Abstract
The grafting of poly(p-dioxanaone) (PPDO) onto β-poly(N-2-hydroxyethyl)-DL-aspartamide (PHEA) was achieved, first, by synthesis of polysuccinimide (PSI) from DL-aspartic acid through thermal condensation polymerization, and then, followed by aminolysis of PSI to form PHEA with hydroxyl end groups, and finally the PPDO was grafted onto PHEA through these hydroxyl end groups by ring-opening polymerization (ROP) of PDO in the presence of tin octoate [Sn(Oct)2] and Candida Antarctica Lipase B (Novozyme 435) catalysts. Evidence of grafting was obtained by comparing Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), X-ray diffraction (XRD) analysis, polarized optical microscopy (POM), proton nuclear magnetic resonance (1H–NMR) spectroscopy, and solubility characteristics of PHEA with PPDO-grafted copolymers (PHEA-g-PPDO). The FT-IR spectroscopic analysis reveals the formation of ester linkage between PPDO and hydroxyl end groups of PHEA. TGA analysis shows a quite different thermal profile for grafted polymers. DSC and XRD results reveal the existence of graft copolymer in two different crystalline forms. POM shows banded spherulitic morphology for the graft copolymers. The grafting of PPDO was further confirmed by 1H–NMR. Eventhough, graft copolymer was obtained by both these methods, the graft copolymer obtained by enzyme catalyzed ROP shows higher crystalline order, better spherulitic morphology, and solubility characteristics than that of the graft polymer obtained by organometal catalyzed ROP.
1. Introduction
The extensive use of bioresistant synthetic polymers is considered to be one cause of environmental pollution. The current demand for easy disposal of polymers has instigated many investigations on the synthesis and evaluation of novel biodegradable polymers. These polymers are expected to find applications in medicine (sutures, implants), pharmacy (drug release), and agriculture (mulch, food packaging) Citation[1]. Although several polymers have been identified as degradable in model aqueous media or in animal bodies, the family of aliphatic polyesters appears at the moment to be the most attractive and promising Citation[2–5], where long aliphatic chain promotes biodegradability by imparting flexibility to the polymer chains.
Biodegradable aliphatic polyesters, such as polylactide (PLA), poly(ϵ-caprolactone) (PCL), copolymer of lactide and glycolide (PLGA), poly(β-hydroxybutyrate) (PHB), and poly(p-dioxanaone) (PPDO) have attracted attentions due to their excellent biocompatibility and biodegradability. These biodegradable aliphatic polyesters have been widely used in biomedical and pharmaceutical applications such as surgery repair materials and drug delivery systems Citation[6–8]. Among these aliphatic polyesters, PPDO is a biodegradable, aliphatic poly(ether–ester) usually prepared by ring-opening polymerization (ROP) with organometallic catalysts such as Sn(Oct)2. Due to the presence of ether bond and methylene groups in the molecular structure, PPDO has greater flexibility compared with other frequently used biodegradable aliphatic polyesters such as PLA and PGA. PPDO has been widely been used as a biodegradable monofilament suture material. As one of the biodegradable and biocompatible aliphatic polyesters, PPDO has high flexibility and good tensile strength Citation[9]. This can be used not only as medical materials but also as films, molded products, laminates, foams, nonwoven materials, adhesives, and coatings Citation[10–12]. However, high cost and discontinuous degradation rate are the key factors limiting wider applications of PPDO, for example, controlled release of drug. Graft copolymerization Citation[13–19] is a versatile method to modify the PPDO for providing functionality to the resulting polymers, and for regulating polymer properties. However, there are few reports about graft copolymerization of PPDO with other polymers Citation[20–22].
The PPDO can be grafted on to other polymers by ROP. For ROP, various catalysts have been employed including metal catalyst such as Sn(Oct)2, AlEt3, and derivatives of Ti, Zr, and Hg Citation[23–25]. To minimize harmful effects of metallic residues in medically applied PPDO, some nontoxic catalysts have also been investigated, including diethyl zinc, zinc lactate, and recently enzymatic catalysis Citation[9,26,27]. Enzymatic polymerization in vitro has become an area of increasing research activity as a new environment-friendly methodology for material synthesis. In contrast to ‘chemical organometallic catalysts’, the green biocatalyst enzyme is a promising alternative due to its special properties, such as nontoxicity, recyclability, high enantio-, region- and chemo-selectivities, and so on Citation[28,29]. For example, Lipase B from Candida Antarctica Lipase B immobilized on an acrylic macroporous resins (Novozyme-435) has been proven to be an active biocatalyst in homopolymer syntheses via both ROP of lactone Citation[30,31].
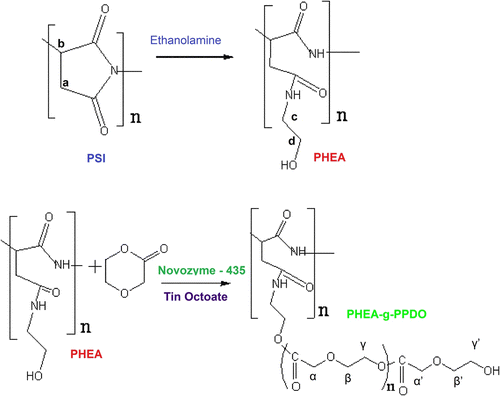
In our ongoing efforts to polymerize p-dioxanone on different substrates Citation[32–35], here we report the polymerization of PDO on hydroxyl terminated PHEA using either Sn(Oct)2 or Novozyme-435 as catalyst with the objective to compare the results. The synthetic strategy involves conversion of DL-aspartic acid into polysuccinimide (PSI) in the presence of polyphosphoric acid under reflux conditions in tetramethylene sulfolane solvent. Then the PSI was opened up by reacting with ethanolamine in dimethyl formamide to obtain α,β-Poly(N-2-hydroxyethyl)-DL-aspartamide (PHEA), which was used for grafting of p-dioxanone by ROP using both organometallic and enzyme catalysts. The PHEA is a totally biodegradable polymer and its potential applications in biological and pharmaceutical area have been explored recently Citation[36,37]. The introduction of aliphatic polyester onto PHEA by graft polymerization would lead to the controlled degradability and physical properties. The molecular structures of the obtained graft copolymers were characterized carefully via FT-IR, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), X-ray, OM, and NMR techniques.
2 Experimental
2.1 General information and materials
DL-aspartic acid (SIGMA, USA), phosphoric acid (Duksan Pharmaceutical Co Ltd, Korea), tetramethylene sulfolane (Aldrich, USA), and methanol (DC chemical Co Ltd, Korea) were used as received for the synthesis of PSI. Ethanolamine (Junsei, Japan), N,N-dimethyl formamide (DMF, Junsei, Japan), ethyl ether (Junsei, Japan), and acetone (DC chemical Co Ltd, Korea) were used as received for the synthesis of PHEA. Dimethyl sulpoxide(DMSO, TEDIA Co, USA), toluene, and chloroform (DC chemical Co Ltd, Korea) were used as received. The p-dioxanone monomer (Samyang company, Korea) was purified according to the procedure described by Raquez et al. Citation[38,39]. Tin octoate [(Sn(Oct)2] from Aldrich was used after dissolving it in pure and dry toluene. Novozyme-435(LC2002070) was a gift from Novozymes A/S, Denmark and used as received.
2.2 Synthesis of PSI
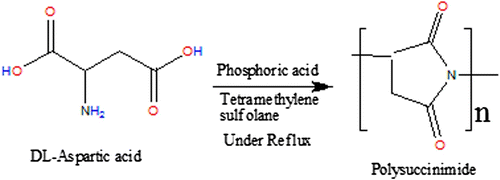
PSI was synthesized according to the procedure described Citation[40] as follows: A suspension of DL-aspartic acid (25.0 g, 0.188 mol) and phosphoric acid (0.9 ml, 9.4 mmol) catalyst in the tetramethylene sulfolane solvent (80 g) was refluxed under nitrogen atmosphere. Water formed in the reaction mixture was removed using a Dean-Stark trap apparatus with a reflux condenser. After 4.5 h, the solvent was removed; the precipitate was washed with methanol and then with water several times until it was neutral. The residue was washed with methanol and dried at 85 °C under reduced pressure. Schematic representation of PSI synthesis is shown in Figure .
2.3 Synthesis of poly(hydroxyethylaspartamide) (PHEA)
A typical procedure for the preparation of PHEA is as follows: 3.88 g PSI was dissolved in 100 mL DMF and then 0.40 mL ethanolamine was added at 0 °C for a period of 10 min, then the reaction flask was moved into water bath at 25 °C and stirred for another 5 h. The solution was then precipitated in ethyl ether. The precipitate was washed with acetone for three times and dried at 25 °C under vacuum.
2.4 Synthesis of PHEA-g-PPDO [tin octoate]
Under nitrogen atmosphere, 0.16 g PHEA was charged into a rigorously dried 50 mL flask and then was degassed at 60 °C in a vacuum line for 2 h, purging three times with dry nitrogen. Then 1.633 g (0.1 mol) PDO was charged into flask. The flask was then immersed into a preheated oil bath at 60 °C for about 30 min to obtain a clear homogeneous melt system of PDO and PHEA. Then the metal catalyst tin octoate (0.5 ml), was injected under nitrogen at 60 °C and the reaction was allowed to proceed for 8 h at 70 °C.
2.5 Synthesis of PHEA-g-PPDO (Novozyme 435)
0.16 g PHEA was charged into a rigorously dried 50 mL flask and then was degassed at 60 °C in a vacuum line for 2 h, purging three times with dry nitrogen. Then under nitrogen atmospheres, 1.633 g (0.1 mol) PDO, 0.108 g (Novozyme-435), and 4.3 mL toluene were charged into flask. The flask was then immersed into a preheated oil bath at 60 °C for about 30 min to obtain a clear homogeneous solution. Then the reaction was allowed to proceed for 8 h at 70 °C.
2.6 Extraction and purification of PHEA-g-PPDO
When the reaction time reached 8 h, the polymerization was stopped by fast cooling to room temperature. After cooling, the unreacted monomer (PDO) and the homopolymer (PPDO) were removed by extracting with toluene and 1,2-dichloroethane, respectively Citation[22]. After drying the solvent, the product was washed with water to remove the water soluble PHEA, until a constant weight was reached.
2.7 Characterization techniques
Molecular weight and molecular weight distribution data of the polymers were determined by gel permeation chromatography (GPC) using tetrahydrofuran (THF) as eluent with a flow rate of 1.0 mL/min at 35 °C through three Waters μ-styragel columns in series and a waters 515 refractive index (RI) detector. The column system was calibrated by a set of standard monodisperse polystyrenes. The data analysis was accomplished on a waters millennium 32 systems. FT-IR spectra of the samples were recorded on a Perkin Elmer (Spectrum 1000) FT-IR spectrophotometer in the range of 4000–400 cm−1 at a resolution of 4 cm−1 with a maximum of 100 scans. The spectral calibration of the instrument was made using polystyrene film at regular intervals of time. DSC measurements were made using TA instruments (2010 DSC) from ambient temperature to 350 °C in a nitrogen atmosphere at a heating rate of 10 °C/min. TGA analysis was carried out in SCINCO (TGA 1000) series thermal analyzer system at a heating rate of 20 °C/min from ambient to 800 °C under nitrogen atmosphere. The X-ray diffraction measurements were performed using Bede D3 system (Bede scientific Instruments Ltd, Durham, UK) equipped with high flux (18 Kw) direct drive Rigaku rotating anode source of Cu Kα radiation with a resolution of 12 arc seconds. The morphology of the samples was studied with a Zeiss MC-80 polarizing microscope equipped with a Linkam TP-91 hot-stage and a camera system. Measurements of nuclear magnetic resonance (NMR) spectra were conducted on a FT-NMR (Inova 400NB, USA) Spectrometer with DMSO-d 6 as solvent, operating at 400 MHz. Chemical shifts (in parts per million, ppm) were reported down field from 0.00 ppm using trimethylsilane TMS as internal standard.
3 Results and discussion
With the objective to graft PPDO onto PHEA, here we report the ROP of p-dioxanone in the presence of organomettalic catalysts [tin octoate, Sn(Oct)2] and enzyme catalyst (Novozyme 435) as per the scheme described in Figure . The grafting of PPDO onto PHEA was achieved, first by synthesis of PSI and PHEA, then, followed by ROP of PDO onto the PHEA backbone with organomettalic and enzyme catalysts. First, PSI was synthesized by acid catalyzed polycondensation of DL-aspartic acid using Phosphoric acid as catalyst. Then, PHEA with hydroxyl end groups was prepared by aminolysis of PSI with ethanolamine. Next, the ROP of p-dioxanone (PDO) onto PHEA was carried out with metal and enzyme catalysts. During the course of graft polymerization, an amount of PPDO homopolymer was inevitably produced besides the target graft copolymer. Before the characterization, the PPDO homopolymer, unreacted monomer (PDO), unreacted polymer (PHEA), and the catalyst impurities were removed by using appropriate solvents, and characterized by solubility test, FT-IR, DSC, TGA, XRD, OM, and NMR techniques.
3.1 Solubility characteristics and molecular weight analysis of PPDO-g-PHEA
The simplest method to prove the formation of PHEA-g-PPDO is based on the solubility difference between the graft copolymer and the nongrafted homopolymer. The solubility details of PSI, PHEA, PHEA-g-PPDO (Tin), and PHEA-g-PPDO (Enzy) are tabulated in Table . In acetone, all the polymers were not soluble. In water, only PHEA was soluble. In DMF, both PSI and PHEA were soluble, but both PPDO-grafted polymers were not soluble. In DMSO, all the polymers were completely soluble. Even though, it is a simple test, it gives strong evidence about the conversion and purity of the polymers. For example, PSI which was not soluble in water, became completely soluble after aminolysis, but after grafting with PPDO again became insoluble, indicating the formation of grafting with PPDO. PHEA and PPDO were soluble in water and DMF, respectively. When the reaction product was extracted with DMF and alternatively with water, an insoluble solid still remained. A physical mixture of PHEA and PPDO was treated in the same way and was found to dissolve completely. Therefore, it is proved that the resulted graft copolymer was not a simple physical mixture, but some chemical bonds must exist between the PHEA and PPDO macromolecules. Even though the graft copolymer obtained by both these methods, Tin octoate catalyzed ROP and Novozyme-435 catalyzed ROP, was soluble in DMSO, there was some difference in the transparency of these solutions. The solution formed by dissolution of PHEA-g-PPDO (Enzy) polymer shows better transparency that the solution obtained by the dissolution of PHEA-g-PPDO (Tin) polymer.
Table 1. Solubility data of PSI, PHEA, PHEA-g-PPDO (Tin) and PHEA-g-PPDO (Enzy).
In order to demonstrate the graft polymerization of PDO onto PHEA, the molecular weight of the purified graft copolymer was determined by GPC. This was accomplished by removing the unreacted monomer (PDO) and the homopolymer (PPDO) from the final reaction product by extracting with toluene and 1, 2-dichloroethane, respectively Citation[22]; then the product was washed with water to remove PHEA. Then the residue was dissolved in DMSO and filtered. And the DMSO solvent from filtrate was removed to obtain the grafted polymer. This purified product was used to determine the Mw, Mn, and polydispersity. Table shows the Mw, Mn, and polydispersity index values. From the table, it is clear that the polydispersity index of grafted polymer is higher than that of homopolymer (PHEA). And between the metal (Tin octoate) and enzyme catalysts, the enzyme catalyzed graft polymer shows higher molecular weight and polydispersity index.
Table 2. Molecular weight characteristics of PHEA, PHEA-g-PPDO.
3.2 Structural analysis of PHEA-g-PPDO by FT-IR
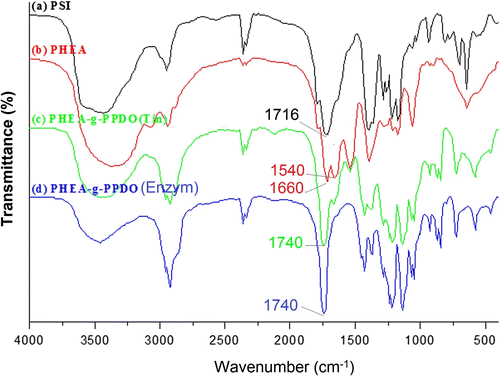
The grafting of PPDO with PHEA was further confirmed by the difference between the FT-IR spectra of PHEA and that of graft copolymers (PHEA-g-PPDO). The infrared (IR) spectra of PSI, PHEA, PHEA-g-PPDO (Tin), and PHEA-g-PPDO (Enzy) samples are shown in the Figure (a–d). The IR peak characteristic of ester link that appeared at 1740 cm−1 for PPDO-grafted PHEA (Figure ) is due to the presence of saturated ester group Citation[41,42]. This is the link between the hydroxyl groups of PHEA and keto group of PPDO. The formation of this ester link in the grafted polymer provides substantial evidence for graft polymerization of PDO onto PHEA, which is absent in the PHEA spectrum (Figure ). The FT-IR spectrum of PHEA (Figure ) shows characteristic peaks at 1660 cm−1 (amide I) and 1540 cm−1 (amide II). The absence of IR band at 1716 cm−1 (Figure ) for PHEA indicates the successful conversion of succinimide units into the amide units. The appearance of imide peak at 1716 cm−1 (Figure ) shows the formation PSI. The appearance of IR peak characteristic of ester link at 1740 cm−1 proves the successful grafting of PPDO onto PHEA by both organometallic as well as enzyme catalyzed ROP.
3.3 Thermal analysis of PHEA-g-PPDO by DSC analysis
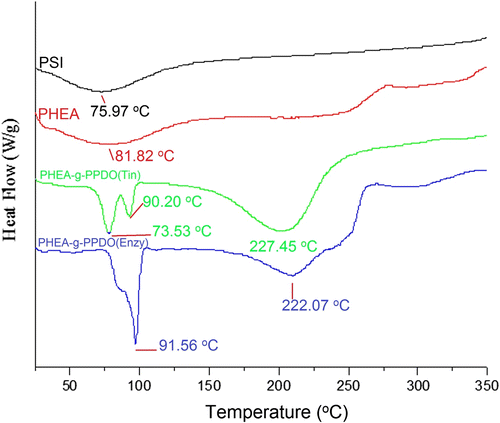
DSC is a unique technique to analyze the molecular architecture of these grafts copolymers and their interaction behavior. As we know, graft polymers have different thermal transitions compared to their linear counter parts owing to their molecular architecture. The DSC thermograms of PSI, PHEA, PHEA-g-PPDO (Tin), and PHEA-g-PPDO spacde (Enzy) are shown in Figure . PSI (Figure ) and PHEA (Figure ) show wide and flat transition peaks with a peak maximum at 75.97 and 81.82 °C respectively, whereas the PPDO-grafted products [PHEA-g-PPDO(Tin), PHEA-g-PPDO(Enzy)] show sharp multiple endothermic peaks. In case of Tin octoate catalyzed grafting, the polymer shows two transitions, the first one is at 73.53 and 90.20 °C, and the second one is at 227.45 °C. In case of enzyme catalyzed grafting, the copolymer shows two transitions the first one is at 79.87 and 91.56 °C and the second one is at 222.07 °C. The sharp multiple endothermic peaks are associated with crystallization behavior of PPDO Citation[21,43]. PSI is heterocyclic amorphous polymer does not show either sharp T g or T m (Figure ). However, it shows a very wide transition starting from 25 to 125 °C with peak maximum at 75.97 °C. The aminolysis of PSI with hydroxylethylamine has widened and intensified this transition with peak maxima at 81.82 °C (Figure ). From Figure , it is clear that PHEA-g-PPDO shows multiple endothermic peaks because of the heterogeneous nature Citation[21,43]. The phenomenon of multiple melting (T m) peaks may be due to its particular molecular architecture, in which the hydrophobic PPDO chains were grafted onto heterocyclic and hydrophilic PHEA, which has changed the phase morphology and overall crystallinity of both polymers. Eventhough the graft copolymer obtained by both, organometal catalyzed ROP and enzyme catalyzed ROP, these methods show sharp crystalline transition peaks, wide difference exists between both the polymers in terms of peak position and intensity. The peak intensity (sharpness) and higher transition temperature (91.56 °C) indicate higher crystalline order for enzyme catalyzed polymer than that of organometal catalyzed polymer.
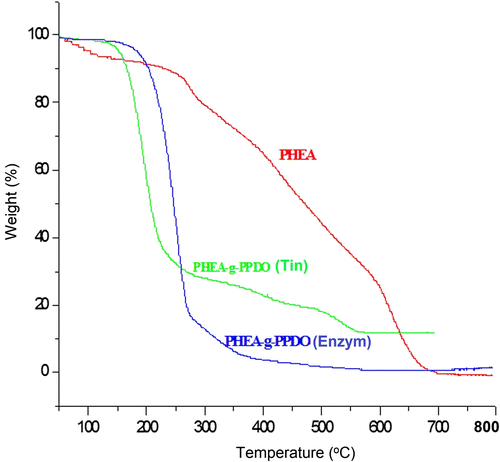
3.4 Thermal analysis of PHEA-g-PPDO by TGA
The grafting of PPDO with PHEA was further confirmed and supported by the difference between the TGA spectra of PHEA and that of graft copolymer (PHEA-g-PPDO). Typical TGA thermograms obtained for PHEA, PHEA-g-PPDO (Tin), and PHEA-g-PPDO (Enzy) are shown in Figure and the percentage of weight loss at different temperatures is given in Table . The TGA thermogram of PHEA shows a weight loss of around 8% between 50 and 150 °C. This weight loss may correspond to the loss of bound and unbound water associated with the hydroxyl terminated PHEA molecules Citation[44,45]. Whereas, PPDO-grafted PHEAs [PHEA-g-PPDO (Tin), PHEA-g-PPDO (Enzy)] do not show such a weight loss corresponding to bound and unbound water especially in the temperature zone between 50 and 150 °C. This may be because the grafting of PPDO with PHEA takes place through the hydroxyl end groups of PHEA, to the extent that grafting of the hydroxyl groups are reduced in the PHEA molecule. So, due to the conversion of hydroxyl groups into an ester link, the weight loss corresponding to bound and unbound water is reduced. Even though it is a simple analysis, it gives a strong evidence for grafting of PPDO onto PHEA. On that basis, the comparison between the Tin octoate catalyzed ROP and enzyme catalyzed ROP revealed that the enzyme catalyzed ROP is an efficient one, because in PHEA-g-PPDO (Enzy), the weight loss due to moisture starts at a higher temperature than that of PHEA-g-PPDO (Tin). This is the first evidence for the grafting from TGA analysis, the second one is; after the moisture loss, the PHEA, which does not have weak ester links show better thermal stability compared to the PPDO-grafted PHEA, where PPDO is linked with PHEA through ester link. This poor thermal stability of grafted products shows the presence of ester link and the second evidence for the grafting PPDO with PHEA in both organometal as well as enzyme catalyzed ROP. Apart from this, the degradation pattern of the PHEA is much different from the degradation profile of the grafted products. TGA analysis confirms the grafting of PPDO onto PHEA based on the wider difference in thermal stability between the grafted polymers and PHEA. The graft copolymers obtained by both these methods, Tin octoate catalyzed ROP and Novozyme-435 catalyzed ROP, were poor in thermal stability compared to PHEA. Between these graft polymers, the graft polymer obtained by ROP using enzyme catalyst shows more weight loss after 250 °C compared to the graft copolymer obtained by the ROP using organometal catalyst. This higher weight is due to the higher percentage of PPDO attached to the PHEA-g-PPDO (Enzy). It shows that enzyme catalyzed mechanism is better than the organometal catalyzed mechanism.
Table 3. TGA data of PHEA, PHEA-g-PPDO (Tin) and PHEA-g-PPDO (Enzy).
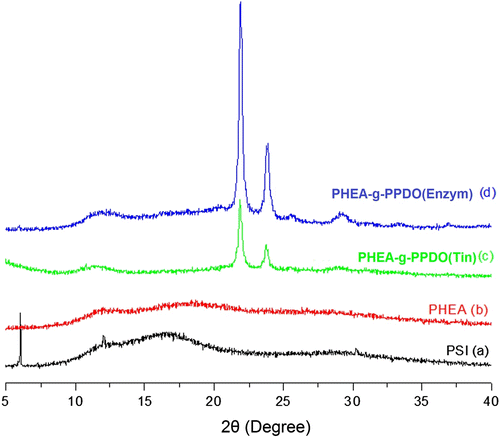
3.5 Structural analysis of PHEA-g-PPDO by X-ray diffraction patterns
The grafting was also supported by XRD (Figure ) results. The X-ray diffraction pattern of the grafted PHEA shows two crystalline peaks between 2θ = 20° and 24° (due to PPDO grafts at the PHEA backbone), while no such peaks are visible in the XRD of the PHEA itself. While the PHEA grafted with PPDO by ROP using organometallic catalyst (Tin octoate) shows two crystalline peaks at 2θ = 21.90o, 23.72o (Figure ), the PHEA grafted with PPDO by ROP using enzyme catalyst (Novozyme-435) also shows two crystalline peaks, but at little different positions at 2θ = 21.96o, 23.82o (Figure ). The graft copolymer obtained by both of these methods shows two XRD peaks indicating that the graft copolymers exist in two different crystalline forms. However, there is some difference between these two methods in terms of intensity and peak position. Comparative analysis reveals that the graft copolymer obtained by the enzyme catalyzed method shows higher crystalline order and intensity than that of organometal catalyzed ROP method. This is inconsistent with the TGA and DSC results.
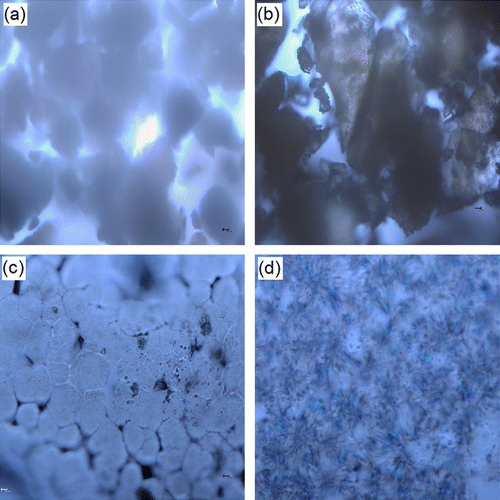
3.6 The spherulitic morphology of PHEA-g-PPDO
When PHEA-g-PPDO was crystallized from melt, it forms banded spherulites with well-defined Maltese crosses. Figure shows optical micrographs of PSI, PHEA, PHEA-g-PPDO (Tin), and PHEA-g-PPDO (Enzy) after isothermal crystallization at 110 0C for 1 min. From the figure, we can observe that the grafted polymers, PHEA-g-PPDO (Tin) and PHEA-g-PPDO (Enzy), exhibits spherulitic morphology with regular concentric rings (Figure ), while this is not to be found in either PHEA (Figure ) or PSI (Figure ). PSI and PHEA, both being amorphous polymers, do not undergo crystallization and do not show spherulitic formation. This clearly demonstrates that crystallinity of grafted polymer in comparisons with PHEA and PSI due to the attachment of PPDO chains. PHEA-g-PPDO (Tin) optical microscope is much different from PHEA-g-PPDO (Enzy). This may be due to the structural difference. The graft copolymer obtained by ROP using enzyme has good spherulitic morphology than that of the graft copolymer obtained using organometallic catalyst. This result is well supported by the DSC, TGA, and XRD results.
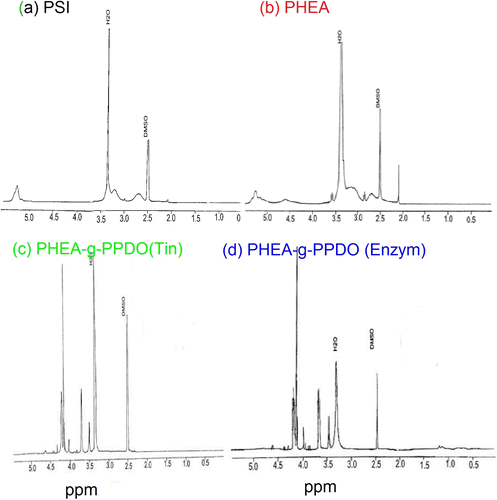
3.7 Structure analysis of PHEA-g-PPDO by 1H–NMR
The grafting of PPDO with PHEA was also supported by the difference between the NMR spectra of PHEA and that of graft copolymers (PHEA-g-PPDO). The PPDO-grafted PHEA (Figure ) shows signals corresponding to attached PPDO at Hα (4.17 ppm), Hβ (3.77 ppm), Hγ (4.30 ppm), Hα’ (3.68 ppm), Hβ’ (3.73 ppm), which is much different from the NMR signals of p-dioxanone at Hα (4.26 ppm), Hβ (3.78 ppm), Hγ (4.40 ppm) Citation[21]. The typical proton resonances of the p-dioxanone (3.78–4.40 ppm) unit are broadened and shifted up to 0.1 ppm compared to the monomer. PSI prepared by polycondensation of aspartic acid (Figure ) shows signals at (a) 2.50–3.20 ppm (m, 2H) and (b) 5.00–5.14 ppm (s, 1H), and was assigned to methene and methyne protons, respectively (Figure ) Citation[45]. The aminolysis of PSI with ethanolamine yielded (PHEA) and which (Figure ) shows additional signals corresponding to the ethanolamine groups at and assigned as (c) 2.84 ppm (m, 2H) (–NH–CH2–CH2–OH) and at (d) 3.53 ppm (m, 2H) (–NH–CH2–CH2–OH) (Figure ) Citation[46].
4 Conclusion
A new graft copolymer composed of PHEA was successfully synthesized by grafting PPDO on to PHEA backbone using metal Tin octoate and enzyme catalysts (Novozyme 435). The grafting of PPDO onto PHEA was proved by FT-IR, DSC, TGA, XRD, OM, 1H–NMR, and solubility techniques. The FT-IR spectroscopic analysis reveals the formation of ester linkage between PPDO and hydroxyl terminated PHEA. The DSC results indicate heterogeneous nature of graft polymers in the form of multiple melting peaks. TGA analysis reveals the reduced thermal stability for graft copolymers. POM revealed the spherulitic morphology for the graft copolymers. The grafting of PPDO was further confirmed by 1H–NMR and X-ray diffraction patterns. Thus, the obtained PHEA-g-PPDO was an amphoteric hybrid with hydrophilic amide groups and hydrophobic PPDO side chains. These are noteworthy in that they exhibited new thermal transition and changed solubility. Thus, the graft copolymers are expected to have biocompatibility, biodegradability, and functionality, and will have wide potential applications in many fields. It can also be used as compatibilizer to blend hydrophilic and hydrophobic polymers.
Acknowledgment
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2011-0026998) and Hannam University research fund in 2012.
References
- Huang SJ. Biodegradable polymers. Encyclopedia Polym. Sci. Eng. 1985;2:220–243.
- Vert M, Li MS, Spenlehauer G, Guerin PJ. Biosorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992;3:432–446.
- Doi , Y , Kunioka , M , Nakamura , Y and Soga , K . 1988 . Nuclear magnetic resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate . Macromolecules. , 81 : 2722 – 2728 .
- Tokiwa , Y and Suzuki , T . 1977 . Hydrolysis of polyesters by lipase . Nature. , 270 : 76 – 78 .
- Kawabata , N . 1993 . Removal of microorganisms from water using pyridinium-type of polymers . Kobunshi ronbunshu. , 50 : 809 – 820 .
- Doi , Y . 1990 . Microbial Polyester , New York , NY : Publisher .
- Inotte Y, Yoshie N. Structure and physical properties of bacterially synthesized polymers. Prog. Polym. Sci. 1992;17:571–610.
- Hayashi , P . 1994 . Biodegradable polymers for biomedical uses . Prog. Polym. Sci. , 19 : 663 – 702 .
- Yang , KK , Wang , XL and Wang , YZ . 2002 . Poly(p-dioxanone) and its copolymers . Sci. Polym. Rev. , C43 : 373 – 398 .
- Doddi N, Versfelt CC, Wasserman D. United States patent US 4032 988(A1); 1977.
- Lipinsky ES, Sinclair RG, Browing JD. United States patent US 5, 767, 222; 1997.
- Forschner RC. Patent Appl. WO 9 721 753 Al. 1997.
- Yalan , G and Kayan , A . 2012 . Ringopening polymerization of isopropylglycidylether(IPGE) with new catalysts of Ti, Sn, Al-alkoxides and comparision of its reactivity . Des. Monomers Polym. , 15 : 405 – 416 .
- Yu , X , Fu , Z , An , H , Wu , D , Han , Y and Song , H . 2010 . Synthesis and characterization of photochromic copolymer grafting azoaromatic chromospheres of pullulan . Int. J. Polym. Mater. , 60 : 40 – 50 .
- An , J , Wang , W and Wang , A . 2012 . Preparation and swelling behavior of a pH responsive psyllium-g-poly(acrylic acid)/atapulgite super absorbent nanocomposite . Int. J. Polym. Mater. , 61 : 906 – 918 .
- Elsawy , MA , Saad , GR and Elsabee , MZ . 2011 . Grafting of N-isopropyl acrylamide onto bacterial polyhydroxybutyrate/hydroxyvalerate copolymers . Polym. Plast. Technol. Eng. , 50 : 1055 – 1063 .
- Jayamand , M . 2012 . Synthesis and characterization of syndiotactic polystyrene-graft-poly(methyl methacrylate) via free radical polymerization . Polym. Plast. Technol. Eng. , 51 : 514 – 520 .
- Li , W , Yao , Y , Yuvan , Y , Meng , Y and Xie , L . 2012 . Synthesis and characterization of linear low density polyethylene grafted glycerol monolauric acid monotaconoic acid diester . Polym. Plast. Technol. Eng. , 51 : 620 – 625 .
- Saad , GR , Elsawy , MA and Elsabee , MZ . 2012 . Preparation, characterization and anti-bacterials activity of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-g-poly(N-vinylpyrrolidone) copolymers . Polym. Plast. Technol. Eng. , 51 : 1113 – 1121 .
- Wang XL,Yang KK, Wang YZ. Synthesis and nuclear magnetic resonance analysis of starch-g-poly(1,4-dioxan-2-one) copolymers. J. Polym. Sci., Part A. Polym. Chem. 2004;42: 3417–3422.
- Sugih , AK , Picchioni , F and Heeres , HJ . 2009 . Experimental studies on the ring opening polymerization of p-dioxanone using an Al(O’Pr)3-monosaccharide initiator system . Eurp. Polym. J. , 45 : 155 – 164 .
- Wang XL, Yang KK, Wang YZ, Chen DQ, Chen SC. Crystallization and morphology of starch-g-poly(1,4-dioxan-2-one) copolymers. Polymer. 2004;45:7961–7968.
- Nishida , H , Yamashita , M , Endo , T and Tokiwa , Y . 2000 . Equilibrium polymerization behavior of 1,4-Dioxan-2-one in bulk . Macromolecules. , 33 : 6982 – 6986 .
- Schultz HS. United States patent US 3,063,967.
- Bagget JM, Horvath JW, Wilson BW. United States patent US 3,391,126.
- Al-Azemi , TF and Bisht , KS . 1999 . Novel functional polycarbonate by lipase-catalyzed ring opening polymerization of 5-methyl 5-benzyloxycarbonyl-1,3-dioxan-2-one . Macromolecules. , 32 : 6536 – 6540 .
- Nishida H, Yamashita Y, Nagashima M, Endo T, Tokiwa YJ. Synthesis of metal-free poly(1,4-dioxan-2-one) by enzyme catalyzed ring opening polymerization. J. Polym. Sci., Part A. Polym. Chem. 2000;38:1560–1567.
- Kobayashi , S , Uyama , H and Kimura , S . 2001 . Enzymatic polymerization . Chem. Rev. , 101 : 3793 – 3818 .
- Gross , RA , Kumar , A and Kalra , B . 2001 . Polymer synthesis by in vitro enzyme catalysis . Chem. Rev. , 101 : 2097 – 2124 .
- Bisht , KS , Deng , F , Gross , RA , Kaplan , DL and Swift , G . 1998 . Ethyl glucoside as a multifunctional initiator for enzyme catalyzed regioselective lactone ring opening polymerization . J. Am. Chem. Soc. , 120 : 1363 – 1367 .
- Kumar , A and Gross , RA . 2000 . Candida antartica lipase B catalysed polycaprolactone synthesis: effects of organic media and temperature . Biomacromolecules. , 1 : 133 – 138 .
- Yoon KR, Chi YS, Lee KB, Lee JK, Kim DJ, Koh YJ, Joo SW, Yun WS, Choi IS. Surface-initiated ring opening polymerization of P-dioxanone from gold and silicon surfaces. J. Mater. Chem. 2003;13:2910–2914.
- Yoon KR, Koh Y-J, Choi IS. Formation of silica/poly(p-dioxanone) microspheres by surface initiated polymerization. Macromol. Rapid Commun. 2003;24:207–210.
- Yoon KR, Chi YS, Lee KB, Joo SW, Yun WS, Choi IS. Surface-initiated, enzymatic polymerization of biodegradable polyesters. Adv. Mater. 2003;15:2063–2066.
- Yoon , KR , Kim , Y and Choi , IS . 2005 . Mechanistic study on Sn(oct)2 catalysed ring opening polymerization of p-dioxanone by surface initiated polymerization and X-ray photoelectron spectroscopy . J. Polym. Res. , 11 : 265 – 268 .
- Yamakawa , I , Kawahara , M , Watanabe , S and Miyake , Y . 1989 . Sustained release of insulin by double layered implant using poly(DL-Lactic acid) . J. Pharm. Sci, , 79 : 505 – 509 .
- Mendichi , R , Giammona , G , Cavallaro , G and Schieroni , AG . 2000 . Molecular characterization of α,β-poly(N-hydroxyethyl)-DL-aspartamide by light scattering and viscometry studies . Polymer. , 41 : 8649 – 8657 .
- Raquez JM, Degee P, Narayan R, Dubois P. Coordination-insertion ring opening polymerization of 1,4-dioxan-2-one and controlled synthesis of diblock copolymers with e-caprolactone. Macromol. Rapid Commun. 2000;21:1063–1071.
- Raquez JM, Degee P, Narayan R, Dubois P. Some thermodynamic, kinetic and mechanistic aspects of the ring opening polymerization of 1,4-dioxan-2-one initiated by Al(O’Pr)3 in bulk. Macromolecules. 2001;34:8419–8425.
- Doll , KM , Shogren , RL , Holser , RA , Willet , JL and Swift , G . 2005 . Swift G. polymerization of L-aspartic acid to polysuccinimide and copoly(succinimide aspartate) in super critical carbon dioxide . Lett. Org. Chem. , 2 : 687 – 689 .
- Chowdhury P, Samui S, Kundu T, Nandi MM. Graft polymerization of methyl methacrylate onto guar gum with ceric ammonium sulfate/dextrose redox pair. J. Appl. Polym. Sci. 2001;82:3520–3525.
- Williams DH, Fleming I. Spectroscopic methods in organic chemistry. 4th ed. Tata McGraw Hill: New Delhi; 1988, p. 47.
- Pezzin APT, Ekenstein GORAV. Melt behavior, crystallinity and morphology of poly(p-dioxanone). Polymer. 2001;42:8303–8306.
- Cao YX, Qing J, Sun F, Zhou, Lin S. Graft copolymerization of acrylamide onto carboxymethyl starch. Eur. Polym. J. 2002;38:1921–1924.
- Zhang LM, Tan YB. Graft copolymerization of 2-(dimethylamino)ethyl methacrylate onto carboxymethylated cellulose. Macromol. Mater. Eng. 2000;280/281:59–65.
- Chen , H , Chen , T , Hu , J and Wang , C . 2005 . Nano aggregates of biodegradable amphiphilic poly(hydroxyethyl aspartamide-co-propylaspartmide) grafted with poly(DL-lactide) . Colloids Surf. A. , 268 : 24 – 29 .