Abstract
Chalcone moiety containing epoxy resin (ECH) has been synthesized by condensing 0.5 mol 1,3-bis(4-hydroxy phenyl) prop-2-ene-1-one and 2.2 mol epichlorohydrin using 1 mol sodium hydroxide in 70 ml water as a catalyst and isopropyl alcohol as a solvent at reflux temperature for 20 min. ECH used in the study had 263 epoxy equivalent, = 809,
= 275, and
= 2.94. ECH has been cured using 5–25% triethyl amine as a hardener at 100 °C. ECH and ECH-T-20 (cured) have been characterized by Fourier transform infrared, 1Proton nuclear magnetic resonance, differential scanning calorimetric, and thermogravimetric analysis. ECH and ECHT-20 are thermally stable up to about 225o and 288o, respectively, and followed multistep degradation. Jute and glass composites have been fabricated by hand layup technique at 135 °C under 27.58 MPa pressure for 4 h. JECHT-20 and GECHT-20 possess, respectively, 38.7 and 288.6 MPa tensile strength; 26 and 296.3 MPa flexural strength; >8.9 and >21.7 kV/mm electric strength; and 1.1 × 1012 and 2.1 × 1013 Ω cm volume resistivity. Fairly good thermal, mechanical, and electrical properties of the resin and composites may find their industrial importance for various applications.
1. Introduction
Epoxy resins are well known for their extensive industrial applications – such as coatings, adhesives, constructions, reinforced plastics, insulation materials for electric devices and matrices for advanced composites, molding compounds and encapsulation materials in advanced electronic components, in land, marine, and space transportations, automobile and electrical components, rehabilitation, pollution control equipments, adhesives, laminates, printed circuit boards, molds for casting and composite materials, in the aerospace and aircraft industries, in electronic packaging, etc. – due to their high thermal resistance, superior electrical and mechanical properties (high strength and modulus), excellent moisture, solvent and chemical resistance, low shrinkage after cure, good adhesion to many substrates, and good processability Citation[1–13]. The characteristic excellent physico-chemical properties of epoxy resins make them suitable for an increasing number of engineering applications such as those requiring high strength and stiffness, good dielectric behavior, resistance to chemicals, low shrinkage during cure, etc.
Epoxy resins can be cross-linked with a variety of functionalized compounds that contain hydroxyl, carboxyl, and amine groups Citation[14]. The properties of the final products depend on the quantity and type of crosslinking agent. Thus, it is very important to choose the right quantity of crosslinking agent Citation[15]. Some of the epoxy compounds are useful for the synthesis of photosensitive polymers for coating and adhesive applications. Among many promising photosensitive groups, the chalcone derivatives possess high photo-crosslinking efficiency Citation[16]. The 4,4′-disubstituted chalcones possess a quasi-two-dimensional charge transfer characteristics and used for organic second-order nonlinear optical polymers for practical device applications due to ease in device fabrication and low cost Citation[17]. For advanced microelectronic packaging technology, the epoxy resins should have improved combined physical, mechanical, and thermal properties namely good thermal stability, low dielectric constant and dissipation factor, low water absorption, low coefficient of thermal expansion, low internal stress, high mechanical strength, and low modulus Citation[18–21].
Literature survey revealed that epoxy resin containing chalcone moiety Citation[16] has been studied for the synthesis of photosensitive polymers. To the best of our knowledge no work has been reported on physico-chemical study of this type of resin, which encouraged us to take up synthesis, spectral and physico-chemical study of the chalcone moiety containing epoxy resin(I) and its fiber reinforced composites: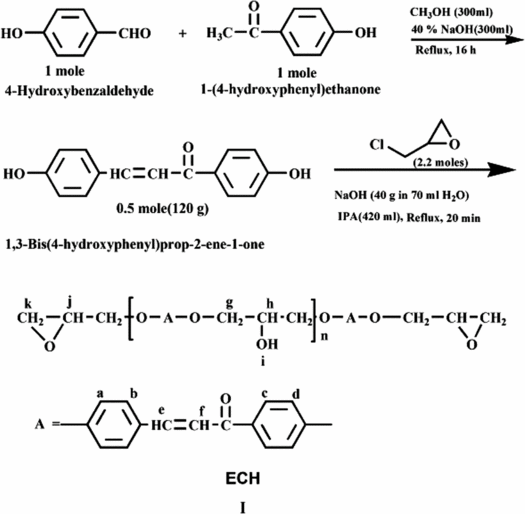
2 Experimental
2.1 Reagents and materials
Solvents and chemicals used were of laboratory grade and purified prior to their use Citation[22]. Woven jute fabric (Brown jute, Corchorus capsularis) used in the present study was collected from a local market and silane-treated E-glass fabric was collected from Unnati Chemicals Ahmedabad.
2.2 Synthesis of 1,3-bis(4-hydroxyphenyl)prop-2-en-1-one (II)
1,3-Bis(4-hydroxyphenyl)prop-2-en-1-one was synthesized according to scheme mentioned below and crystallized three times from methanol–water system. The yield and melting point of yellow-colored chalcone were 55% and 125 °C, respectively.
2.3 Synthesis of chalcone moiety-containing epoxy resin
Epoxy resin of 1,3-bis(4-hydroxyphenyl)prop-2-en-1-one was synthesized by condensing 1,3-bis(4-hydroxyphenyl)prop-2-en-1-one and epichlorohydrin. A 2 L three-neck flask equipped with a mechanical stirrer and condenser was placed into a thermostat bath. To this flask, 0.5 mol (120 g) 1,3-bis(4-hydroxy phenyl)prop-2-en-1-one was dissolved in 420 ml isopropyl alcohol and 1 mol NaOH in 70 ml water was added to this solution with stirring. The temperature of the resultant solution was brought to reflux temperature and then 2.2 mol (196 ml) epichlorohydrin was added with stirring and refluxed for 20 min and then cooled to room temperature. Yellow-colored solid resin was isolated from water, filtered, washed well with distilled water and finally with cold methanol and dried at room temperature. The resin is soluble in common organic solvents such as 1,4-dioxane, tetrahydrofuran (THF), methylethylketone (MEK), dichloromethane, 1,2-dichloro ethane, N,N-dimethylformamide, dimethylsulfoxide, etc. Here after resin is designated as ECH and was purified three times from MEK system prior to its use.
2.4 Curing study
ECH was cured using varying proportion of triethylamine (TEA) (5–25 wt.%) as a hardener. Thus, into five different wide mouth test tubes, 2 g ECH was dissolved in 10 ml MEK and 5, 10, 20, and 25% TEA. The test tubes were placed in water bath at 80 °C, the solvent was allowed to evaporate slowly with stirring and then temperature was raised to 100 °C. The curing time was started counting, when temperature was reached to constant 100 °C in a thermostat bath. The gel time of each of the sample was noted at the time at which viscous mass all of a sudden converted into solid and infusible foamed mass. The observed gel times for 5, 10, 20, and 25% TEA were 110, 80, 17, and 18 min, respectively. All the samples were further post cured at 135 °C for 30 min in order to cure traces of residual uncured resin. Cured resins were found to be insoluble in common solvents. ECH cured using 20 wt.% TEA is here after designated as ECHT-20 and used for further characterization.
2.5 Fabrication of jute/glass-ECH composites
For the preparation of jute/glass composites (Table ) required quantities of ECH (70% of fabric) and TEA were dissolved into a 500 ml beaker containing 200–250 ml MEK at room temperature. The resultant solution was heated at 80 °C with stirring for 40 min. The solution was allowed to cool to room temperature and was applied to jute/glass fabrics (20 cm × 20cm) with a smooth brush and solvent was allowed to evaporate at room temperature. Impregnated fabrics were staked one over the other and kept between two Teflon sheets. These Teflon sheets were kept between two preheated stainless steel plates and pressed under 27.58 MPa pressure at 100 °C for 2 h and at 135 °C for 4 h and 12 h at room temperature.
Table 1. Details of fabrication jute/glass-ECH composites.
2.6 Measurements
IR (KBR pellets) spectra of ECH and ECHT-20 were scanned on a Shimadzu Fourier transform infrared (FTIR)-8400 spectrometer over the frequency range from 4000 to 400 cm−1. Proton nuclear magnetic resonance (1HNMR) spectrum of ECH was scanned on a Bruker AVANCE II (500 MHz) spectrometer by using CdCl3 as a solvent and TMS as an internal standard. Epoxy equivalent of ECH was determined according to pyridine-pyridinium chloride method. Molecular weights and polydispersity index of ECH were determined by gel permeation chromatography using Perkin Elmer GPC (series 200) and using tetrahydrofuran as a solvent and standard polystyrene mixed beads at 30 °C. The machined composite samples were conditioned at room temperature (50% RH) for 24 h in a testing laboratory. The tensile strength (ISO/R 527-1996 Type-I) and flexural strength (ASTM D-790-2003), electric strength (IEC-60243-Pt-1-1998) and volume resistivity (ASTM D-257-2007) measurements were made on a Shimadzu Autograph Universal Tensile Testing Machine, Model No. AG-X Series at a speed of 10 mm/min, Universal Tensile Testing Machine, Model No. 1165, a high voltage tester (Automatic Electric – Mumbai) in air at 27 °C by using 25/75 mm brass electrodes and a Hewlett Packard high resistance meter in air at 25 °C after charging for 60 s at 500 V DC applied voltage, respectively. Measurements were carried out in triplicate and the mean values were considered. Differential scanning calorimetric (DSC) and thermogravimetric analysis (TGA) measurements were done on a Shimadzu DSC60 and Perkin Elmer TGA (Model Pyris-I) at 10 °C/min heating rate in nitrogen atmosphere.
3 Results and discussion
3.1 Epoxy equivalent and molecular weight
Observed epoxy equivalent value of ECH is 264 (average of three measurements). Weight average molecular weight, number average molecular weight, and molecular weight distribution of ECH were 809, 275, and 2.94, respectively.
3.2 Spectral analysis
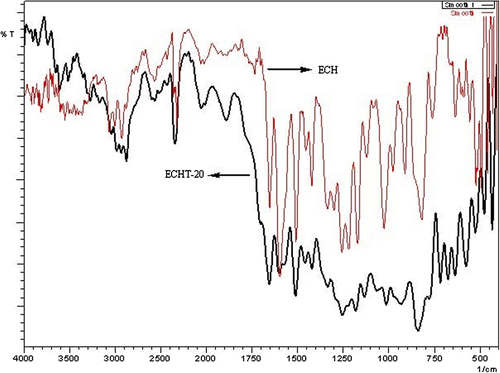
FTIR(KBr) spectra of ECH and ECHT-20 are presented in Figure . Characteristic IR absorption peaks (cm−1) for ECH are 3064-3003 (=CH str.), 1653(=CO and C=C str. of chalone), 1258 (C–O–C str.), 1220 (O–H def.), 1028 (C–OH str.), 912 and 821 (C–O str. of epoxy ring) and for ECHT-20 they are 3611 (O–H str.), 3042 (=CH str.), 1703 (C=O str.), 1655(C=C str. of chalcone), 1337 (C–N str. and C–OH str.), 1256(C–O–C str.), and 1134 (C–OH str.) besides normal modes of aliphatic, aromatic, and olefinic groups. In cured sample peak intensity due to epoxide ring stretching is drastically reduced, while O–H stretching increased considerably confirming formation of network structure.
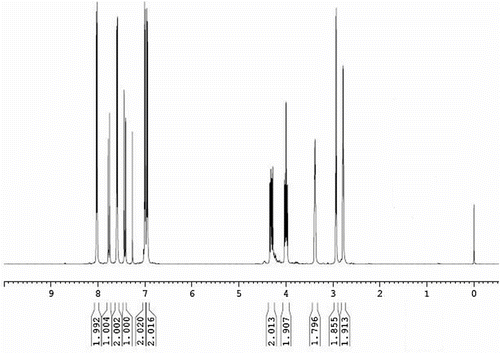
1HNMR (CDCl3) spectrum of ECH is presented in Figure . Different types of protons, their chemical shifts (ppm) and multiplicities are assigned as follows: 2.797–2.791[d, H(k)], 2.970–2.921 [q, H(j)], 3.406–3.367 [m, H(h)], 4.034–3.967 and 4.346–4.279 [m, H(g)], 6.962–6.945 [d, H(a), J = 8.5], 7.029–7.008[d, H(d), J = 10.5], 7.443–7.412[d, H(f), J = 15.5], 7.603–7.586[d, H(b), J = 8.5], 7.783–7.752 [d, H(e), J = 15.5] and 8.034–8.017 [d, H(c), J = 8.5]. Residual chloroform is appeared at 7.267 ppm. Thus, IR and NMR spectral data supported proposed structure of ECH.
3.3 Thermal analysis
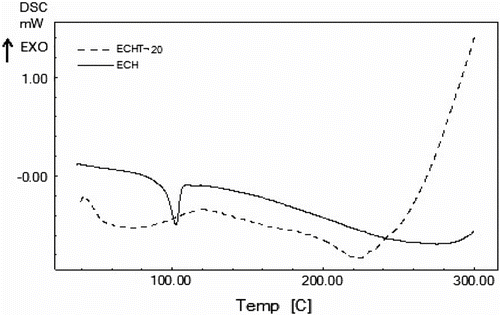
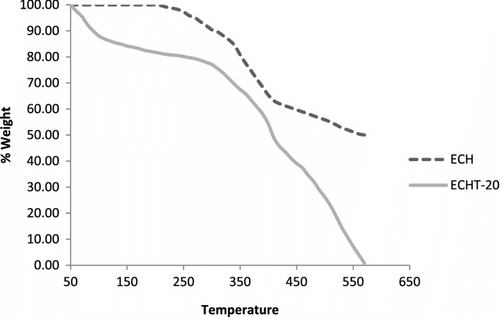
DSC thermograms of ECH and ECHT-20 are presented in Figure . Endothermic transition at 102 °C is assigned as melting transition of ECH and it is disappeared in case of ECHT-20 but a broad endothermic peak is observed at 223 °C. This transition is probably due to some physical change in the cured resin and it is further supported by no weight loss in its TGA thermogram (Figure ) at 225 °C. From Figure , it is observed that ECH followed apparently two-step decomposition, while ECHT-20 followed apparently three-step decomposition reactions. ECH and ECHT-20 are thermally stable up to about 225 and 288 °C, respectively. ECH involved 8.5 and 25.4% weight loss over temperature ranges from 225–300o and 330–430 °C, respectively, and left 47.2% residue at 600 °C. ECHT-20 involved 17, 17, and 33% weight loss over the temperature ranges from 288–377o, 377–425, and 454–550 °C, respectively. Thus, thermal stability of TEA cured resin has increased considerably as compared to neat resin. Ether and imine linkages are weak linkages in the cured resins. Selective degradation starts from such weak points and result in the formation of free radicals. These free radicals may undergo a series of reactions such as rearrangements, branching, crosslinking to form new compounds, which further degrade at elevated temperatures into either low molecular weight substances or highly thermally stable cross-linked polymer. A 47.2% residue left at 600 °C in case of ECH suggested formation of highly thermally cross-linked products, while ECHT-20 has degraded into low molecular weight substances. Thus cured resins possess fairly good thermal stability (225–288 °C).
3.4 Mechanical and electrical properties
Tensile strength, flexural strength, electric strength, and volume resistivity data of JECHT-20 and GECHT-20 are reported in Table from which it is observed that both the composites possess good tensile and flexural properties. For JECHT-20 flexural strength is decreased, while for GECHT-20 it is increased to some extent. In case of electric strength measurements flashover was observed above 8.9 and 21.7 kV/mm, respectively, for JECHT-20 and GECHT-20. Both the composites possess fairly good volume resistivity. Fairly good mechanical and electrical properties are indicative of rigid and polar nature of these composites. Mechanical and electrical properties of composites depend upon various factors such as amount, type, and arrangement of fibers within the composites as well as on the interactions between matrix and reinforcing agent, humidity, interfacial bonding, fillers, compatibilizers, impact modifier, mode of testing, degree of crosslinking, temperature, sample preparation, loading conditions, rate of loading, morphology, molecular architecture, molecular weight, impurities, geometry of electrodes, electrode material, sample thickness, structure, presence of polar groups in the polymer chains and fibers, etc. Citation[23–26]. Thus, fairly good mechanical and electrical properties of the composites signify their importance for low load bearing housing units and in electrical and electronic appliances.
Table 2. Mechanical and electrical properties of jute/glass-ECH composites.
4 Conclusions
ECH is found soluble in common organic solvents. A considerable enhancement in thermal stability is observed, when ECH is cured using TEA (288 °C) as compared to thermal curing of ECH (225 °C). Both types of composites are found to have good mechanical and electrical properties signifying their industrial applications in construction, electrical, and electronic industries.
Acknowledgments
Authors are thankful to Director SICART – V.V. Nagar for testing facilities and Department of Science & Technology, New Delhi for major research project grants (SERC Sl. No. 1272, 15-06-2010).
References
- Wu , CC and Lee , WJ . 2010 . Synthesis and properties of copolymer epoxy resins prepared from copolymerization of bisphenol A, epichlorohydrin, and liquefied dendrocalamus latiflorus . J. Appl. Polym. Sci. , 116 : 2065 – 2073 .
- Tian W, Yang ZG, Sun JY, Zheng J. Synthesis of high purity o-cresol novolac epoxy resins. International Conference on Electronic Packaging Technology and High Density Packaging 2008. ICEPT-HDP 2008; 2008 Jul 28–31; Shanghai, p. 1–5.
- Shokrall , SA and Al-Muaikel , NS . 2010 . Thermal properties of epoxy (DGEBA)/phenolic resin (novolac) blends . The Arabian J. Sci. Eng. , 35 : 7 – 14 .
- Bajpai , P and Bajpai , M . 2010 . Development of a high performance hybrid epoxy silicone resin for coatings . Pigm. Resin Technol. , 39 : 96 – 100 .
- Rong , M and Zeng , H . 1996 . Polycarbonate/epoxy semi-interpenetrating polymer network: 1. Preparation, interaction and curing behaviour . Polymer. , 37 : 2525 – 2531 .
- Jones , RR . 1993 . “ Composite materials ” . In Chemistry and technology of epoxy resins , Edited by: Ellis , B . 256 – 302 . London : Chapman & Hall . Chapter 8
- Shau , MD and Wang , TS . 1996 . Syntheses, structure, reactivity, and thermal properties of new cyclic phosphine oxide epoxy resins cured by diamines . J. Polym. Sci., Part A: Poly. Chem. , 34 : 387 – 396 .
- Xie , MR , Wang , ZG and Zhao , YF . 2001 . Synthesis and properties of a novel, liquid, trifunctional, cycloaliphatic epoxide . J. Polym. Sci., Part A: Polym. Chem. , 39 : 2799 – 2804 .
- Lin , CH , Huang , JM and Wang , CS . 2002 . Synthesis, characterization and properties of tetramethyl stilbene-based epoxy resins for electronic encapsulation . Polymer. , 43 : 2959 – 2967 .
- Tao , Z , Yang , S , Ge , Z , Chen , J and Fan , L . 2007 . Synthesis and properties of novel fluorinated epoxy resins based on 1,1-bis(4-glycidylesterphenyl)-1-(3′-trifluoromethylphenyl)-2,2,2-trifluoroethane . Euro. Polym. J. , 43 : 550 – 560 .
- Sua , WF , Lee , YC and Pan , WP . 2002 . Thermal properties of phthalic anhydride- and phenolic resin-cured rigid rod epoxy resins . Thermochim. Acta. , 392–393 : 395 – 398 .
- Bledzki , AK and Gassan , J . 1999 . Composites reinforced with cellulose based fibres . Prog. Polym. Sci. , 24 : 221 – 274 .
- Saheb , DN and Jog , JP . 1999 . Natural fiber polymer composites: a review . Adv. Polym. Tech. , 18 : 351 – 363 .
- Wu , S and Soucek , MD . 1998 . Kinetic modelling of crosslinking reactions for cycloaliphatic epoxides with hydroxyl- and carboxyl-functionalized acrylic copolymers: 1. pH and temperature effects . Polymer. , 39 : 5747 – 5759 .
- Liu , YL , Hsiue , GH , Chiu , YS , Jeng , RJ and Perng , LH . 1996 . Phosphorus-containing epoxy for flame retardant. I. Synthesis, thermal, and flame-retardant properties . J. Appl. Polym. Sci. , 61 : 613 – 621 .
- Choi , DH , Oh , SJ , Cha , HB and Lee , JY . 2001 . Photochemically bifunctional epoxy compound containing a chalcone moiety . Eur. Polym. J. , 37 : 1951 – 1959 .
- Tao , XT , Watanabe , T , Kono , K , Deguchi , T , Nakayama , M and Miyata , S . 1996 . Synthesis and characterization of poly(aryl ether chalcone)s for second harmonic generation . Chem. Mater. , 8 : 1326 – 1332 .
- Lin , CH , Chiang , JC and Wang , CS . 2003 . Low dielectric thermoset. I. Synthesis and properties of novel 2,6-dimethyl phenol-dicyclopentadiene epoxy . J. Appl. Polym. Sci. , 88 : 2607 – 2613 .
- Li , H , Wang , L , Jacob , K and Wong , CP . 2002 . Syntheses and characterizations of thermally degradable epoxy resins. III . J. Polym. Sci., Part A: Polym. Chem. , 40 : 1796 – 1807 .
- Moon , KS , Choi , HD , Lee , AK , Cho , KY , Yoon , HG and Suh , KS . 2000 . Dielectric properties of epoxy-dielectrics-carbon black composite for phantom materials at radio frequencies . J. Appl. Polym. Sci. , 77 : 1294 – 1302 .
- Lin , LL , Ho , TH and Wang , CS . 1997 . Synthesis of novel trifunctional epoxy resins and their modification with polydimethylsiloxane for electronic application . Polymer. , 38 : 1997 – 2003 .
- Vogel , AI , Tatchell , AR , Funis , BS , Hannaford , AJ and Smith , PWG . 1998 . Vogel’s textbook of practical organic chemistry , 5th ed. , London : Addison Wesley Longman .
- Dutta , S , Karak , N and Baruah , S . 2010 . Jute-fiber-reinforced polyurethane green composites based on Mesua ferrea L. seed oil . J. Appl. Polym. Sci. , 115 : 843 – 850 .
- Ray , D , Sarkar , BK , Das , S and Rana , AK . 2002 . Dynamic mechanical and thermal analysis of vinylester-resin-matrix composites reinforced with untreated and alkali-treated jute fibres . Compos. Sci. Technol. , 62 : 911 – 917 .
- Mavani , SI , Mehta , NM and Parsania , PH . 2006 . Performance evaluation of alkali and acrylic acid treated–untreated jute composites of mixed epoxy–phenolic resins . J. Appl. Polym. Sci. , 101 : 2363 – 2370 .
- Patel , VA and Parsania , PH . 2010 . Performance evaluation of alkali and acrylic acid treated untreated jute composites of mixed epoxy-phenolic resins . J. Reinf. Plast. Compos. , 29 : 725 – 730 .