Abstract
Network homo- and copolymers were prepared by photo- and thermal radical polymerizations of urethane dimethacrylate bearing photo-initiating Irgacure unit (IrDM) at the same molecule, and their thermal, optical, and mechanical properties were investigated. In the photopolymerization, the resulting IrDM homopolymer indicated the highest glass transition temperature and thermal stability among homo- and copolymers of IrDM and MMA studied. In particular, IrDM homopolymer has the excellent environmental stability, and the coloration of IrDM copolymers and poly(MMA) observed by, heating seems to arise from the additional reactions of free initiator fragment and monomer remained unreacted in the system. For optical properties, all their polymers represented high transparency and refractive index (RI), but the mechanical properties much depended on the contents of IrDM unit in the copolymer. In the thermal copolymerization of IrDM and MMA using benzoyl peroxide (BPO) as an initiator, the resulting copolymers have still a photo-initiating unit at the bridge in the network structure, and post photo-irradiation to the polymer changed the mechanical performances. In addition, the copolymers of IrDM with 2-hydroxy methacrylate and acrylic acid (AA) were also prepared and their unique functionalities were investigated.
1. Introduction
Multi-functional polymers have served as unique materials in the fields of nano-composites, adhesives, coatings, printing inks, and so forth Citation[1–5], and they have been prepared by polymer blending, polymer networking, copolymerization, and polymerization of multi-functional monomers. Preparation of such polymers by the radical polymerization of functional monomer, in particular, has attracted much attention because of ease of molecular design and handling, and economical advantage Citation[6]. Among functional monomers, methacrylate-based specific monomers have often been employed in such a polymerization since methacrylates are a popular molecule and easily available, and they can often generate the polymer having characteristic physical properties, i.e. strong adhesiveness, transparency, flexibility, and easiness of processing Citation[7,8]. Thus, various types of methacrylate-based monomers modified by the functional units including epoxide, bisphenol, phthalic acid, and urethane moieties have so far been synthesized and polymerized by a radical initiator Citation[5,9,10]. It has been pointed out, however, that the products are more or less colored by exposing to air, and the coloration is not so effective not only for physical property but also for processability, colorability, esthetics, and optical property Citation[11]. Such coloration, for instance, has been believed to be due to an autoxidation Citation[12], but the chemical species inducing the coloration have never been specified. Some authors speculated a free initiator fragment remained in a polymer matrix to be one of the chemical species inducing the coloration Citation[13,14]. For the effect of the free initiator, it can be readily proved by analyzing the dependence of the coloration on the initiating systems. For instance, the coloration may be reduced in the initiating system where the initiator is bound in a polymer chain since the side reaction inducing the coloration is depressed by the reduction of initiator reactivity due to the restricted mobility. Unfortunately, however, the polymerization of the methacrylate bearing an initiating unit at the same molecule has little been known.
In the present study, in order to examine the effect of initiator on the coloration and to obtain an environmentally-stable polymer, we designed the dimethacrylate monomer bearing a photo-initiating Irgacure unit, i.e. Irgacure dimethacrylate (IrDM), and polymerized it without initiator or using photo and thermal initiators.
2 Experimental
2.1 Materials
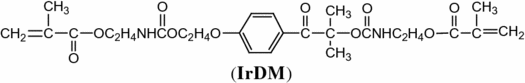
IrDM was prepared by adding dropwise the benzene solution (100 mL) of 2-isocyanatoethyl methcrylate (Showa Denko) (155.15 g, 1.0 mol) to the 4-methyl-2-pentanone (350 mL) solution of Irgacure 2959 (BASF) (112.15 g, 0.5 mol) below 40 °C in the presence of a small amount of dibutyltin dilaurate (0.07 g) in a 1 L four necked round bottom flask and further continuous reaction for 12 h at 80 °C. Complete reaction was confirmed by no detectable isocyanate absorption (2270 cm−1) in IR spectrum of the reaction mixture. Purification of the product was performed by pouring the reaction mixture into benzene to remove insoluble Irgacure 2959 and catalyst and by evaporating the benzene from IrDM. IrDM (while powder); mp: 84–85 °C, yield: 260.35 g (97.4%). IR (neat): 3325 (ν N–H), 1730 (ν C=O), 1640 cm−1 (ν C=C). 1H-NMR (CDCl3, TMS, ppm): 1.67 (s, 6 h), 1.93 (s, 6H), 3.32 (dt, J = 5.0, 6.0 Hz, 2 h), 3.52 (dt, J = 5.0, 6.0 Hz, 2 h), 4.01 (t, J = 6.0 Hz, 2 h), 4.19 (t, J = 6.0 Hz, 2 h), 2 h), 4.23 (t, J = 6.0 Hz, 2 h), 4.43 (t, J = 6.0 Hz, 2 h), 5.05 (t, J = 5.0 Hz, 1 h), 5.12 (t, J = 5.0 Hz, 1 h), 5.59 (d, J = 5.0 Hz, 1 h), 6.10 (d, J = 5.0 Hz, 1 h), 6.88 (d, J = 8.0 Hz, 2 h), 8.05 (d, J = 8.0 Hz, 2 h).
Commercial grade (Wako Chemical) monomers including methyl methacrylate (MMA), (AA), and 2-hydroxyethyl methacrylate (HEMA) were purified by ordinary methods. (BPO) used as a thermal initiator was purified by reprecipitation from chloroform solution to methanol. CDCl3 used for NMR measurement was purchased from Acros Organics and used without further purification.
2.2 Preparation of polymer
IrDM and comonomers, for instance, were mixed and degassed by heating at 85 °C under vacuum for 10 min, and the photopolymerization was carried out under the irradiation of 365 nm LED lamp (Nichia Co. NC4U13x) at ambient temperature for a given time in the mold (50 mm × 25 mm × 2 mm) made from Tefron plates clamped and sealed with 2 mm thick Pyrex glass (Edmund Optics) which was coated with polypropylene film to avoid a sticking to the specimen. The homopolymerization of IrDM under LED irradiation was also carried out at ambient temperature, i.e. below melting point of IrDM, in the manner similar to the copolymerization. In this case, IrDM which was once melted over 85 °C would no longer return to the original white solid even after cooling to ambient temperature, and it maintained a homogeneous high viscous transparent fluid during the polymerization. In the thermal copolymerization of IrDM with MMA and AA (BPO, 1 wt.%) was added as an initiator into the degassed monomers, and then the reaction mixture was heated at 80 °C for 5 h in a constant temperature oven. Photo-irradiation of the thermally prepared copolymer of IrDM was carried out under the irradiation of 365 nm LED lamp at ambient temperature for 5 h. Poly(MMA) used for the reference experiments was prepared by the photopolymerization of MMA in bulk with Irgacure 2959 (4.9 wt.%) initiator under the irradiation of 365 nm LED lamp at ambient temperature for 5 h.
2.3 Measurements
1H-NMR spectra were recorded on JEOL EX-400 (400 MHz) spectrometer at 20 °C in CDCl3, in which TMS was used as an internal standard. FT-IR spectra were measured in the 4000–400 cm−1 region on Jasco FT/IR-230 spectrometer in neat or KBr pellet for the molecular structure determination of reagents, monomers, and polymers at ambient temperature. Electron spin resonance (ESR) spectrum was recorded using a polymer film on JEOL-1XG spectrometer equipped with an X-band microwave unit and 100 kHz field modulation at ambient temperature. Coupling constant (a) and g-value were determined on the basis of Mn2+ standard sample. Transmittance (T) in the UV spectrum of the polymer was recorded using a film sample with thickness of 50 ± 1 μm on Ocean Optics S2000 spectrophotometer. The RI (n D) of polymers was measured with an Atago Abe refractometer, in which the matching oil used in this system is 1.6570.
Shore D hardness was measured using Ueshima UF shore’s durometer. The mechanical properties were evaluated with Shimadzu model AG-50kND at 25 °C using the specimen with the size of 50 mm × 25 mm × 2 mm. Thermogravimetrical analysis (TGA), differential TGA (DTG), and differential scanning calorimetry (DSC) were performed at a heating rate of 10 °C/min with Seiko TG/DTA220 and Seiko DSC22, respectively under a nitrogen stream (30 mL/min).
3 Results and discussion
3.1 IrDM polymers prepared by photo-irradiation
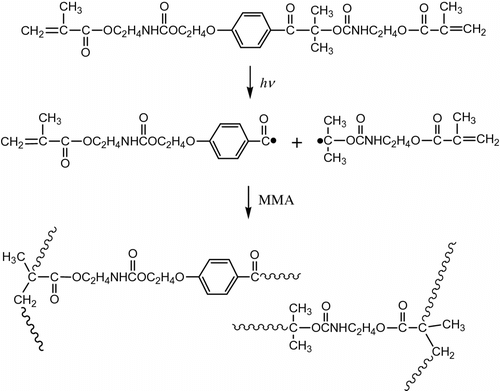
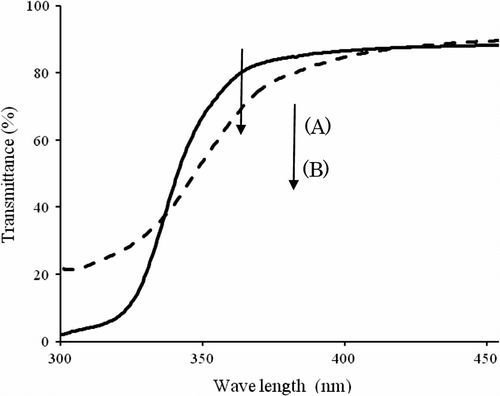
Homopolymerization of IrDM and the copolymerization with MMA were carried out in bulk under the irradiation of 365 nm LED lamp at ambient temperature for the reaction time of 0.5, 1, 3, and 5 h, in which IrDM content in the monomer feed in the copolymerization was limited to be less than 20 wt.% because of poor solubility of IrDM in MMA. Both homo- and copolymerizations proceeded smoothly without additional initiator according to Scheme , and it gave quantitatively a polymer even after the reaction time of 3 h. The resulting polymers were transparent, and insoluble in most of the organic solvents including acetone, diethyl ether, toluene, and tetrahydrofuran because of the cross-linked structure (Scheme ), but they became soluble in organic solvents after the decomposition of the urethane bond in the bridged chain of the network polymer by the reaction with H2SO4. Table represents the optical properties of the polymers obtained, in which theoretical (RI) Citation[15] was also listed for comparison. It is obvious that all polymers obtained represent the transmittance as high as over 97% in the visible regions at 470 and 600 nm, and the measured RI is similar to the calculated RI, but the former tends to be higher than the latter because of the increased density of polymer by cross-linking. It is noted that the transparency of poly(MMA) film prepared by the photopolymerization using Irgacure2959 initiator turned to yellow under the heating at 100 °C for 100 h in air in contrast to PIrDM100 and PIrDM20 which were still transparent. Such a coloration is demonstrated by the red shift of the absorption band around 360∼400 nm of poly(MMA) as seen in the UV spectra in Figure , which may be due to the appearance of some new coloring product having the absorption band around 360∼400 nm which corresponds to a complementary color region of yellow. This is indicative of the usefulness of the initiator-binding to the polymer chain to maintain the stability and transparency.
Table 1. Transmittance and RI of PIrDM and poly(MMA).
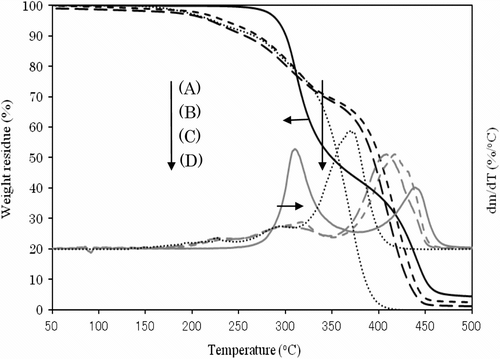
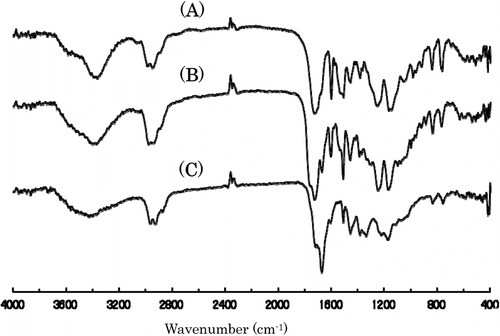
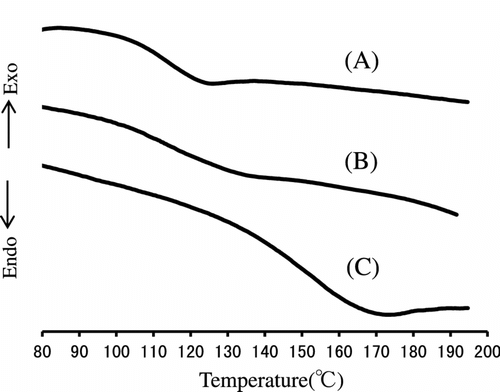
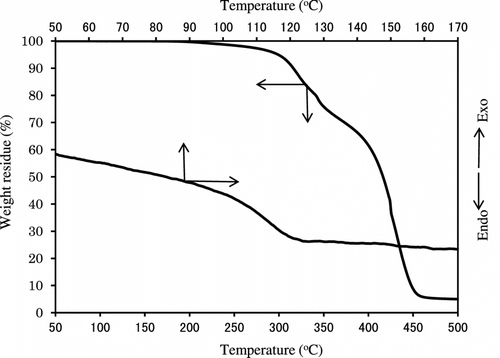
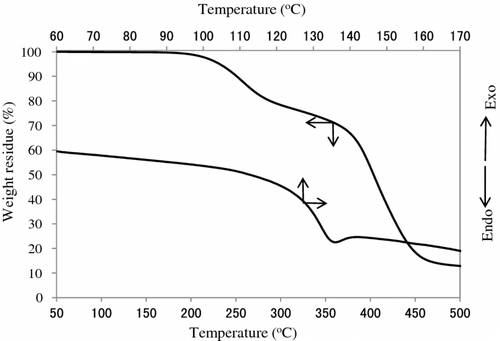
Figure shows the variation of the TGA and DTG curves with the copolymer composition. All polymers other than poly(MMA) represent two step degradations having DTG peaks of about 310 and 410∼440 °C. Table lists the weight loss temperatures in 5, 10, and 50% losses of polymer. It is obvious that the weight loss in the first step at 310 °C of DTG increases with increasing IrDM contents in the polymer, but that in the second step at 410∼440 °C of DTG becomes low in PIrDM100. Therefore, the degradation seems to start from the bond scission of the bridged urethane unit, and follow the elimination of MMA unit. This is supported by the IR spectral change of PIrDM100 pyrolyzed at 290 and 410 °C for 5 min. As seen in Figure , the absorptions due to the NH stretching at 3350 cm−1 and the bending at 1545 cm−1 of the urethane moiety are reduced in an early stage, and then the C=O stretching band around 1735 cm−1 due to the ester moiety decreases continuously, suggesting the degradation of the bridged urethane unit in the first step and then the ester unit in the second step. Glass transition temperature (Tg) which was estimated by the DSC curves shown in Figure is also listed in Table .
Table 2. Glass transition and weight loss temperatures of PIrDM and poly(MMA).
All PIrDM have the T g value higher than poly(MMA) (104 °C), and the T g of the copolymers is located in between the T g of each homopolymer. It is noted that even for PIrDM10 which contains only 2 mol-% (10 wt.%) cross-linked IrDM unit, the T g value becomes high. In particular, PIrDM100 has extremely high T g value in spite of containing the elastic urethane unit Citation[16], probably because of the poor mobility of chain segment by some suitable chain stacking.
Mechanical properties of PIrDM are summarized in Table . Young’s modulus and Shore D hardness are lowest in PIrDM100, and they are increased with increasing MMA contents in the copolymer. This might be due to the elastic, but slightly rigid character of the phenyl urethane moiety Citation[16]. Such a high T g and softness observed for PIrDMA100 will serve, for instance, as the advanced adhesive and sealing materials for new electronic devices like high power LED light. IrDM was also easily copolymerized with other acrylates including (HEMA), which can be expected to produce the polymer with both hydrophilic and hydrophobic segments at the same polymer Citation[17]. Figure shows the TGA and DSC curves of poly(IrDM-co-HEMA) (HEMA: 80 wt.%) obtained under the irradiation of 365 nm LED lamp at ambient temperature for 3 h. As clearly seen, the copolymer with HEMA shows the TGA and DSC (T g = 120 °C) curves similar to poly(IrDM-co-MMA), but the former has a hydrophilic character due to the hydroxyl group to form a glossy surface by exposing to water as expected Citation[18] in contrast to the latter.
Table 3. Mechanical properties and surface hardness of PIrDM and poly(MMA).
3.2 IrDM polymers prepared using thermal initiator
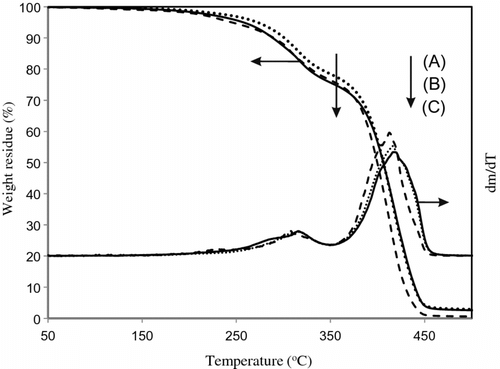
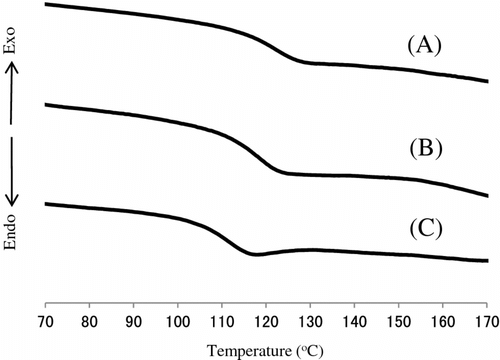
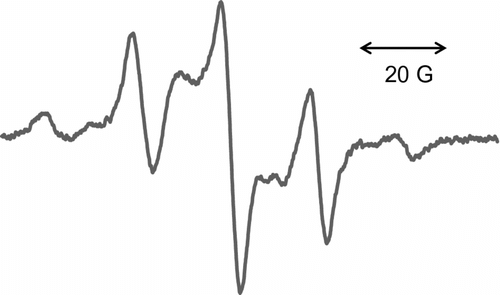
Thermal copolymerization of IrDM (10 and 20 wt.%) and MMA using BPO at 80 °C gave a copolymer, t-PIrDM10 and t-PIrDM20 respectively, in over 97% yield for the reaction time of 5 h although small amounts of IrDM monomer are unfortunately still remained unreacted in the resulting rigid network polymer matrix. In comparison with , the shape of TGA curves of poly(IrDM-co-MMA) are quite similar to each other, and even to the post photo-irradiated t-PLrDM20 shown in curve C in Figure and in Scheme . The T g value, however, becomes lower in the thermal polymers, i.e. t-PIrDM20 (T g = 128 °C) and t-PIrDM10 (122 °C) rather than the photopolymers, i.e. PIrDM20 (138 °C) and PIrDM10 (125 °C), as can be determined from the DSC curves in Figure . Moreover, the color of t-PIrDM20 was changed from transparency to yellow by photo-irradiation and the T g fairly reduced from 128 to 116 as seen in the curves A and C in Figure because of a destruction of the network structure and a generation of flexible branched chains. Figure shows the ESR spectrum of the photo-irradiated t-PIrDM20. The spectrum consists apparently of nine broad lines (a = 11.5G, g = 2.0035) which are composed of nine lines with a = 22.5 (CH3) and 11.5 G (CH2) and five lines with a = 22.5 (CH3) and 23.0 and 3.1 G due to an unequivalent β-CH2 group. This spectrum can be assigned to the propagating poly(MMA) radical Citation[19] which is generated by the addition of free BPO fragment radical to the MMA monomer remained in t-PIrDM20 network. The signal can be easily detected even after a long period of darkness since the diffusion or the mobility of the propagating radical is suppressed and the termination is much restricted in such a rigid matrix as the radical trapped in a heterogeneous system Citation[20] and adsorbed on silica gel Citation[21]. While, such a radical was not observed for the photo-polymerized PIrDM20. Therefore, the coloration may be attributed in part to the unreacted MMA monomer and free unbounded-BPO initiator remained in the system.
Scheme 3 Schematic representation of thermal copolymerization of IrDM and MMA and following photo-decomposition of copolymer.
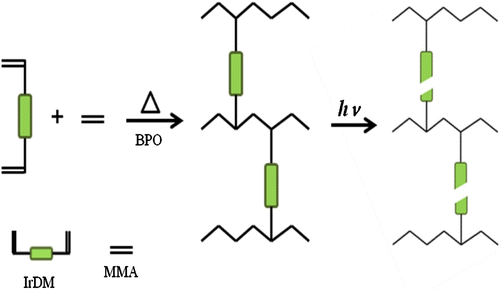
Table summarizes the variation of the optical, thermal, and mechanical properties of t-PIrDM20 with photo-irradiation. In particular, the values of stress at break and Young’s modulus in the three-point bending test extremely declines by the photo-irradiation, which might be due to the destruction of the network structure as depicted in Scheme . IrDM was also thermally copolymerized with hydrophilic AA monomer by BPO at 80 °C to obtain an amphiphilic copolymer. The copolymerization was carried out in higher content of AA (90 wt.% in monomer feed) in dimethylsulfoxide since the copolymerization in bulk or in lower contents of AA, e.g. 80 wt.%, brought about the precipitation of the resultant polymer during the polymerization. The resulting poly(IrDM-co-AA) represented a characteristic IR absorption at 1715 cm−1 due to the carboxylic group of the AA unit, and the TGA and DSC curves shown in Figure are similar to those of poly(IrDM-co-HEMA), in which Tg of poly(IrDM-co-AA) was determined to be 134 °C.
Table 4. Change of properties of t-PIrDM20 by photo-irradiation.
4 Conclusions
Network homo- and copolymers were synthesized through a radical polymerization using Irgacure-bound dimethacrylate (IrDM), MMA, HEMA, and AA monomers. All polymers obtained represented high transparency and RI, and IrDM homopolymer was outstanding in terms of glass transition temperature and thermal stability. Coloration to yellow, in particular, was not observed in the photo-polymers of IrDM even by heating at 100 °C in contrast to poly(IrDM-co-MMA) and poly(MMA) which were prepared with the initiator of BPO and free Irgacure 2959. This indicates that the coloration is attributed to the additional reaction of the initiator fragment and monomer remained freely in the system. It was consequently demonstrated that the unique functionality and thermal stability could be easily endowed to a polymer by the polymerization using the initiator-bounded dimethacrylate and by combining the photo- and thermal polymerizations.
Acknowledgment
The authors are grateful to Dr K. Masaki of Tokushima Prefectural Industrial Technology Center for help with physical properties measurements.
References
- Tanaka , H . 1992 . Recent developments in radical stabilization in polymers and in polymerization . Prog. Polym. Sci. , 17 : 1107 – 1152 .
- Peinado , C , Alonso , A , Salvador , EF , Baselga , J and Catalina , F . 2002 . Following in situ photoinitiated polymerization of multifunctional acrylic monomers by fluorescence and photocalorimetry simultaneously . Polymer. , 43 : 5355 – 5361 .
- Pan , G , Wu , L , Zhang , Z and Li , D . 2002 . Synthesis and characterization of epoxy-acrylate composite latex . J. Appl. Polym. Sci. , 83 : 1736 – 1743 .
- Inan , TY , Ekinci , E , Kuyulu , A and Gungor , A . 2002 . Preparation of novel UV-curable methacrylated urethane resins from a modified epoxy resin and isocyanatoethylmethacrylate (IEM) . Polym. Bull. , 47 : 437 – 444 .
- Kagawa , E , Kume , S and Tanaka , H . 2009 . Preparation and properties of IPN materials containing bisphenol A acrylate and an epoxide hybrid unit . Des. Monomers Polym. , 12 : 497 – 510 .
- Tanaka , H . 2003 . Captodative modification in polymer science . Prog. Polym. Sci. , 28 : 1171 – 1203 .
- Jehl , D , Widmaier , JM and Mayer , GC . 1983 . The transparency of polyurethane-poly(methyl methacrylate) interpenetrating and semiinterpenetrating polymer networks . Eur. Polym. J. , 19 : 597 – 600 .
- Barszczewska , R and Izabela , M . 2009 . Structure-property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA . Dent. Mater. , 25 : 1082 – 1089 .
- Puzin , YuI , Kraikin , VA , Galinurova , EI , Egorov , AE , Prokudina , EM and Monakov , Y . 2001 . Quantitative determination of phthalides in molecules of vinyl polymers and their role in radical polymerization . J. Appl. Chem. , 74 : 292 – 297 .
- Hermant , I , Danyanidu , G and Mayer , GC . 1983 . Transition behavior of polyurethane-poly(methyl methacrylate) interpenetrating polymer networks . Polymer. , 24 : 1419 – 1424 .
- Krajicek , DD . 1977 . The future of complete prosthodontics . J. Prosthetic Dentistry. , 37 : 126 – 132 .
- Sonnerskog , S . 1959 . Initiating mechanism of color formation and decomposition in poly(vinyl chloride) . Acta Chem. Scand. , 13 : 1634 – 1638 .
- Regel , W and Schneider , C . 1981 . Poly(maleic anhydride) – synthesis and proof of structure . Macromol. Chem. Phys. , 182 : 237 – 242 .
- Ham DO, Lankshear B, McGowan CB. Decay mechanisms of fluorescers in polymer hosts. In: Book of Abstract (Am. Chem. Soc. Nat. Meet.); 1998; Boston. PHYS-458.
- Van Krevelen , DW . 1972 . Properties of polymers , New York , NY : Elsevier .
- Choi , MC , Han , M , Kim , I and Ha , CS . 2010 . Highly transparent polymer substrates for flexible displays using semi-interconnected interpenetrating polymer networks . J. Nanosci. Nanotech. , 10 : 6829 – 6833 .
- Kim , JY , Shin , DH , Ryu , JH , Choi , GH and Suh , KD . 2004 . Synthesis of nanophase-separated poly(urethane-co-acrylic acid) network films and their application for magnetic nanoparticle synthesis . J. Appl. Polym. Sci. , 91 : 3549 – 3556 .
- Nair , PD and Krishnamurthy , VN . 1991 . Hydrophobic-hydrophilic interpenetrating polymer networks for biomedical applications . Polym. Sci. , 2 : 1003 – 1007 .
- Kamachi , M , Kohno , D , Liaw , DJ and Katsuki , S . 1978 . ESR study of the propagating radical in the polymerization of MMA in frozen state . Polym. J. , 10 : 69 – 75 .
- Tanaka , H , Sato , T and Ota , T . 1980 . Long-lived polymer radicals. 2. An ESR study on the reactions of the propagating polymer radicals of N-methylacrylamide and N-methylmethacrylamide with vinyl monomers at room temperature . Macromol. Chem. , 181 : 2421 – 2431 .
- Tanaka , H , Kameshima , T , Sato , T and Ota , T . 1987 . Decomposition of dimethyl 2,2’-azodiisobutyrate in the presence of silica gel and zeolites . Macromol. Chem. Rapid Commun. , 8 : 229 – 232 .