Abstract
A trifunctional epoxy acrylate (Tri-EA) was synthesized via trifunctional epoxy and acrylic acid, the structure of the monomer was characterized by FTIR and 1H NMR. Photopolymerization kinetics of Tri-EA – under different conditions such as varying photoinitiator type and concentration and light intensity and different functional reactive diluents, i.e. trimethylol propanetriacrylate (TMPTA), tripropylene glycol diacrylate (TPGDA), and 2-Hydroxyethyl acrylate (HEA) – was studied by real-time infrared spectroscopy (RTIR). The cured films were characterized with thermogravimetric analysis (TGA), dynamic mechanical analysis (DMA), and universal testing machine. The results showed that different functional reactive diluents had a significant effect on kinetics and properties of the cured Tri-EA systems. Compared with difunctional epoxy acrylate (di-EA), Tri-EA showed higher glass transition temperatures (T g), higher thermal stability, and better adhesive strength while the polymerization extent and rate was lower.
1. Introduction
UV-curable formulations contain oligomers or prepolymers, which consisted of reactive sites based on a functional acrylate group to meet the volatile organic compounds compliance Citation[1,2]. There are several types of acrylated prepolymers with different back-bone structures such as epoxy acrylates (EAs), urethane acrylates, polyester acrylates, polyether acrylates, and acrylated oils Citation[3–10]. Possessing both good properties of epoxy and acrylic resins, EAs were widely employed in UV-curing systems, such as composite material, coatings, and adhesives Citation[1,4]. The wide use of EAs based on bisphenol diglycidyl ether and epoxy novolac was due to their versatile chemistry to tailor make products with good thermal properties, adhesion, hardness, and chemical resistance Citation[1,5,11,12].
The cure process is free radical polymerization and results in three dimensional network formations. Generally, the types of EAs and curing conditions have to be considered. On the one hand, due to the high viscosity of oligomers or prepolymers, reactive diluents are incorporated in the formulations to make thin-film applications possible. Structure and length of oligomers and the functionality of diluents can affect the cross-linking reactions and also the physical and mechanical properties of the cured films. On the other hand, cure conditions directly influence on the acrylate double bond conversion and the extent of conversion directly controls the cross-link density, which appears to be related to properties of the coat Citation[13].
Acrylated epoxy oligomers owning epoxy backbone were capable of a rational-designed formulation that provided good coating properties after curing. Cheng Citation[14,15] synthesized a new kind of trifunctional epoxy resin. It is notable for its excellent thermal resistance with 4,4′-diamino-diphenyl sulfone (DDS) or 4,4-diaminodiphenylmethane (DDM) as hardener. The glass transition temperatures (T g) of TMBPBTH-EPOXY/DDM and TMBPBTH-EPOXY/DDS tested by dynamic mechanical thermal analysis (DMTA) were 240.2 and 290.1 °C, respectively. When this new kind of trifunctional epoxy resin was reacted with crylic acid, this trifunctional epoxy acrylate (Tri-EA) is expected to have good properties, e.g. thermal stability, mechanical properties, and adhesive strength. Then, the photopolymerization kinetics of trifunctional EA with different functionality of diluents was monitored by real-time infrared spectroscopy (RTIR) under different UV-curing conditions. Adhesion strength was obtained by using a universal testing machine. The thermal and mechanical properties of Tri-EA were studied by TGA and DMA, respectively.
2 Experimental
2.1 Materials
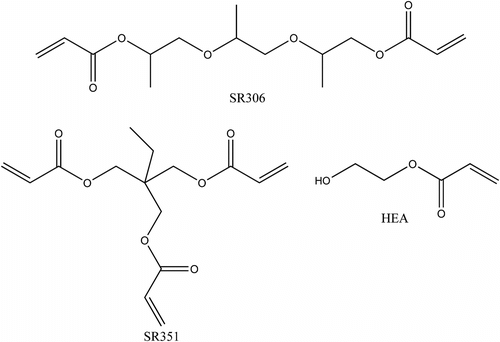
Trifunctional epoxy TMBPBTH-EPOXY was synthesized in laboratory. Bisphenol A epoxy resin (CYD-127, 180–190 g/eq) was supplied by Yueyang Baling Huaxing Petrochemical Ltd (China). Acrylic acid (MA, AR) was purchased from Beijing chemical plant (Beijing, China), Benzyl trimethyl ammonium chloride (BTAC) was obtained from Tianjin Jinke Fine Chemical Institute (Tianjin, China); trimethylol propanetriacrylate (TMPTA, SR351) and tripropylene glycol diacrylate (TPGDA, SR306) were donated by Sartomer. Another reactive diluent 2-Hydroxyethyl Acrylate (HEA) was supplied by Beijing Oriental Chemical Factory. The structures of the reactive diluents are given in Chart . The photoinitiator 2-hydroxyl-2-methyl-1-phenylpropane-1-one (Darocur1173), benzophenone (BP), 1-hydroxycyclohexyl phenyl ketone (184), and 2-isopropylthioxanthone (ITX) were donated by Runtec Chemistry (Changzhou, China). Ethyl-4-N,N-dimethy-laminobenzoate (EDMAB) was obtained from Aldrich (Mil-waukee, WI).
2.2 Instrumentation
2.2.1 NMR
The 1H NMR spectra was recorded on a BrukerAV600 unity spectrometer operated at 600 MHz, with CDCl3 as solvent and tetramethylsilane (TMS) as the internal standard.
2.2.2 FTIR
RTIR was obtained on a Nicolet 5700 instrument (Nicolet Instrument, Thermo Company, USA). Series RTIR was used to determine the conversions of double bond. The blend of monomer and photoinitiator was applied between two KBr crystals (The casting knife applied leads to a film thickness of 25 μm) and irradiated by UV spot light source (EFOX Lite, 50 W miniature acr lamp, with 5 mm crystal optical fiber, Canada) at room temperature. The light intensity on the surface of samples was detected by UV Radiometer (Beijing Normal University, China). For each sample, the series RTIR runs were repeated three times. Conversion data were obtained by monitoring the decay of the acrylate double bond C=C peak at ∼810 cm−1 Citation[16]. On irradiation, the decrease of the C=C absorption peak area from 796 to 818 cm−1 accurately reflects the extent of the polymerization. The degree of conversion (DC) can be expressed as follows:
where A 0 is the initial absorbance around 810 cm−1and At is the absorbance value at irradiation time t. The polymerization rate (R p) was determined from the slope of the initial linear portion of the conversion–time curves as follows:
where [M]0 is the initial concentration of C=C double bonds (mol−1)
2.2.3 DMA
Samples (the details are given in Table ) for DMA were irradiated by the same UV light source for 15 min to ensure their complete curing. Mechanical properties were measured with dynamic mechanical analysis (DMA 242C, NETZSCH, Germany). The samples were of uniform size (35 mm × 5 mm × 0.8 mm). The temperature scan was performed over a temperature range from −50 to 200 °C with a ramping rate of 5 °C per min (frequency 1 Hz) by using extension mode. The loss and storage modulus, as well as the loss tangent (tanδ, ratio of loss to storage modulus), were recorded as a function of temperature. The (T g) was taken to be the maximum of the loss tangent vs. temperature curve.
Table 1. Properties of cured films.
2.2.4 TGA
The decomposition temperature of UV-cured films was measured by TA (Q500) thermal analyzer at a heating rate of 20 oC/min and at a test range of 30–600 oC under a nitrogen purge (50 mL/min). The sample weight was about 5–10 mg.
2.3 Measurement of adhesion strength
100 μmv-thick sample was uniformly spread on the surface of glass Citation[17]. The dimension of one piece of glass was 5 mm × 20 mm × 50 mm. Then, the pieces of glass were overlapped in 10 mm in which the sample was spread and the area of bonding was 10 mm × 20 mm. It was irradiated by UV light under light intensity 30 mW/cm2 for 10 min. After UV curing, he samples were tested by using a universal testing machine (Model 1185, Instron, USA) with a crosshead speed of 5 mm/min at room temperature. Five samples were measured for each experiment and the average of these values was recorded.
2.4 Synthesis
2.4.1 Synthesis of Tri-EA
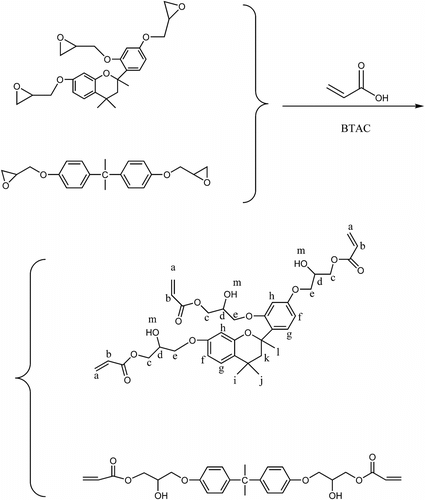
A mixture of 46.82 g of TMBPBTH-EPOXY (0.1 mol), toluene (300 mL), and a small amount of hydroquinone monomethyl ether 0.14 g (0.0011 mol) as inhibitor was placed into a 500 mL four-necked bottle equipped with stirrer, dropping funnel, thermometer, and condenser and was heated to 90 oC. A mixture of 28.2 g (0.39 mol) acrylic acid and BTAC 0.7 g (0.0038 mol) was added slowly. After dropping, the reaction was kept at 90 oC for 6 h and at 100 oC for about 3 h. FTIR was used to monitor the process of the reaction. The reaction was completed until the absorbance peak of TMBPBTH-EPOXY at 910 cm−1 disappeared. Subsequently, the mixture was dissolved in 600 mL toluene and washed three times with sodium hydroxide aqueous solution (2.5 wt%) and deionized water to remove the acrylic acid residue and inhibitor. Then, toluene was removed by rotary evaporation. The product was obtained and the yield was 78.5%. The synthesis process is shown in Scheme .
1H NMR (DMSO), δ (ppm), 5.81–6.38 (6H, (a, b)), 3.95–4.15, 4.17–4.38 (15H, c–e), 7.07–7.08, 6.54–6.59, 6.39–6.45 (6H, f–h),1.11–1.17 (3H, i), 0.56–0.60 (3H, j), 1.77–1.85, 2.97–3.01 (2H, k), 1.52–1.62 (3H, l), 2.08–2.09 (3H, m).
IR: 3457 cm−1 (–OH), 2957, 2856 cm−1 (CH), 1723 cm−1 (C=O), 1635 cm−1 (C=C), 810 cm−1 (=C–H).
2.4.2 Synthesis of EA
A mixture of 40.82 g (0.12 mol) of CYD-127, and a small amount of hydroquinone monomethyl ether 0.14 g (0.0011 mol) as inhibitor was placed into a 500 mL four-necked bottle equipped with stirrer, dropping funnel, thermometer and condenser, heated to 80 oC Citation[18]. A mixture of 18.35 g (0.25 mol) acrylic acid and BTAC 0.41 g (mol) was added slowly. After dropping, the reaction was kept at 80 oC for 6 h and at 90 oC for about 4 h. FTIR was used to monitor the process of the reaction. The reaction was completed until the absorbance peak of CYD-127 at 915 cm−1 disappeared. Subsequently, The mixture was dissolved in 500 mL chloroform and washed three times with sodium hydroxide aqueous solution (2.5 wt%) and deionized water to removed the acrylic acid residue and inhibitor. Then, chloroform was removed by rotary evaporation. The product was obtained and the yield was 80.3%.
IR: 3443 cm−1 (–OH), 2965, 2873 cm−1 (CH), 1725 cm−1 (C=O), 1634 cm−1 (C=C), 810 cm−1 (=C–H)
1H NMR (CDCI3), δ (p pm), 7.12–7.14, 6.80–6.82 (8H, Ar–H), 4.24–4.27, 3.94–3.98 (4H,O–CH2–CH), 4.31–4.35 (2H,–CH–OH), 4.36–4.39, 4.16–4.18 (4H, –CH2–OC(=O)), 2.06–2.12 (2H, –OH), 1.90 (6H, –CH3), 6.43–6.47, 6.13–6.19, 5.86–5.89 (6H, –CH = CH2).
3 Results and discussion
3.1 Photopolymerization kinetics
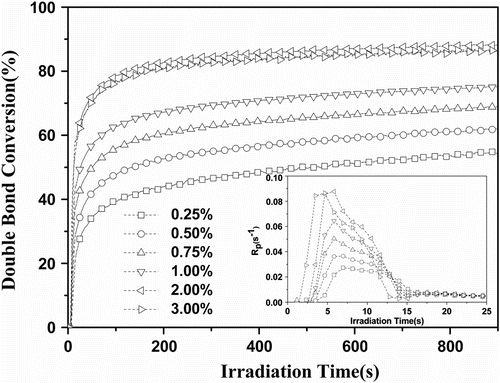
The concentration of the photoinitiator is an important factor influencing on the final conversion and curing rate. Generally, at low initiator concentrations, the photopolymerization rate tended to increase as the amount of the photoinitiator increases. However, increasing the photoinitiator concentration did not always lead to an increase in the cure rate. Figure shows the results of the photopolymerization of Tri-EA/SR306 initiated by 1173 when the light intensity was 25 mW/cm2. There was an optimum cure rate (0.088 s−1 which was obtained at 2.0 wt% 1173). Further increase in the concentration of 1173 did not produce a corresponding increase in cure rate. This might be attributed to the light absorption process with higher photoinitiator concentrations consisting of the light screening of the initiator itself and its photolysis products and the absorptivity of the photoinitiator.
Figure 1 Effect of photoinitiator concentration on photopolymerization of 75%Tri-EA + 25% SR306. Inset: R p plots (I = 25 mW/cm2).
Radical photoinitiators were generally divided into two classes according to the process by which the initiating radicals were formed. Compounds that underwent unimolecular bond cleavage upon irradiation were termed type I photoinitiators. If the excited-state photoinitiator interacted with a second molecule (a coinitiator) to generate radicals in a bimolecular reaction, the initiating system was termed a type II photoinitator Citation[19,20]. The effects of different photoinitiators on the photocuring of Tri-EA/SR306 are shown in Figure . Four photoinitiator systems were investigated. The final DCs with 1173, 184, BP/EDMAB, and ITX/EDMAB as the initiators were 75.3, 69.1, 68.8, and 73.7%, respectively, and the maximum R
p (, per second) were 0.0645, 0.0515, 0.0306, and 0.0635 s−1, respectively.
Figure 2 Effect of different photoinitiators on the photopolymerization of 75%Tri-EA + 25% SR306. Inset: R p plots (I = 25 mW/cm2).
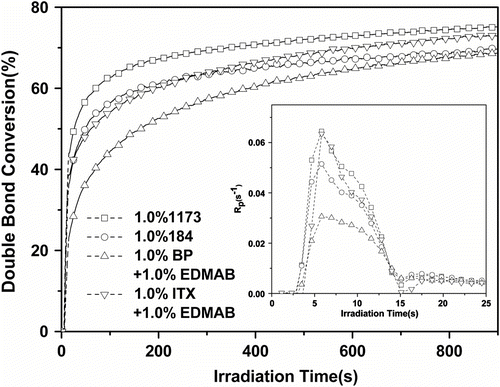
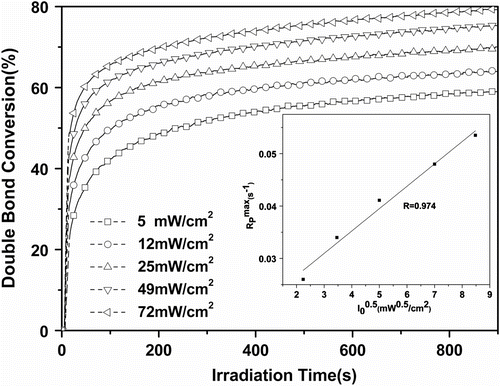
Figure shows the effect of light intensity on the conversion and curing rate of Tri-EA/SR306 when 1.0 wt% 1173 was used as the initiator. The final double bond conversion of Tri-EA/SR306 increased from 59.1 to 80.2% when the light intensity varied from 5 to 72 mW/cm2. The obtained results indicate that and final DC increase with the increase in light intensity. It could be attributed to the increase in radical species and a temporary excess of free volume, which increased the mobility of unreacted double bonds and allowed higher degrees of conversion to be reached than in volume equilibrium systems Citation[21].
Figure 3 Effect of the light intensity on the photopolymerization of 75%Tri-EA + 25% SR306. Inset: linear regressions with R = 0.974 (1173 = 1.0%).
The photopolymerization rate equations could be expressed as follows:
where [M]0 is the monomer concentration, kp
and kt
are kinetic rate constants, is the quantum yield, ϵ is the photoinitiator molar extinction coefficient, I
0 is the light intensity of the UV radiation, and [A]0 is the photoinitiator concentration.
At a constant concentration of monomer [M]0, photoinitiator [A]0, a linear plot of Rp
vs.
I
0
0.5 should be obtained. Figure presents the plot of the maximum rate of photopolymerization vs. I
0
0.5. It was a straight line (R = 0.974), which meant that the relationship of the Rp
and the light intensity I
0
0.5 matched the equation well.
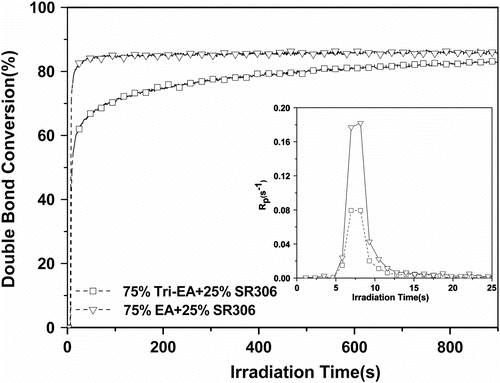
The reactive diluent used to lower the formulation viscosity played a key role, for it acted both on the cure speed and on the polymerization extent, as well as on the properties of the cross-linked polymer formed Citation[22]. Figure shows that a decrease in the functionality of the reactive diluent accelerated the curing rate of Tri-EA mixture. And Table shows that HEA gave better dilution to Tri-EA than other diluents. It suggests that the initial resin viscosity is a major factor in controlling polymerization kinetics rate and the final conversion of Tri-EA mixture at a given (initial) viscosity range.
Figure 4 Conversion vs. irradiation time plot for Tri-EA with different functional reactive diluents. Inset: plots (1173 = 1.0%, I = 25 mW/cm2).
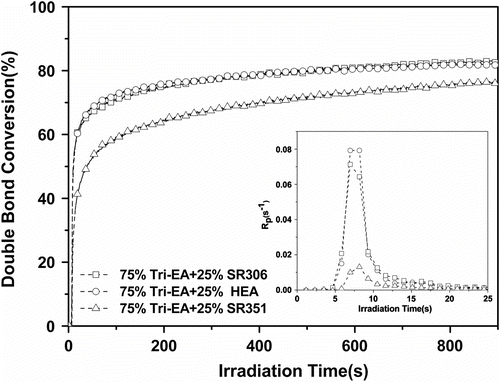
The reactivity of the UV-curable resin depends on the functionality and chemical structure of the acrylated oligomer used to cure, as well as on its viscosity, which affects the propagation and termination rate constants. Figure shows comparison of R p and conversion for Tri-EA/SR306 and EA/SR306. The Tri-EA/SR306 system exhibited lower R p max (0.0713 s−1) and final conversion (83.1%) than that of the EA/SR306 system (0.182 s−1 and 85.7%). Tri-EA resins contain stiffer center core structure than the bisphenol A core structure of EA. Moreover, the number of hydroxyl groups on Tri-EA is more than that on EA. This made Tri-EA/SR306 system possess stronger hydrogen bonding which affected the initial viscosity of mixture through its hydroxyl groups than that EA/SR306 system. According to the above results, Tri-EA which has a stiffer center core structure and higher viscosity than EA could be responsible for limiting to maxima rate and conversion.
3.2 Mechanical properties
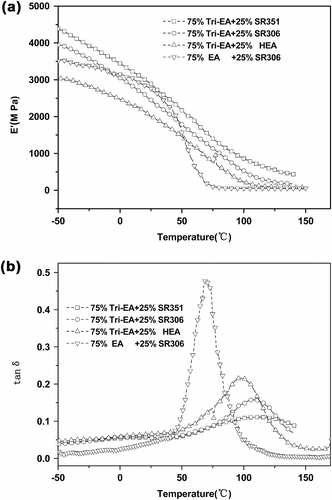
The mechanical properties of the photopolymers were measured by DMA. Figure show typical profiles recorded by DMA for UV-cured samples through the monitoring of the variation of the storage modulus and the tensile loss factor with an increase in the temperature. The chemical structure and functionality of the monomer used as a reactive diluent and the oligomer employed had a great influence on mechanical properties. For Tri-EA with different functional reactive diluent systems, the glass-transition temperatures and storage modulus of the cured samples increased as the functionality of the reactive diluent increased. For di- and tri-epoxy acrylate with same reactive diluent SR306, the T g and storage modulus of the Tri-EA systems was greater than that of EA systems.
The behavior of the storage modulus as a function of temperature provides clues regarding the nature of the polymer network being evaluated. The cross-linking density (ρ) of the cured specimens was calculated from the equilibrium storage modulus in the rubber region over the a-relaxation temperature according to the rubber elasticity theory, as follows Citation[4,23–25]:
where G′ was the storage modulus in the rubbery plateau, R was the gas constant, and T was the absolute temperature at Tα + 30 °C temperature (K) (Tα is the a-relaxation temperature). The ρ is summarized in Table . An increase in the functionality of the reactive diluents added to Tri-EA was shown to increase cross-link density. Tri-EA/SR351 obtained the most cross-linking density 434.67 mmol/cm3. It makes the cured polymer harder, but less flexible and more brittle. Comparing Tri-EA and EA with same reactive diluents SR306 showed Tri-EA/SR306 had higher cross-linking density. It was as expected because the more rigid the network, the more restricted the mobility of the segments and thus, the higher the T g.
The pencil hardness of coatings, which affect the abrasion and scratch resistance, depended on the T g as well as cross-link density. Table shows that the Tri-EA system possessed higher hardness when compared with the two-functional EA/SR306 system. This was because Tri-EA system had higher T g and cross-link density than that of EA/SR306 system.
3.3 The thermal stability
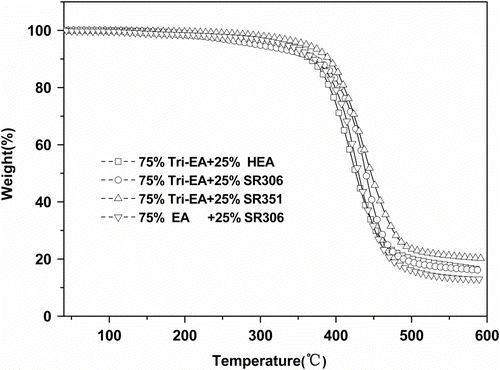
The thermal stability of polymer was greatly influenced by the structure, chemical composition, kind and concentration of remaining polar groups, cohesive energy between molecular chains, molecular chain rigidity, different interaction parameters, and other chemical structural factors like steric strain, conformational arrangements of groups, etc. Citation[23,26,27]. Figure and Table shows the TGA measurements of different cured films in N2. The temperature corresponding 10% weight loss, T d,10%, was taken as an index of thermal stability Citation[28,29]. For Tri-EA with different functional reactive diluents systems, it could be seen that the thermal stability of Tri-EA exhibited a maximum with Tri-EA/SR351 system. The thermal stability was controlled by two factors: the rigid moieties and cross-linking density. For Tri-EA systems, cross-link density increased with the increase of the reactive diluents functionality. And therefore, Tri-EA/SR351 system obtained best thermal stability. Comparing Tri-EA and EA with same reactive diluents SR306 showed Tri-EA/SR306 had better thermal stability than that of EA/SR306 because Tri-EA had the more rigid moieties and higher cross-linking density than that of EA.
Table 2. TGA of cured flims.
3.4 Adhesive strength
The adhesive strength at the interface was decided depending on three factors Citation[30]: specific interaction like electrostatic interaction (hydrogen bond and van der Waals force), effect of polymerization shrinkage when coatings are cured, and other factors (an anchor effect at the interface). Figure showed that average adhesion strength of Tri-EA containing HEA, SR306, and SR351 on glass substrate is 3.74, 2.51, and 1.65 MPa, respectively. Shrinkage, or volume reduction, of the resins, which occurs during the curing process would affect its adhesion strength because high shrinkage might induce a considerable amount of internal stress that pulls the adhesive resin away from the substrate. Polymerization shrinkage could increase with the increase of the reactive diluents functionality. The hydroxyl groups are able to form hydrogen bonds on the glass substrate. Appropriate amount of hydroxyl groups of monomer was also a critical factor to the adhesion strength of photo-curable resins. Reactive diluents HEA contained hydroxyl group. Tri-EA containing HEA possesses the highest adhesion strength. This could be mainly due to the lowest degree of shrinkage and largest hydroxyl group.
Figure 8 The adhesive strength of different cured systems in glass (A) 75%Tri-EA + 25% HEA, (B) 75%Tri-EA + 25% SR306, (C) 75% Tri-EA + 25% SR351, and (D) 75% EA + 25% SR306.
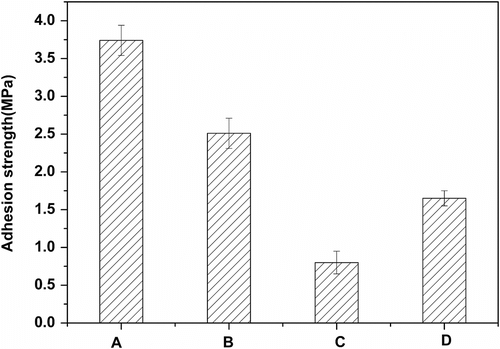
Comparison of adhesion strength for EA and Tri-EA with SR306 was also shown in Figure . Tri-EA /SR306 showed higher adhesion strength in comparison with EA/SR306. The Tri-EA contained three hydroxyl groups, which is one hydroxyl group more than EA. The hydroxyl groups are able to form hydrogen bonds on the glass substrate. Tri-EA /SR306 could form more hydrogen bonds on the glass substrate than the EA/SR306. Therefore, one can estimate that more hydroxyl groups on the glass substrate were responsible for the better adhesion.
4 Conclusion
A Tri-EA was successfully synthesized via trifunctional epoxy and acrylic acid. The results showed that different functional reactive diluents had a significant effect on kinetics and properties of the cured systems. Though those of Tri-EA had lower cure speed and the polymerization extent than EA, the cured Tri-EA films exhibited higher T g, higher hardness, higher thermal stability, and better adhesive strength than that of difunctional epoxy acrylate (EA).
Acknowledgments
This work was supported by the Open Fund from State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology. This study was also supported by the Natural Science Foundation of Jiangsu Province (BK2010190), Key Laboratory for Green Chemical Process of Ministry of Education (GCP201002).
References
- Bajpai , M , Shukla , V and Kumar , A . 2002 . Film performance and UV curing of epoxy acrylate resins . Prog. Org. Coat. , 44 : 271 – 278 .
- Zhang , JY , Windall , G and Boyd , IW . 2002 . UV curing of optical fibre coatings using excimer lamps . Appl. Surf. Sci. , 186 : 568 – 572 .
- Oprea , S , Vlad , S , Stanciu , A and Macoveanu , M . 2000 . Epoxy urethane acrylate . Eur. Polym. J. , 36 : 373 – 378 .
- Chattopadhyay , D , Panda , SS and Raju , K . 2005 . Thermal and mechanical properties of epoxy acrylate/methacrylates UV cured coatings . Prog. Org. Coat. , 54 : 10 – 19 .
- Kardar , R , Ebrahimi , M , Bastani , S and Jalili , M . 2009 . Using mixture experimental design to study the effect of multifunctional acrylate monomers on UV cured epoxy acrylate resins . Prog. Org. Coat. , 64 : 74 – 80 .
- Pelletier , H , Belgacem , N and Gandini , A . 2006 . Acrylated vegetable oils as photocrosslinkable materials . J. Appl. Polym. Sci. , 99 : 3218 – 3221 .
- Xu , G and Shi , W . 2005 . Synthesis and characterization of hyperbranched polyurethane acrylates used as UV curable oligomers for coatings . Prog. Org. Coat. , 52 : 110 – 117 .
- Zhu , SW and Shi , WF . 2002 . Synthesis and photopolymerization of hyperbranched polyurethane acrylates applied to UV curable flame retardant coatings . Polym. Int. , 51 : 223 – 227 .
- He , Y , Zhou , M , Wu , B , Jiang , Z and Nie , J . 2010 . Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment . Prog. Org. Coat. , 67 : 264 – 268 .
- Li , Z , Xiao , M and Nie , J . 2008 . Synthesis and photopolymerization of 2-(acryloyloxy) ethyl pyrrolidine-1-carboxylate . Des. Monomers Polym. , 11 : 235 – 242 .
- Kagawa , E , Kume , S and Tanaka , H . 2009 . Preparation and properties of IPN materials containing bisphenol A acrylate and an epoxide hybrid unit . Des. Monomers Polym. , 12 : 497 – 510 .
- Xiao , M , He , Y and Nie , J . 2008 . Novel bisphenol A epoxideacrylate hybrid oligomer and its photopolymerization . Des. Monomers Polym. , 11 : 383 – 394 .
- Kannurpatti , AR , Anseth , JW and Bowman , CN . 1998 . A study of the evolution of mechanical properties and structural heterogeneity of polymer networks formed by photopolymerizations of multifunctional (meth) acrylates . Polymer. , 39 : 2507 – 2513 .
- Cheng , J , Li , J and Yang , WT . 2009 . Curing behavior and thermal properties of trifunctional epoxy resin cured by 4,4′diaminodiphenylmethane . J. Appl. Polym. Sci. , 114 : 1976 – 1983 .
- Cheng , J , Li , J and Zhang , J . 2009 . Curing behavior and thermal properties of trifunctional epoxy resin cured by 4,4′-diaminodiphenyl sulfone . Express Polym. Lett. , 3 : 501 – 509 .
- Esen , DS , Karasu , F and Arsu , N . 2011 . The investigation of photoinitiated polymerization of multifunctional acrylates with TX-BT by photo-DSC and RT-FTIR . Prog. Org. Coat. , 70 : 102 – 107 .
- Li , H , Niu , R , Yang , J , Nie , J and Yang , D . 2011 . Photocrosslinkable tissue adhesive based on dextran . Carbohydr. Polym. , 86 : 1578 – 1585 .
- Pan , G , Wu , L , Zhang , Z and Li , D . 2002 . Synthesis and characterization of epoxy-acrylate composite latex . J. Appl. Polym. Sci. , 83 : 1736 – 1743 .
- Arsu , N and Aydın , M . 1999 . The effect of amines on the polymerization of methyl methacrylate . Die Angew. Makromol. Chem. , 266 : 70 – 74 .
- Temel , G , Enginol , B , Aydin , M , Balta , DK and Arsu , N . 2011 . Photopolymerization and photophysical properties of amine linked benzophenone photoinitiator for free radical polymerization . J. Photochem. Photobiol., A , 219 : 26 – 31 .
- Shi , SQ and Nie , J . 2008 . Dimethacrylate based on cycloaliphatic epoxide for dental composite . Dent. Mater. , 24 : 530 – 535 .
- Decker , C . 1996 . Photoinitiated crosslinking polymerisation . Prog. Polym. Sci. , 21 : 593 – 650 .
- Hill , LW . 1997 . Calculation of crosslink density in short chain networks . Prog. Org. Coat. , 31 : 235 – 243 .
- Park , SJ and Jin , FL . 2004 . Thermal stabilities and dynamic mechanical properties of sulfone-containing epoxy resin cured with anhydride . Polym. Degrad. Stab. , 86 : 515 – 520 .
- Xiao , M , Li , Z and Nie , J . 2011 . Synthesis and photopolymerization of 2‐(acryloyloxy) ethyl piperidine‐1‐carboxylate and 2‐(acryloyloxy) ethyl morpholone‐4‐carboxylate . J. Appl. Polym. Sci. , 119 : 1978 – 1985 .
- Chattopadhyay , D , Rohini Kumar , D , Sreedhar , B and Raju , K . 2004 . Thermal stability and dynamic mechanical behavior of acrylic resin and acrylic melamine coatings . J. Appl. Polym. Sci. , 91 : 27 – 34 .
- Krongauz , VV and Ling , MTK . 2009 . Photo-crosslinked acrylates degradation kinetics . J. Therm. Anal. Calorim. , 96 : 715 – 725 .
- Pan , G , Du , Z , Zhang , C , Li , C , Yang , X and Li , H . 2007 . Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety . Polymer. , 48 : 3686 – 3693 .
- Snow , AW and Buckley , LJ . 1997 . Fluoromethylene cyanate ester resins. Synthesis, characterization, and fluoromethylene chain length effects . Macromolecules. , 30 : 394 – 405 .
- Nakayama , N and Hayashi , T . 2008 . Synthesis of novel UV-curable difunctional thiourethane methacrylate and studies on organic–inorganic nanocomposite hard coatings for high refractive index plastic lenses . Prog. Org. Coat. , 62 : 274 – 284 .