogel beads were obtained by the suspension copolymerization of two crosslinking agents, GDMA and GDGDA. Poly(GDMA-co-GDGDA) microbeads were highly hydrophilic due to hydroxyl functionality from both crosslinking agents. In addition, the bifunctional forms of the same microbeads were also obtained by including functional monomers in the same recipe. The hydrophilicity and functionality of the microbeads were changed by adjusting the feed ratio of the monomers or by using different monomers. Mono and bifunctional hydrogel beads were characterized in terms of equilibrium swelling ratio, functional group content, average size and size distribution, and microbead yields. Poly(GDMA-co-GDGDA) microbeads can be used in biotechnological and biomedical applications such as separation of biomacromolecules, tissue engineering, drug targeting, solid phase extraction, DNA diagnostic assays, and gene delivery. Poly(GDMA-co-GDGDA) microbeads are promising materials as sorbent or microcarrier because of their easy chemical derivatization, the hydrophilicity, and the functionality.
Experimental
Materials
The monomers of glycerol dimethacrylate (GDMA, technical grade, 85%, mixture of isomers), glycerol 1,3-diglycerolate diacrylate (GDGDA, technical grade), 2-(dimethylamino) ethyl methacrylate (DMAEM), and methacrylic acid (MAA) were supplied from Aldrich Chemical Co. (Milwaukee, WI, USA) and used without further purification. Benzoyl peroxide (BPO, 97% active compound, Aldrich Chemical Co.) and polyviny1alcohol (PVA, 87–89% hydrolyzed, molecular weight: 85.000–146,000, Aldrich Chemical Co.) were utilized as initiator and stabilizer, respectively, and were used without further purification. Cyclohexanol (Cyc-OH; Aldrich Chemical Co.) was included in the polymerization recipe as a diluent and used without further purification. Ethyl alcohol used as solvent in washing of microbeads was a product of Birpa Co. (Istanbul, Turkey). Distilled–deionized water was used in all experiments.
Production of microbeads
A suspension polymerization procedure was proposed to prepare spherical and swellable poly(GDMA-co-GDGDA) microbeads. In a typical procedure, the continuous medium was prepared by dissolving 500 mg PVA in 50 mL of distilled water. The monomer phase was prepared by mixing Cyc-OH (4 mL), GDMA (2 mL), and GDGDA (2 mL) in a test tube. The initiator, BPO (80 mg), was dissolved within the monomer phase. The monomer phase was transferred into the continuous medium in a magnetically stirred and glass-sealed polymerization reactor (100 mL) placed in a water bath equipped with a temperature control system. The reactor was heated to 85 °C in 30 min while stirring. The polymerization was conducted at 85 ± 1 °C for 4 h. The reactor content was cooled down to room temperature. In order to remove the diluent and any possible unreacted monomer, poly(GDMA-co-GDGDA) microbeads were cleaned by the following procedure. The microbeads were allowed to settle and the dispersion medium was decanted. The microbeads were resuspended in ethyl alcohol. The new dispersion was stirred for about 1 h at room temperature and the microbeads were isolated by decanting the liquid part. Microbeads were washed twice with ethyl alcohol and three times with distilled–deionized water using the same procedure. In order to obtain poly(GDMA-co-GDGDA) microbeads with different sizes and swellabilities, the GDMA/GDGDA volume ratio, cyclohexanol concentration, and the stirring rate were varied. Bifunctional microbeads carrying hydroxyl–carboxyl or hydroxyl–amine groups were also prepared by including amine and carboxyl carrying functional monomers, DMAEM and MAA, respectively, in the suspension copolymerization proposed. The recipes for copolymerization are tabulated in Table . In these runs, the initiator and stabilizer concentrations, the polymerization temperature and the polymerization time were constant as given above.
Table 1. Experimental conditions for suspension copolymerization of GDGDA with GDMA.
Average size and size distribution of microbeads
The average size and the size distribution of the microbeads were determined by optical microscopy. The microbeads equilibrated in distilled water were evaluated with an optical microscope (Olympus, Japan) mostly at a magnification of 125×. To determine the average size and size distribution of microbeads, at least three different photographs were obtained for each sample. For this purpose, approximately 100–200 microbeads were counted on each photograph. Then, the number average diameter and the volume fraction of each size were calculated. The plots showing the size distribution of the beads were plotted by taking into account the size range values defined in the Tyler Standards Citation[29]. The number average diameter (Dp ) of the microbeads was calculated according to the Equation (1):
where Ni is the number of particles of diameter D i (μm).
The standard deviation (SD) from average diameter was calculated according to the Equation (2):
where NT is the total number of particles.
The coefficient of variation (CV) for size distribution was also calculated based on the Equation (3):
Equilibrium swelling behavior of microbeads
Equilibrium swelling ratios (ESRs) of microbeads were determined by volumetric method. Certain amounts of well dried microbeads (approximately 1000 mg) were filled into a cylindrical glass tube (5 mm of internal diameter and 100 mm of height) and the bed height obtained with the dried microbeads was measured (h 0). Then, the tube was filled with distilled water (different pH values) and the microbeads were allowed to swell at room temperature for 24 h (i.e. predetermined equilibrium swelling time) with occasional shaking. At the end of this period, the bed height with the swollen microbeads was again measured (h 1). ESR of the microbeads was calculated using the Equation (4):
Microbead yield
The copolymer or terpolymer microbeads were extensively washed and dried in a vacuum oven at 50 °C for 48 h. The microbeads were weighed in an electronic balance. The microbead yield was calculated by the following expression:
where W p and W m are the weight of dry microbeads and the total weight of monomers initially charged to the reactor, respectively.
Results and discussion
In this study, mono and bifunctional hydrogel beads in the size range of 25–460 μm were synthesized by a new suspension copolymerization technique. Two crosslinking agents with hydroxyl functionality, GDMA and GDGDA, were included in the proposed copolymerization. In addition, the bifunctional forms of the same microbeads were also obtained by including functional monomers in the same recipe. The hydrophilicity and functionality of the microbeads were changed by adjusting the feed ratio of the monomers or by using different monomers. Some selected physical properties of ingredients used in the suspension polymerization were determined and are given in Table . As seen here, the crosslinking agents with hydroxyl groups (i.e. GDMA and GDGDA) are more viscous with respect to water and functional monomers (i.e. MAA and DMAEM). Particularly, GDGDA is the most viscous component of the selected suspension polymerization system. The solubility parameter of GDMA is close to the functional monomers. The solubility parameter of the other crosslinking agent GDGDA could not be obtained from the literature. However, it should be higher than that of GDMA since GDGDA contains three hydroxyl groups when GDMA contains only one hydroxyl group.
Table 2. The physical properties of ingredients used in the suspension polymerization system.
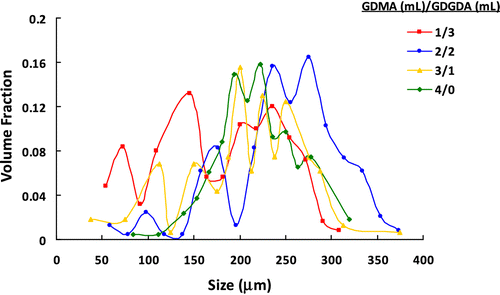
The GDMA and GDGDA contain one and three hydroxyl functionalities, respectively. For this purpose, the gel beads with high hydroxyl contents (i.e. highly hydrophilic in nature) were aimed by increasing the relative amount of GDGDA in the initial monomer mixture. The effect of monomer composition on the size distribution of poly(GDMA-co-GDGDA) beads is given in Figure and Table . Additionally, the yields of poly(GDMA-co-GDGDA) microbeads obtained by changing GDMA/GDGDA ratio were also given in Table . As seen here, the microbead yield was almost quantitative in every experiment and exhibited no significant change with the GDMA/GDGDA volume ratio. As observed in Table , the average size raised with increasing GDMA/GDGDA ratio. A factor for providing an increment in the average size may be change of interfacial tension by the GDMA concentration. GDGDA consists of three hydroxyl groups; thus, GDGDA is more polar when compared to GDMA. Far less soluble in water than GDGDA, GDMA is preferentially situated in the droplet phase. As a result, in the surface tension of the droplet phase, higher GDMA concentration most likely caused a reduction, which in turn yielded an increase in the interfacial tension between the continuous medium and the droplet phase. The decrease in the droplet phase viscosity or in the interfacial tension involves a decrease in the average size, in accordance with the common mathematical model of suspension polymerization. The average size is straightforwardly proportional to the interfacial tension between the two immiscible phases; accordingly, an increase in the interfacial tension with increasing GDMA feed concentration results in a raise in the average size Citation[33,24].
Table 3. The effect of GDMA/GDGDA volume ratio on the microbead yield, average size, and size distribution of poly(GDMA-co-GDGDA) beads.
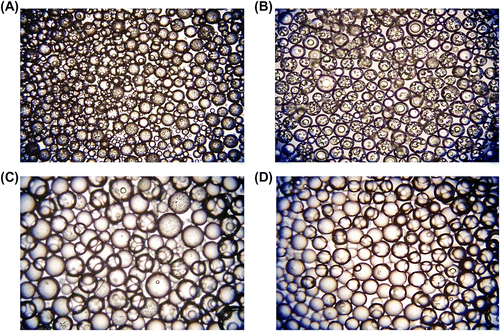
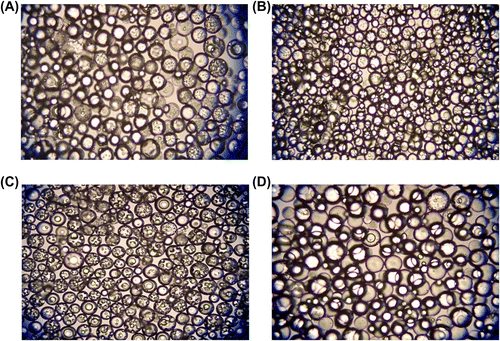
As seen in Table , the CV for size distribution decreased also with increasing GDMA/GDGDA ratio. In this set, poly(GDMA-co-GDGDA) beads with the average sizes ranging between 180 and 248 μm were obtained. The size range for each batch lied between 50 and 370 μm (Figure ). The optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different GDMA/GDGDA ratios are shown in Figure . As seen here, polydisperse (shown in Figure ) and spherical poly(GDMA-co-GDGDA) microbeads were produced with different GDMA/GDGDA ratios. The microbeads had a transparent view under optical microscope. This view indicated that the beads had gel-type microporosity.
Figure 2 Optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different GDMA/GDGDA ratios. GDMA/GDGDA ratios (mL/mL) are: (A) 1/3, (B) 2/2, (C) 3/1 and (D) 4/0. Original magnification: 125×.
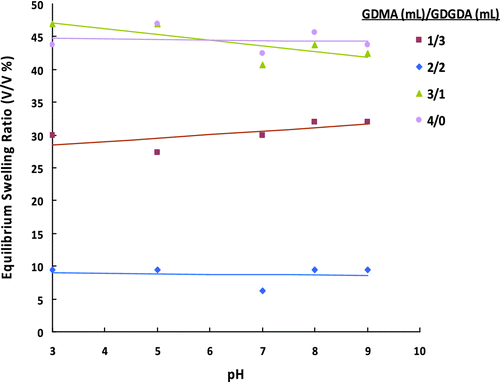
The equilibrium swelling ratios of poly (GDMA-GDGDA) gel beads synthesized with different GDMA/GDGDA volume ratios at different pHs are presented in Figure . It is well known that the swelling of hydrophilic microbeads depends on factors such as the structure of the material, the degree of crosslinking, the chain length of the crosslinking agent, the hydrophilic/hydrophobic balance, the shape and dimension of the system, and so on. The gel beads synthesized with higher GDGDA content exhibited higher equilibrium swelling ratios. Higher hydroxyl content of GDGDA with respect to GDMA should be probably responsible for this behavior. ESR exhibited no appreciable change with pH since no charged groups are present on the beads.
Figure 3 ESRs of poly(GDMA-GDGDA) gel beads synthesized with different GDMA/GDGDA volume ratios at different pHs.
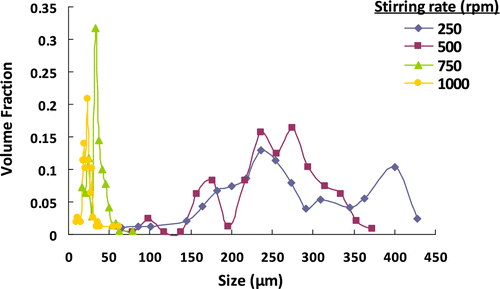
The effect of stirring rate on the size distribution of poly(GDMA-co-GDGDA) beads is given in Table and Figure . The microbead yields with different stirring rates are also given in Table . As seen here, no significant effect of stirring rate on the microbead yield was observed, and slightly increased with increasing stirring rate. Table indicates that the average size significantly decreased and CV for size distribution remained roughly constant with increasing stirring rate. In this set, poly(GDMA-co-GDGDA) beads with the average sizes ranging between 24 and 425 μm were obtained. As shown in Figure , the size distribution was also relatively narrower with the higher stirring rates, while a relatively wide size distribution was obtained with low stirring rates (i.e. 250 and 500 rpm). As explained in the related literature for different suspension polymerization systems Citation[21,34,35], the average size reduced with increasing stirring rate. Also, for most practical purpose, the stirring rate can provide a relatively convenient means of particle size control.
Table 4. The effect of stirring rate on the microbead yield, average size, and size distribution of poly(GDMA-co-GDGDA) beads.
The optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different stirring rates are presented in Figure . These findings indicated that the stirring rate was an effective variable to control the average size of gel beads in the suspension polymerization technique proposed. The equilibrium swelling ratios of poly(GDMA-co-GDGDA) gel beads at different pHs are shown in Figure .
Figure 5 Optical micrographs of poly(GDMA-co-GDGDA) beads with different stirring rates. Stirring rates: (A) 250, (B) 500, (C) 750, and (D) 1000 rpm, respectively. Original magnification: 125×.
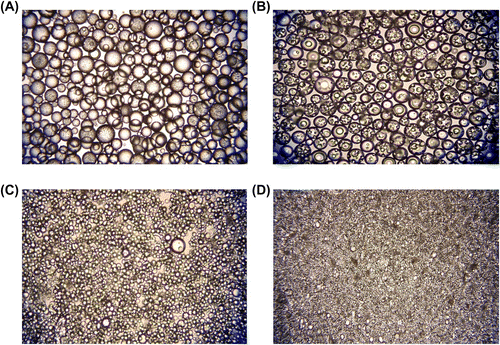
Figure 6 ESRs of poly(GDMA-co-GDGDA) gel beads synthesized with different stirring rates at different pH values.
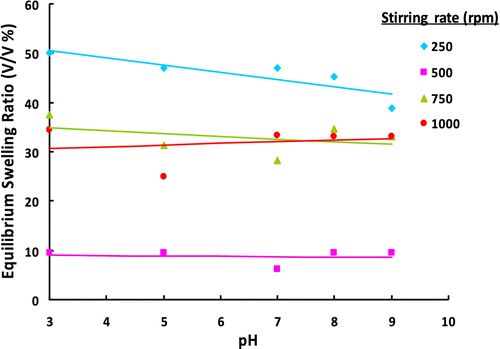
As mentioned above, equilibrium swelling ratio also showed no appreciable change. However, it was interesting that the beads obtained with higher stirring rates (i.e. the beads with lower average size) exhibited higher equilibrium swelling ratios. This finding should be attributed to lower degree of crosslinking in the beads with lower average size.
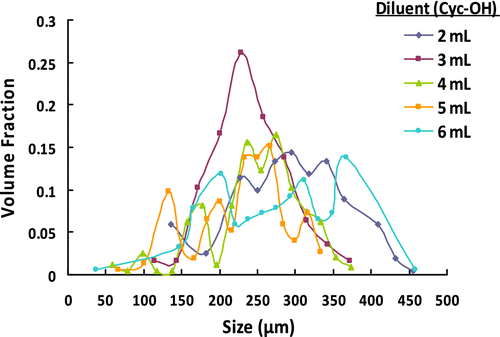
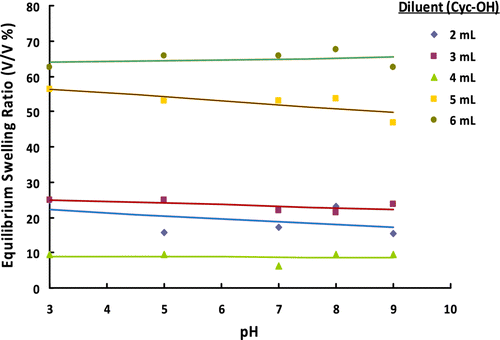
Both monomers involved in the suspension polymerization process are water-soluble. To obtain a stable suspension including an aqueous-based continuous medium and a disperse phase containing monomers, the introduction of a diluent capable of dissolving both monomers, but that is insoluble in water was necessary. For this purpose, Cyc-OH having sufficiently lower surface tension value relative to water was used as a diluent in the suspension polymerization proposed. The effect of amount of diluent on the size distribution of poly(GDMA-co-GDGDA) beads is given in Table and Figure . The microbead yields are also given in Table . As seen in Table , no significant change was observed in microbead yields with different volumes of diluent. However, all microbead yields were almost quantitative.
Table 5. The effect of amount of diluent on the microbead yield, average size, and size distribution of poly(GDMA-co-GDGDA) beads.
Insignificant change was observed for the effect of amount of diluent on the average size and size distribution given in Table 5. However, the narrowest size distribution was obtained with the diluent amount of 3 mL. The optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different amounts of diluent are shown in Figure .
Figure 8 Optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different amounts of diluent. Amounts of diluent: (A) 2, (B) 3, (C) 4, and (D) 5 mL respectively. Original Magnification 125×.
The equilibrium swelling ratios of poly(GDMA-co-GDGDA) gel beads obtained with different amounts of diluent at different pHs are shown in Figure . The gel beads synthesized with larger amount of diluent exhibited significantly higher equilibrium swelling ratios. At a constant monomer concentration, swellability of the microbeads raised by increasing amount of the diluent, likewise the general tendency previously published ones in the related open literature Citation[36,37]. This finding could be explained by higher gel-type microporosity of the beads synthesized with higher amount of diluent.
Figure 9 ESRs of poly(GDMA-co-GDGDA) gel beads synthesized with different amounts of diluent at different pH values.
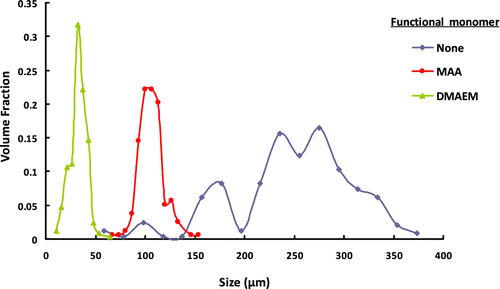
The effect of functional monomer type on the size distribution of terpolymer beads including GDMA, GDGDA, and functional monomer are given in Table and Figure . The microbead yields of terpolymer are also given in Table 6. As seen in Table , lower microbead yields were obtained in the presence of functional monomers. This behavior should be probably explained by the formation of some water-soluble polymer dominantly including the functional monomer. The highest average size and the broader size distribution were obtained in the absence of functional monomer (Table ). The introduction of MAA into the suspension polymerization recipe resulted in a significant decrease both in average size and in CV for size distribution while the introduction DMAEM into the suspension polymerization recipe resulted in a significant decrease in average size. The average particle size of poly(GDMA-co-GDGDA) microbeads was 248 μm. On the other hand, the average particle size values of poly(GDMA-GDGDA-MAA) and poly(GDMA-GDGDA-DMAEM) terpolymer microbeads were determined as 106 and 33 μm, respectively. In other words, lower average size values were found for the microbeads synthesized using functional monomers. The average size of the monomer droplets (and, hence, that of the resulting particles) is directly proportional to the volume ratio of the droplet phase to suspension medium, the viscosity of the droplet phase, and the interfacial tension between the two immiscible phases Citation[21]. The presence of functional monomers (DMAEM and MAA) probably reduces the interfacial tension between the droplet phase and the aqueous phase. On the other hand, the viscosity values of monomers are given in Table . As seen here, the viscosities of functional monomers are reasonably lower with respect to those of GDMA and GDGDA. One can easily conclude that the presence of functional monomers markedly reduces the viscosity of droplet phase. Lower droplet phase viscosity and lower interfacial tension between the droplet phase and the aqueous phase provide lower droplet size leading to the final microbeads with lower size.
Table 6. The effect of functional monomer type on the microbead yield, average size, and size distribution of terpolymer beads including GDMA, GDGDA, and functional monomer.
Figure 10 The effect of functional monomer type on the size distribution of terpolymer microbeads including GDMA, GDGDA, and functional monomer.
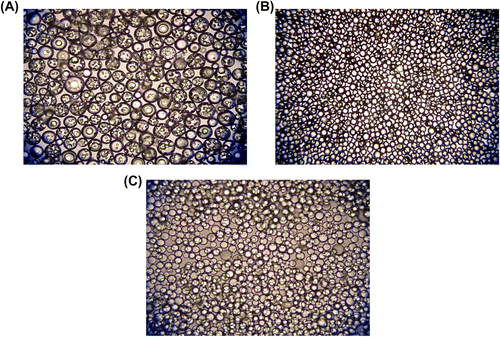
The optical micrographs showing the size distribution of plain, poly(GDMA-GDGDA-MAA), and poly(GDMA-GDGDA-DMAEM) microbeads are given in Figure . As seen here, poly(GDMA-GDGDA-MAA) microbeads were obtained with narrower size distributions.
Figure 11 Optical micrographs of terpolymer beads synthesized with different functional monomers. Functional monomers: (A) None, (B) MAA, and (C) DMAEM. Original Magnification 125× for (A) and (B) and 500× for (C).
The MAA content of poly(GDMA-GDGDA-MAA) terpolymer gel beads was determined as 0.70 mmol/g dry beads by potentiometric titration. In the presence of an amine functionalized functional monomer, the average particle size significantly decreased with respect to poly(GDMA-co-GDGDA) particles synthesized in the absence of functional monomer. The DMAEM content of poly(GDMA-GDGDA-DMAEM) terpolymer gel beads was determined as 0.21 mmol/g dry beads by elemental analysis.
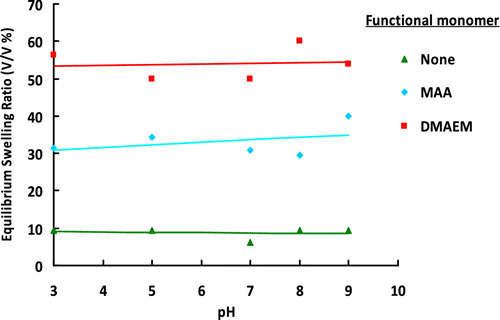
The equilibrium swelling ratios of plain, poly(GDMA-GDGDA-MAA), and poly(GDMA-GDGDA-DMAEM) microbeads are shown in Figure . Higher equilibrium swelling ratios were observed with the functional microbeads with respect to the plain microbeads sample. However, insignificant change in equilibrium swelling ratio with pH was observed for both types of functional beads. This behavior could be explained by the high crosslinking degree of both microbead types.
Conclusion
In this study, hydrophilic poly(GDMA-co-GDGDA) microbeads were synthesized due to hydroxyl functionality from both crosslinking agents. Poly(GDMA-co-GDGDA) microbeads can be another alternative for hydrophilic polymer-based microspheres because of the ease of derivatization, the water swellability, and the similarity of molecular structure to a widely used biocompatible material (i.e. 2-hydroxyethyl methacrylate-based hydrogels). These microbeads can be used in biomedical and biotechnological applications due to their controllable hydrophilicity and functionality properties. In addition, the proposed methodology allows the synthesis of bifunctional gel beads carrying anionic and cationic moieties. The presences of hydroxyl–carboxyl and hydroxyl–amine functionalities on the swellable hydrogel beads allow the synthesis of new resins in hydrophilic form.
References
- Kahovec , J , Jelinkova , M and Coupek , J . 1987 . Chemical modification of macroreticular 2-hydroxyethyl methacrylate ethylene dimethacrylate copolymers . Polymer Bulletin , 18 ( 6 ) : 495 – 499 .
- Scranton , AB , Mikos , AG , Scranton , LC and Peppas , NA . 1990 . The physical-mechanism for the production of hydrophilic polymer microparticles from aqueous suspensions . Journal of Applied Polymer Science , 40 ( 5–6 ) : 997 – 1004 .
- Kamei , S , Okubo , M and Matsumoto , T . 1987 . Adsorption of trypsin onto poly(2-hydroxyethyl methacrylate) polystyrene composite microspheres and its enzymatic activity . Journal of Applied Polymer Science , 34 ( 4 ) : 1439 – 1446 .
- Krizova , J , Spanova , A , Rittich , B and Horak , D . 2005 . Magnetic hydrophilic methacrylate-based polymer microspheres for genomic DNA isolation . Journal of Chromatography A , 1064 ( 2 ) : 247 – 253 .
- Peixoto , LS , Melo , PA , Nele , M and Pinto , JC . 2009 . Expanded core/shell poly(vinyl acetate)/poly(vinyl alcohol) particles for embolization . Macromolecular Materials and Engineering , 294 ( 8 ) : 463 – 471 .
- Rodriguez , C , Castro , E , Martin , A , Marin , JR , Berganza , J and Cuevas , JM . 2011 . Magnetic poly(styrene/divinylbenzene/acrylicacid)-based hybrid microspheres for bio-molecular recognition . Micro and Nano Letters , 6 ( 6 ) : 349 – 352 .
- Gan , ZH , Jim , TF , Li , M , Yuer , Z , Wang , SG and Wu , C . 1999 . Enzymatic biodegradation of poly(ethylene oxide-b-epsilon-caprolactone) diblock copolymer and its potential biomedical applications . Macromolecules , 32 ( 3 ) : 590 – 594 .
- Lee , DW , Hwang , SJ , Park , JB and Park , HJ . 2003 . Preparation and release characteristics of polymer-coated and blended alginate microspheres . Journal of Microencapsulation , 20 ( 2 ) : 179 – 192 .
- Armelin , E , Meneguzzi , A , Ferreira , CA and Aleman , C . 2009 . Polyaniline, polypyrrole and poly(3,4-ethylenedioxythiophene) as additives of organic coatings to prevent corrosion . Surface and Coating Technology , 203 ( 24 ) : 3763 – 3769 .
- Qu , JB , Huang , YD , Jing , GL , Liu , JG , Zhou , WQ , Zhu , H and Lu , JR . 2011 . A novel matrix derivatized from hydrophilic gigaporous polystyrene-based microspheres for high-speed immobilized-metal affinity chromatography . Journal of Chromatography B , 879 ( 15–16 ) : 1043 – 1048 .
- Tan , L , Cao , LJ , Yang , M , Wang , G and Sun , D . 2011 . Formation of dual-responsive polystyrene/polyaniline microspheres with sea urchin-like and core-shell morphologies . Polymer , 52 ( 21 ) : 4770 – 4776 .
- Liu , QQ , Wang , L , Xiao , AG and Yu , HJ . 2010 . The spherical cleavage behavior of polydivinylbenzene during suspension polymerization . Designed Monomers and Polymers , 13 ( 4 ) : 369 – 375 .
- Vilchis , L , Guyot , A , Rios , L and Villalobos , MA . 2004 . Co-polymers of acrylic acid and methyl methacrylate as stabilizers for suspension polymerizaton of styrene . Designed Monomers and Polymers , 7 ( 6 ) : 521 – 539 .
- Uguzdogan , E , Denkbas , EB and Kabasakal , OS . 2010 . The use of polyethyleneglycol methacrylate-co-vinylimidazole (PEGMA-co-VI) microspheres for the removal of nickel(II) and chromium(VI) ions . Journal of Hazardous Materials , 177 ( 1–3 ) : 119 – 125 .
- Ebraheem , KAK and Hamdi , ST . 1997 . Synthesis and properties of a copper selective chelating resin containing a salicylaldoxima group . Reactive and Functional Polymers , 34 ( 1 ) : 5 – 10 .
- Ma , ZY , Guan , YP and Lu , HZ . 2006 . Affinity adsorption of albumin on cibacron blue F3GA-coupled non-porous micrometer-sized magnetic polymer microspheres . Reactive & Functional Polymers , 66 ( 6 ) : 618 – 624 .
- Ding , S , Floyd , JA and Walters , KB . 2009 . Comparison of surface confined ATRP and SET-LRP syntheses for a series of amino (meth)acrylate polymer brushes on silicon substrates . Journal of Polymer Science Part A: Polymer Chemistry , 47 ( 23 ) : 6552 – 6560 .
- Odabasi , M and Denizli , A . 2001 . Polyhydroxyethylmethacrylate-based magnetic DNA-affinity beads for anti-DNA antibody removal from systemic lupus erythematosus patient plasma . Journal of Chromatography B , 760 ( 1 ) : 137 – 148 .
- Quach , AD , Crivat , G , Tarr , MA and Rosenzweig , Z . 2011 . Gold nanoparticle-quantum dot-polystyrene microspheres as fluorescence resonance energy transfer probes for bioassays . Journal of the American Chemical Society , 133 ( 7 ) : 2028 – 2030 .
- Mallikarjuna , B , Rao , KM , Prasad , CV , Rao , KC and Subha , MCS . 2011 . Development of triprolidine-hydrochloride-loaded pH-sensitive poly(acrylamide-co-acrylamidoglycolic acid) co-polymer microspheres: In vitro release studies . Designed Monomers and Polymers , 14 ( 5 ) : 445 – 459 .
- Arshady , R . 1992 . Suspension, emulsion, and dispersion polymerization – a methodological survey . Colloid and Polymer Science , 270 ( 8 ) : 717 – 732 .
- Sivakumar , M and Rao , KP . 2002 . Synthesis, characterization, and in vitro release of ibuprofen from poly(MMA-HEMA) copolymeric core-shell hydrogel microspheres for biomedical applications . Journal of Applied Polymer Science , 83 ( 14 ) : 3045 – 3054 .
- Reno , F , Traina , V , Gatti , S and Cannas , M . 2011 . Vitamin E triggers poly(2-Hydoxyethyl metacrylate) (PHEMA) embolic potential: A proposed application for endovascular surgery . Journal of Biomaterials Science, Polymer Edition , 22 ( 4–6 ) : 641 – 650 .
- Tuncel , A and Cicek , H . 2000 . 2-Hydroxypropylmetacrylate based mono- and bifunctional gel beads prepared by suspension polymerization . Polymer International , 49 ( 6 ) : 485 – 494 .
- Tuncel , A . 2000 . Suspension polymerization of poly(ethylene glycol) methacrylate: a route for swellable spherical gel beads with controlled hydrophilicity and functionality . Colloid and Polymer Science , 278 ( 12 ) : 1126 – 1138 .
- Saracoglu , B , Uguzdogan , E , Golgelioglu , C and Tuncel , A . 2009 . Synthesis of monodisperse glycerol dimethacrylate-based microgel particles by precipitation polymerization . Industrial and Engineering Chemistry Research , 48 ( 10 ) : 4844 – 4851 .
- Kahraman , AS , Gumusderelioglu , M and Tuncel , A . 2011 . Cellular interactions of monodisperse poly(GDMA) latex particles-containing DMAEM brushes . Colloids and Surfaces A , 384 ( 1–3 ) : 90 – 97 .
- Kim , HW , Chung , MG , Kim , YB and Rhee , YH . 2008 . Graft copolymerization of glycerol 1,3-diglycerolate diacrylate onto poly(3-hydroxyoctanoate) to improve physical properties and biocompatibility . International Journal of Biological Macromolecules , 43 ( 3 ) : 307 – 313 .
- Perry , RH and Chilton , C . 1973 . “ Tyler standads ” . In Chemical Engineers’ Handbook , 5th ed. , Edited by: Chilton , C . 21 – 44 . Tokyo : McGraw-Hill Kogakusha .
- http://www.chemspider.com/Chemical-Structure.67171.html (GDMA); http://www.chemspider.com/Chemical-Structure.3807655.html (GDGDA); http://www.chemspider.com/Chemical-Structure.3951.html (MAA); http://www.chemspider.com/Chemical-Structure.16879.html (DMAEM); http://www.chemspider.com/Chemical-Structure.7678.html (Cyc-OH); http://www.chemspider.com/Chemical-Structure.937.html (H2O).
- Bandup , J and Immergut , EH , eds. 1975 . Polymer handbook , Toronto : Wiley Interscience .
- Aoki , H , Hosoya , K , Norisuye , T , Tanaka , TN , Tokuda , D , Ishizuka , N and Nakanishi , K . 2006 . Basic study of the gelation of dimethacrylate-type crosslinking agents . Journal of Polymer Science Part A: Polymer Chemistry , 44 ( 2 ) : 949 – 958 .
- Arshady , R and Ledwith , A . 1983 . Suspension polymerization and its application to the preparation of polymer supports . Reactive Polymer , 1 : 159 – 174 .
- Uguzdogan , E , Denkbas , EB , Ozturk , E , Tuncel , SA and Kabasakal , OS . 2009 . Preparation and characterization of polyethyleneglycolmethacrylate (PEGMA)-co-vinylimidazole (VI) microspheres to use in heavy metal removal . Journal of Hazardous Materials , 162 ( 2–3 ) : 1073 – 1080 .
- Kesenci , K , Tuncel , A and Piskin , E . 1996 . Swellable ethylene glycol dimethacrylate-hydroxyethylmethacrylate copolymer beads . Reactive & Functional Polymers , 31 ( 2 ) : 137 – 147 .
- Park , TG and Hoffman , AS . 1990 . Immobilization and characterization of beta-galactosidase in thermally reversible hydrogel beads . Journal of Biomedical Materials Research , 24 ( 1 ) : 21 – 38 .
- Okay , O and Funke , W . 1990 . Anionic dispersion polymerization of 1,4-divinylbenzene . Macromolecules , 23 ( 10 ) : 2623 – 2628 .