Abstract
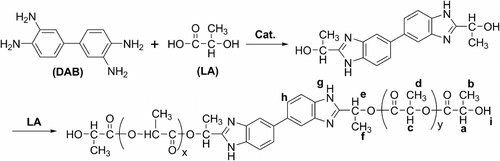
Directly starting from D,L-lactic acid (LA) and 3,3′-diaminobenzidine (DAB), poly(lactic acid-co-3,3′-diaminobenzidine) [P(LA-co-DAB)] as a kind of potential flame retardant is synthesized via melt polycondensation. When the molar feed ratio LA/DAB is 80/1, the optimal synthetic conditions, including type and dosage of catalyst, temperature, and time of copolymerization, are discussed. After the prepolymerization at 140 °C for 8 h, using 0.7 wt.% stannous oxide (SnO) as the catalyst, the melt copolymerization for 6 h at 160 °C gives the polymer with the biggest intrinsic viscosity ([η]) 0.71 dL • g−1. The structure and properties of P(LA-co-DAB)s at different molar feed ratios are systematically characterized by Fourier transform infrared spectroscopy, proton nuclear magnetic resonance (1H NMR), gel permeation chromatography, differential scanning calorimetry, and X-ray diffraction (XRD). With the increase of the molar feed ratio LA/DAB, the crystallinity and [η] of P(LA-co-DAB) increase gradually. When LA/DAB is 120/1, the biggest weight-average molecular weight (M w) is 5900 Da. The results of thermogravimetric analysis show that the decomposition temperature at 5% weight loss (T 5%) and char yield of the copolymers are higher than those of homopolymer poly(D,L-lactic acid). Furthermore, the more DAB in the feed content, the higher char yield. These indicate that P(LA-co-DAB) may play a flame retardant role when it is blended with polylactic acid.
1. Introduction
Polylactic acid (PLA) is a nontoxic, completely biodegradable polymer from lactic acid (LA). PLA has excellent physical and mechanical properties Citation[1–3], but poor thermal stability. Especially, its UL-94 vertical burning test only can achieve horizontal burning rating, and the limiting oxygen index value is low (only 20). There is serious melt dripping during the burning test. Therefore, with the increasingly widespread application of PLA in many fields, including textile industry, food packaging, daily necessities, electronic and electrical devices, automobile and aircraft industries, etc. the modification of PLA in the flame retardancy is becoming a hot research focus recently Citation[4,5].
Generally, PLA’s flame retardancy modification is mainly achieved by physical blending with flame retardants. Among those flame retardants, due to the superiority of high heat resistance, the solid polymeric flame retardants, especially ammonium polyphosphate (APP) Citation[6–9], aroused more and more interests in the past. However, APP also has many drawbacks, e.g. large adding dosage and poor compatibility, which affect the original performance of the PLA materials. Therefore, recently some phosphorus-containing PLA copolymers as novel compatible solid polymeric flame retardants were developed by Wang’s group, and their blending with PLA provided a novel route to permanently flame retard PLA Citation[10,11].
Benzimidazoles can be used as a kind of good flame retardant Citation[12,13], but there are no reports on their application in PLA so far. In the viewpoint of the preparation of benzimidazoles from starting materials, 2-(1-hydroxyethyl) benzimidazole can be obtained by the reaction of o-phenylenediamine with LA Citation[14,15]. And we can obtain 2-substituted benzimidazoles using o-phenylenediamine and different carboxylic acids under the condition of solvent-free and melting state Citation[16,17]. Therefore, if using o-phenylenediamine and LA as starting materials, their reaction under solvent-free and melting state may give 2-(1-hydroxyethyl) benzimidazole, and yield PLA terminated with benzimidazole moiety finally. Thus, it is possible to use the inherent benzimidazole moiety in the polymer to realize the PLA’s flame retardancy modification.
As this design, directly starting from D,L-lactic acid (D,L-LA) and 3,3′-diaminobenzidine (DAB), a novel copolymer, poly(lactic acid-co-3,3′-diaminobenzidine) [P(LA-co-DAB)] is synthesized via the melt polycondensation for the first time herein (Scheme ). After the discussions on the optimal synthetic conditions, the structure and properties of P(LA-co-DAB) are systematically characterized by FTIR, 1H NMR, [η], GPC, DSC, XRD, and TGA. The existence of benzimidazole structure in the center of copolymer chain makes P(LA-co-DAB) be a potential compatible solid polymeric flame retardant for PLA materials.
2 Experimental
2.1 Materials
D,L-LA was purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China), and DAB was purchased from J&K Scientific Ltd (Beijing, China). All other chemicals, including p-toluenesulfonic acid (TSA), stannous chloride (SnCl2), stannous oxide (SnO), zinc chloride (ZnCl2), and zinc oxide (ZnO), were commercially available as analytical grades from Guangzhou Chemical Reagent Factory (Guangzhou, China). All these materials were used without further purification.
2.2 Preparation of P(LA-co-DAB)
According to the previous works on melt copolymerization of LA Citation[18–22], LA and DAB should be prepolymerized before copolymerization. After LA and DAB were uniformly mixed as preplanned molar feed ratio, the mixture was directly dehydrated for 8 h at 140 °C under 4000 Pa in a flask equipped with mechanical stirring and thermometer. After prepolymerization, the selected catalyst was added in according to the weight percentage (wt.%) of dehydrated reactants. The melt copolymerization was carried out at a certain temperature (130–170 °C) and an absolute pressure of 70 Pa for 4–12 h. When the reaction finished, the purification via the dissolution in CHCl3 and the subsequent precipitation by the mixed solvent of methanol and distilled water (CH3OH/H2O) ordinarily produced a white powder after drying in vacuo.
2.3 Characterization
The intrinsic viscosity ([η]) of the copolymer P(LA-co-DAB) was determined with Ubbelohde viscometer (Cannon-Ubbelohde, State College, PA) using chloroform (CHCl3) as solvent at 25 °C. The relative molecular weight and molecular weight distribution of the polymer were determined by gel permeation chromatography (GPC) (Waters 1515 pump, Torrance, CA) with tetrahydrofuran as solvent and polystyrene as reference at 35 °C and a flow velocity 1 mL min−1. Three Styragel HR columns from Japan covering a molecular weight range of 1 × 103–106 Da were used and calibrated using five polystyrene narrow standards from BF Goodrich (Richfield, Ohio). Molecular weight distributions of the samples were calculated by the Millennium 2010 software from Waters and were reported as polystyrene equivalent values.
Infrared spectra were obtained from a Fourier transform infrared (FTIR) spectrometer (Bruker Vector 33, Ettlingen, Germany) by the dichloromethane (CH2Cl2) liquid film method. Proton nuclear magnetic resonance (1H NMR) spectra were recorded with a Varian NMR system 400 MHz (USA) with DMSO-d 6 as solvent and tetramethylsilane (TMS) as internal standard. With a wavelength of 1.5406 × 10−10 m and a scanning scope of 2θ from 5°–50° with Cu Kα radiation, Bruker D8 Advance X-ray diffractometer (Bruker, Germany) was used to investigate the crystallinity of the polymer.
Differential scanning calorimetry (DSC) was performed with a Perkin-Elmer DSC7 thermal analyzer (Perkin-Elmer, Cetus Instruments, Norwalk, CT). The samples for DSC measurements (an average weight of 4 mg) were scanned at a heating rate of 10 °C min−1 under a nitrogen atmosphere (flow velocity 20 mL min−1), then they were cooled to −50 °C for 5 min and heated again to 200 °C. The thermogravimetric analysis (TGA) was performed with STA-409PC thermal gravimetric analysis (Netzsch, Germany) at a heating rate of 10 °C min−1 under an air atmosphere (flow velocity 20 mL min−1).
3 Results and discussion
Using LA and DAB as starting materials, the copolymer P(LA-co-DAB)s with different molar feed ratios (LA/DAB = 20/1, 40/1, 80/1, 120/1, 160/1) are directly synthesized via the melt copolycondensation after the synthetic conditions are discussed. The structure and properties of these P(LA-co-DAB)s are characterized by FTIR, 1H NMR, GPC, DSC, XRD, TGA, and viscosity [η] measurements.
3.1 Optimal synthesis conditions
The influences of catalyst kinds on the synthesis of P(LA-co-DAB) are first investigated. There are many compounds used as catalysts in LA melt polymerization, e.g. proton acids, metal oxides, metal halides, and so on Citation[21–27]. Here, five common compounds are selected as catalysts, and their influences on the reaction are shown in Table . It can be observed that the reaction catalyzed by SnO gives higher [η] than that using other familiar catalysts, such as ZnO, ZnCl2, SnCl2, and TSA. As a second reason, the appearance of product using SnO is white as expected. Therefore, SnO is selected as the catalyst in the following experiments, mainly hoping for a higher molecular weight.
Table 1. The influences of different catalysts on the reaction.a
The influences of catalyst SnO quantity are shown in Table . It can be concluded that the [η] reaches a maximum value when the weight percentage of catalyst SnO quantity is 0.7 wt.% of the prepolymer (run 4). Once the quantity is too small, the reaction is so insufficient after a certain time that the [η] is not high. When the quantity is excessive, short-chain molecules are apt to be formed through the degradation of polymer, which is also catalyzed by the metal catalyst Citation[25,26]. Therefore, the suitable dosage of catalyst SnO is 0.7 wt.%.
Table 2. The influences of catalysts dosage on the reaction.a
The influences of different melt copolymerization temperatures on the reaction are shown in Table . With the increase of the melt polymerization temperature, the product’s [η] is also increased. When the temperature is 160 °C, the [η] reaches the maximum (run 4). These indicate that appropriate higher temperature is beneficial to remove the water from the reaction system. However, if the temperature is too high, partly thermal degradation of the product may take place and the appearance of product changes from white to yellowish when the system is at a high temperature for a long time (run 5). Thus, the appropriate temperature of the melt polymerization should be 160 °C.
Table 3. The influences of melt polymerization temperature on the reaction.a
The influences of melt copolymerization time on the reaction are shown in Table . It is obvious that the suitable time should be 6 h (run 2). When the reaction system is at a high temperature for a long time, the oxidation and thermal degradation of polymer become serious, so the [η] drops. Thus, when the molar feed ratio of LA/DAB is 80/1, the appropriate conditions for the synthesis of the copolymer P(LA-co-DAB) via direct melt copolycondensation are described as follows: catalyst SnO quantity 0.7 wt.%, reaction temperature 160 °C, and reaction time 6 h. In this case, the maximum [η] is 0.71 dL g−1 and the corresponding weight-average molecular weight (M w) is 4800 Da (Table ).
Table 4. The influences of melt polymerization time on the reaction.a
Table 5. The influences of different molar feed ratios on yield, [η] and M n of the copolymers.a
3.2 Structure characterization of P(LA-co-DAB)
Using P(LA-co-DAB) synthesized at the molar feed ratio LA/DAB 80/1 under the above optimal synthetic conditions as a representative (Figure ), the structure of copolymer P(LA-co-DAB) is characterized, respectively, with FTIR (Figure ) and 1H NMR spectroscopy (Figure ).
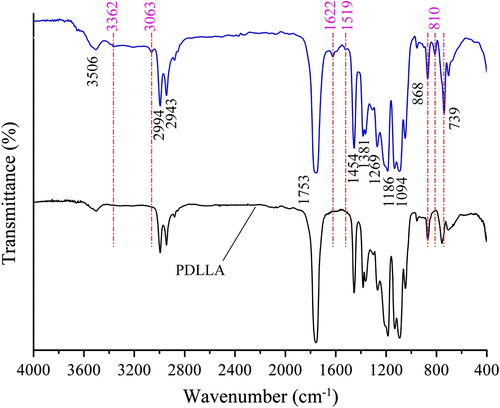
Compared with the homopolymer poly(D,L-lactic acid) (PDLLA) synthesized via direct melt polycondensation Citation[19], it is elucidated that these compounds show many similar absorptions in their FTIR spectra (Figure ). For example, a band at 3506 cm−1 is assigned to stretching vibration of O–H. The bands at 2994, 2943, and 2879 cm−1 are assigned to the saturated C–H stretching vibrations in the PLA chain, whereas their bending vibrations are observed at 1454 and 1381 cm−1. Of course, the strongest absorption of ester carbonyl at 1753 cm−1 and the stronger absorption of C–O–C at 1269, 1186 and 1094 cm−1 are very obvious in Figure . However, the adsorption peak at 3362 cm−1 assigned to the N–H stretching vibration and the bands at 3063, 1622, 1519, 810 cm−1 assigned to the structure of benzene ring are not observed in the PDLLA spectrum, indicating that DAB segment has been introduced into the copolymer. For the bands at 868 and 739 cm−1 usually assigned to the substituted benzene ring, their absorption strength becomes stronger than those corresponding bands in PDLLA, also suggesting the copolymer contains DAB segment (Figure ).
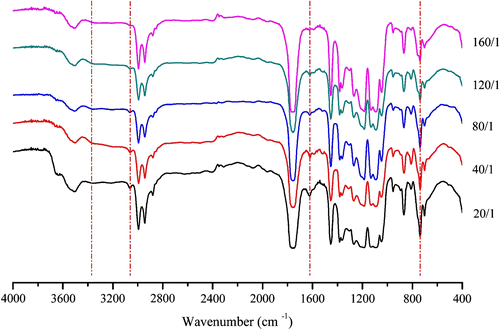
Furthermore, the structural studies on P(LA-co-DAB) copolymers with different molar feed ratios by FTIR (Figure ) show similar features. However, there are some differences in the strength of N–H stretch vibration absorption between the copolymers synthesized as different molar feed ratios. With the increase of LA content, the relative content of terminal N–H becomes less, so its absorption strength becomes smaller. Owing to the same reason, the absorption strength of the bands at 3063 and 1622 cm−1 assigned to the structure of benzene ring also becomes smaller with the increase of LA content.
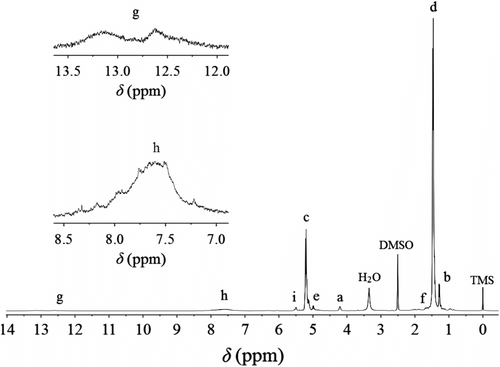
The data of 1H NMR spectrum of P(LA-co-DAB) synthesized as the molar feed ratio LA/DAB 80/1 (Figure ) are obtained as follows. 1H NMR (DMSO-d 6 as solvent and TMS as internal reference), δ, ppm: 1.28 (H b , CH3 in terminal PLA segment), 1.47 (H d , CH3 in PLA chain), 1.68 (H f , CH3 close to benzimidazole segment), 4.21 (H a , CH in terminal PLA segment), 4.99 (H e , CH close to benzimidazole segment), 5.04–5.30 (H c , CH in PLA chain), 5.48 (H i , OH in terminal PLA segment), 7.20–8.32 (H h , Ar–H in benzene ring), and 12.52–13.30 (H g , N–H in the benzimidazole segment). Except for the peaks involving benzimidazole, all data are similar to the reported literature Citation[10].
In a word, the examinations of FTIR and 1H NMR indicate that DAB moiety is incorporated into the polyester backbone, so the obtained products are copolymers indeed. And these conclusions are further confirmed by the following results of GPC test.
3.3 Influences of different molar feed ratios
The influences of different molar feed ratios on [η] and GPC results are shown in Table . Obviously, with the increase of the LA molar feed ratio, not only [η] increases gradually, but also number-average molecular weight (M n), M w and the polydispersity index (PDI, M w/M n) have the correspondingly increasing trend. Meanwhile, both the single peak phenomenon of all GPC curves and the lower PDI value (less than two) show that the product of direct melt copolycondensation from two monomers is indeed a copolymer P(LA-co-DAB) and not a mixture containing any homopolymer PDLLA. However, due to the partial LA conversion into lactide which can easily escape out of the reaction systems during the direct melt copolycondensation Citation[18–22], the tested LA content in the copolymer is lower than that in the feed ratios. For the same reason, the yields (21–38%) of P(LA-co-DAB)s are also slightly lower (Table ).
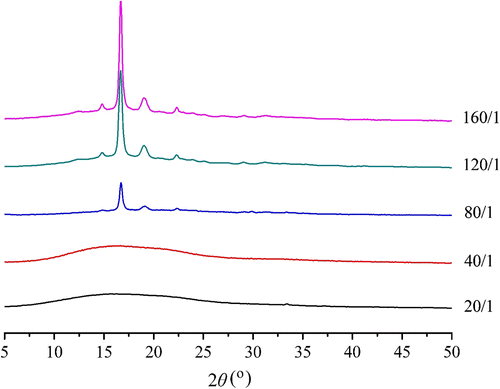
The crystallinity of polymers has great impacts on their physical and biological properties, especially their degradability. The XRD measurement results of the PLA copolymers modified by DAB with different feed molar ratios are shown in the Figure . When the feed molar ratio LA/DAB increases, the XRD absorption peaks of those P(LA-co-DAB)s begin to appear at the 2θ of 16.7° and 19.1°. Compared with the homopolymer PDLLA synthesized via direct melt polycondensation Citation[19], the position of the XRD absorption peaks is basically similar. The analysis results of the crystallinity (X c,%) of those P(LA-co-DAB)s showing absorption peaks are summarized in Table .
Table 6. The influences of different molar feed ratios on XRD results of P(LA-co-DAB)s.a
Obviously, the X c of those P(LA-co-DAB)s is lower than that of PDLLA (Table , runs 3–5). However, the partial crystalline copolymers have bigger crystallite dimension than PDLLA. These results suggest that the introduction of DAB into the PLA chain has an important influence on the crystalline properties of PLA material, even increasing DAB feed amount can make the copolymers become amorphous (Figure ). When the molar feed ratio LA/DAB decreases from 40/1 to 20/1, both relatively lower molecular and shorter PLA chain are disadvantageous for the molecular arrangement. Thus, the amorphous samples are formed.
Therefore, it is possible to adjust the crystalline property and molecular weight of copolymers by changing the molar feed ratio. Similarly, the thermal properties of P(LA-co-DAB) also can be controlled according to the following discussions.
3.4 Thermal properties of P(LA-co-DAB)
Thermal properties of P(LA-co-DAB) are tested by DSC and TGA, and the data of some important temperatures are shown in Table . The results suggest that, with the increase of the LA molar feed ratio, the glass transition temperature (Tg ) of P(LA-co-DAB) gradually decreases due to the relatively longer PLA chain and the corresponding increase of the molecular flexibility. Of course, Tg of P(LA-co-DAB) is slightly lower than that of PDLLA as well, and the reason may be that the copolymers have relatively bigger PDI (Table ) than PDLLA (PDI = 1.25 Citation[19]). Therefore, these results suggest that the introduction of DAB into the PLA chain affects the thermal properties of PLA material. More importantly, it has an important influence on the thermal decomposition temperatures, which is crucial to a good flame retardant.
Table 7. The influences of different molar feed ratios on DSC and TGA results of P(LA-co-DAB)s.a
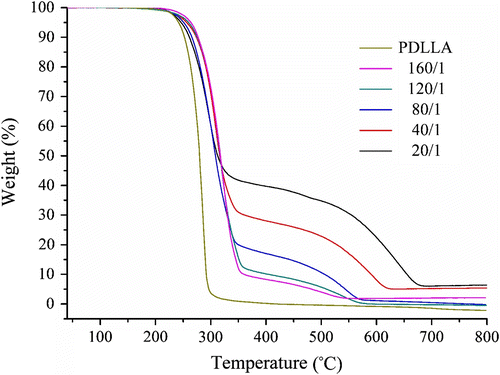
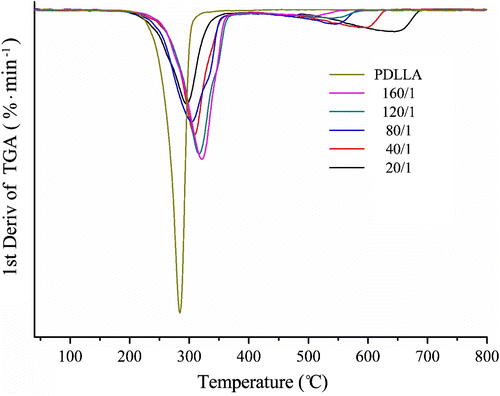
The thermogravimetry (TG) and differential thermogravimetry (DTG) curves of P(LA-co-DAB)s with different feed molar ratios are shown in Figures and , respectively. The two figures show that the copolymers synthesized as different feed molar ratios have similar TG and DTG curves, the weight loss mainly occurs in the high-temperature region. And both, especially two peaks in the DTG curves (Figure ), suggest that the decomposition of P(LA-co-DAB) is a two-step degradation. The first step is the thermal decomposition of PLA chain and the second step is the thermal decomposition of residual copolymer containing benzimidazole moiety.
However, the DTG curve of the homopolymer PDLLA only has one peak, indicating that its decomposition is only one-step degradation and the entire polymeric chain is instantaneously split into small molecular fragments once the thermal decomposition of PDLLA takes place Citation[28]. This difference shows that the copolymer P(LA-co-DAB) is not easy to decompose under common heating conditions. More importantly, it proves that P(LA-co-DAB) may be a potential polymeric flame retardant applied for PLA materials as well.
This conclusion is further confirmed by the data analysis. From Table , it can be seen that, compared with PDLLA, all data of T 5%, T 10% (the decomposition temperatures of 5%, 10% weight loss respectively), T p1 (the corresponding temperature of the first peak in the DTG curve), and T f1 (the final temperature of the first thermal decomposition stage) are basically higher, also indicating that the introduction of benzimidazole structure into the PLA’s chain enhances the thermal stability of the PLA material. From the point of the charring ability (Table ), there is no residue of PDLLA for the complete thermal degradation when the temperature only reaches 375 °C. However, P(LA-co-DAB) can give some residue even when the temperature reaches 500 °C or 600 °C. These suggest that the charring ability of P(LA-co-DAB) is obviously better than PDLLA due to the existence of the nitrogen-containing functional group in the PLA’s chain.
Table 8. The char yield of P(LA-co-DAB)s at different thermal decomposition temperatures.a
The comparison of the thermal decomposition properties of P(LA-co-DAB)s with different feed molar ratios shows that, the thermal stability of P(LA-co-DAB) is not only affected by the molecular weight, but also the content of nitrogen-containing functional group. On the one hand, the first thermal decomposition stage of P(LA-co-DAB) is mainly influenced by the molecular weight. When the M w is bigger, the value of T 5%, T 10%, and T p1 is higher (Table ). This indicates that the better thermal stability is related to the M w of copolymers Citation[11,29]. On the other hand, in the second thermal decomposition stage of P(LA-co-DAB), with the increase of the M w, the value of T p2 (the corresponding temperature of the second peak in the DTG curve) and T f2 (the final temperature of the second thermal decomposition stage) is reduced (Table ), the thermal decomposition of this stage is mainly controlled by the content of nitrogen-containing functional group. Therefore, when the content of nitrogen-containing functional group is increased, the char yield of P(LA-co-DAB) at different decomposition temperatures has an increasing trend (Table ). This also demonstrates that the charring ability of P(LA-co-DAB) is related to the content of nitrogen-containing functional group, especially the char yield is mainly controlled by the content of benzimidazole moiety.
Additionally, compared with inherent flame retardation of phosphorus-containing PLA synthesized by Wang’s group Citation[11], the TG data show that our nitrogen-containing P(LA-co-DAB)s have the higher value for T 5% and T p1 in most cases (Table ), indicating that P(LA-co-DAB) has better thermal stability. At the same time, the char yield of P(LA-co-DAB) at 375 °C and 400 °C is also generally higher (Table ). Especially at the higher temperature of 450 °C, P(LA-co-DAB) obviously has higher char yield. Of course, with the increase of the decomposition temperature, the char yield of P(LA-co-DAB) also has the less decrease trend (Table ). Noticeably, our data are obtained under an air atmosphere, but the literature data are tested under a nitrogen atmosphere Citation[11]. Usually, the thermal stability of polymer in air is somehow lower than that in nitrogen. And the situation that polymer has been exposed in air is more common than in nitrogen during real processing and application Citation[30,31]. Therefore, the difference in testing atmosphere also proves that P(LA-co-DAB) is better from another viewpoint. In a word, P(LA-co-DAB) has better charring ability than the compatible solid polymeric flame retardants reported by Wang’s group Citation[11]. Therefore, if it is blended with PLA, it may have better flame retardant effect for PLA material.
4 Conclusions
Directly starting from LA and DAB, a novel biodegradable material P(LA-co-DAB) containing benzimidazole moiety is synthesized via melt polycondensation as designed for the first time. The structure and properties of P(LA-co-DAB) are systematically characterized by FTIR, 1H NMR, GPC, DSC, XRD, and TGA. As a modified PLA copolymer with better thermal stability and higher char yield, P(LA-co-DAB) could be a potential compatible solid polymeric flame retardant for PLA materials, and its molecular weight and charring ability could be controlled by synthetic conditions and different feed molar ratios.
Acknowledgments
The authors are grateful to the 3rd Talents Special Funds of Guangdong Higher Education (grant number Guangdong-Finance-Education[2011]431), the Natural Science Foundation of Guangdong Province (grant number 5300082), and the Natural Science Foundation of China (grant number 20772035) for the financial support of this work.
References
- Gupta , AP and Kumar , V . 2010 . Synthesis and characterization of novel chain-linked biodegradable polymers . Des. Monomers Polym. , 13 : 65 – 72 .
- Helaly , FM , Essawy , HA and Shabana , MA . 2011 . Elastic polymeric network structure for slow release drug delivery systems . Polym. Plast. Technol. Eng. , 50 : 438 – 441 .
- Zhao , N , Ma , ZG and Xiong , CD . 2012 . Effect of poly(L-lactic acid) on hydrolytic degradation of poly(glycolic acid) . Int. J. Polym. Mater. , 61 : 587 – 595 .
- Fukushima , K , Murariu , M , Camino , G and Dubois , P . 2010 . Effect of expanded graphite/layered-silicate clay on thermal, mechanical and fire retardant properties of poly(lactic acid) . Polym. Degrad. Stab. , 95 : 1063 – 1076 .
- Bourbigot , S and Fontaine , G . 2010 . Flame retardancy of polylactide: an overview . Polym. Chem. , 1 : 1413 – 1422 .
- Ke , CH , Li , J , Fang , KY , Zhu , QL , Zhu , J , Yan , Q and Wang , YZ . 2010 . Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide . Polym. Degrad. Stab. , 95 : 763 – 770 .
- Li , SM , Ren , J , Yuan , H , Yu , T and Yuan , WZ . 2010 . Influence of ammonium polyphosphate on the flame retardancy and mechanical properties of ramie fiber-reinforced poly(lactic acid) biocomposites . Polym. Int. , 59 : 242 – 248 .
- Chen , DK , Li , J and Ren , J . 2011 . Combustion properties and transference behavior of ultrafine microencapsulated ammonium polyphosphate in ramie fabric-reinforced poly(L-lactic acid) biocomposites . Polym. Int. , 60 : 599 – 606 .
- Lin , ZD , Chen , C , Guan , ZX , Xu , BF , Li , X and Huang , ZY . 2012 . Polypropylene/poly(lactic acid) semibiocomposites modified with two kinds of intumescent flame retardants . Polym. Plast. Technol. Eng. , 51 : 991 – 997 .
- Wang , DY , Song , YP , Lin , L , Wang , XL and Wang , YZ . 2011 . A novel phosphorus-containing poly(lactic acid) toward its flame retardation . Polymer. , 52 : 233 – 238 .
- Yuan , XY , Wang , DY , Chen , L , Wang , XL and Wang , YZ . 2011 . Inherent flame retardation of bio-based poly(lactic acid) by incorporating phosphorus linked pendent group into the backbone. J. . Polym. Degrad. Stab. , 96 : 1669 – 1675 .
- Li , Y , Ma , HQ and Wang , YL . 2008 . Progress in the synthesis and application of benzimidazoles and their derivatives . Chin. J. Org. Chem. , 28 : 210 – 217 .
- Mao , ZZ , Li , JX , Xue , FL , Chen , RH and Wang , ZY . 2012 . Research progress in microwave synthesis of benzimidazoles flame retardant materials . Chem. Res. Appl. , 24 : 497 – 502 .
- Katritzky , AR , Aslan , DC , Leeming , P and Steel , PJ . 1997 . Asymmetric induction using chiral 1,2,4-triazole and benzimidazole derivatives . Tetrahedron: Asymmetry. , 8 : 1491 – 1500 .
- Reddy , VM and Reddy , KR . 2010 . Synthesis and antibacterial activity of some novel 6-(1H-benz-[d]imida-zol-2-yl)-8-(5-nitro-2-furyl)-3-(4-pyridyl)-7,8-dihydro[1,2,4]triazolo[3,4 b][1,3,4]thiadiazepines . Chem. Pharm. Bull. , 58 : 1081 – 1084 .
- Thakuria H, Das G. An expeditious one-pot solvent-free synthesis of benzimidazole derivatives. Arkivoc. 2008; 321–328.
- Mamada , M , Pérez-Boli´var , C and Anzenbacher , PJ . 2011 . Green synthesis of polycyclic benzimidazole derivatives and organic semiconductors . Org. Lett. , 13 : 4882 – 4885 .
- Mao , CX , Luo , SH , Wang , QF , Xiong , JF and Wang , ZY . 2012 . Synthesis and characterization of a novel functional biodegradable material, poly(lactic acid-co-borneol) . Des. Monomers Polym. , 15 : 575 – 586 .
- Wang , ZY , Zhao , YM , Wang , F and Wang , J . 2006 . Syntheses of poly(lactic acid-co-glycolic acid) serial biodegradable polymer materials via direct melt polycondensation and their characterization . J. Appl. Polym. Sci. , 99 : 244 – 252 .
- Wang , ZY , Zhao , YM and Wang , F . 2006 . Syntheses of poly(lactic acid)-poly(ethylene glycol) serial biodegradable polymer materials via direct melt polycondensation and their characterization . J. Appl. Polym. Sci. , 102 : 577 – 587 .
- Ye , RR , Wang , ZY , Luo , SH , Yang , LT and Xiao , X . 2011 . Synthesis and characterization of the biomaterial poly(lactic acid-co-Nϵ-carbobenzoyloxy-L-lysine) via direct melt copolymer-ization . J. Appl. Polym. Sci. , 121 : 420 – 426 .
- Ye , RR , Wang , ZY , Wang , QF , Yang , K and Luo , SH . 2011 . Synthesis of biodegradable material poly(lactic acid-co-aspartic acid) via direct melt polycondensation and its characterization . J. Appl. Polym. Sci. , 121 : 3662 – 3668 .
- Takasu , A , Narukawa , Y and Hirabayashi , T . 2006 . Direct dehydration polycondensation of lactic acid catalyzed by water-stable lewis acids . J. Polym. Sci., Part A: Polym. Chem. , 44 : 5247 – 5253 .
- Moon , SI and Kimura , Y . 2003 . Melt polycondensation of L-lactic acid to poly(L-lactic acid) with Sn(II) catalysts combined with various metal alkoxides . Polym. Int. , 52 : 299 – 303 .
- Duan , JF , Du , J and Zheng , YB . 2007 . Synthesis and characterization of a novel biodegradable polymer poly(lactic acid-glycolic acid-4-hydroxyproline) . J. Appl. Polym. Sci. , 103 : 3585 – 3590 .
- Lu , DD , Ren , ZL , Zhou , TH , Wang , SF and Lei , ZQ . 2008 . Synthesis and characterization of amphiphilic biodegradable poly(glutamic acid-co-lactic acid-co-glycolic acid) by direct polycondensation . J. Appl. Polym. Sci. , 107 : 3638 – 3643 .
- Ye , RR , Wang , ZY , Yang , K and Luo , SH . 2010 . Synthesis and characterization of a novel biodegradable material, poly(lactic acid-co-tryptophane) . Des. Monomers Polym. , 13 : 415 – 426 .
- Apreutesei , D , Lisa , G , Hurduc , N and Scutaru , D . 2006 . Thermal behavior of some cholesteric esters . J. Therm. Anal. Calorim. , 83 : 335 – 340 .
- Cam , D and Marucci , M . 1997 . Influence of residual monomers and metals on poly(L-lactide) thermal stability . Polymer. , 38 : 1879 – 1884 .
- Yang , KK , Wang , XL , Wang , YZ , Wu , B , Jin , YD and Yang , B . 2003 . Kinetics of thermal degradation and thermal oxidative degradation of poly(p-dioxanone) . Eur. Polym. J. , 39 : 1567 – 1574 .
- Zeng , JB , Li , YD , Li , SL , Wang , YZ and Yang , KK . 2009 . Thermal and thermo-oxidative degradation of biodegradable poly(ester urethane) containing poly(L-lactic acid) and poly(butylene succinate) blocks . J. Macromol. Sci., Part B: Phys. , 48 : 635 – 649 .