Abstract
α-Amino acids, as one type of the major building blocks of living systems, are the principal components of all naturally occurring peptides and proteins. The aim of this paper was to synthesize and characterize of novel chiral nanostructured poly(amide-imide)s (PAIs) that are biologically active and biodegradable under a natural environment. PAIs were prepared from the polycondensation reaction of optically active diacids monomers containing different natural amino acids with 4,4′-methylene bis(3-chloro-2,6-diethylaniline) in molten tetrabutylammonium bromide as a green solvent. In vitro fungal colonization and weight loss studies showed that the synthetic polymer having alanine amino acid is biologically more active compared to the other components, nontoxic to microbial growth and could be classified as biodegradable compounds. The synthesized PAIs were characterized by different techniques. The field-emission scanning electron microscopy showed that the obtained PAIs were nanostructured polymers.
1. Introduction
Polymers are widely used in our diurnal life in such a way that life without them is not imaginable. The most synthetic polymers are non-biodegradable,Citation[1] and the resistance of synthetic polymers to the degrading action of biosystems is becoming more and more problematic. One of the solutions to resolve this problem is the development of biodegradable polymers. Biodegradable polymers are degraded eventually by microorganisms in the natural environments into carbon dioxide and water Citation[2–5], which is not evident in non-biodegradable ones. Biodegradation of synthetic polymers is an important property in their many applications such as drug delivery, gene transfer, tissue engineering, and regenerative medicine.Citation[6–9]
The synthesis and applications of optically active polymers are topics that nowadays attract much attention. These materials have exhibited many interesting properties such as chiral stationary phases for resolution of enantiomers in chromatographic techniques, chiral liquid crystals in ferroelectrics and nonlinear optical devices. Incorporation of amino acids into a polymeric backbone is useful because the amino acids are naturally occurring compounds, and therefore synthetic polymers based on these amino acids are anticipated to be nontoxic, biocompatible, and biodegradable.Citation[10–13] It seems that poly(amide imide)s (PAIs) with chiral structures based on amino acids are highly biodegradable. Also, PAIs are an important class of material having excellent resistance to high temperatures and favorable balance of other physical and chemical properties.Citation[14–17]
Application of green chemistry principles has already reduced the use of harmful solvents or other toxic ingredients, increased the utilization of renewable resources, and enhanced the design of products for end of life,Citation[18] so that they may be cost-effectively broken apart for reuse or recycling of their raw materials in the nature. Recently, water and ionic liquids (ILs) have been identified and are fascinating attention for organic as well as polymer synthesis as green solvent.Citation[19,20] ILs have attracted substantial interest in recent years. Their often unique property profiles are extremely low volatility, good thermal stability, wide liquid temperature range, nonflammability, good dissolving ability, low viscosity, negligible vapor pressure, and high ionic conductivity.Citation[21–29]
The aim of this study is the use of natural amino acid building blocks for efficient synthesis of novel biodegradable and chiral nanostructured PAIs, using molten tetrabutylammonium bromide (TBAB) as green medium. PAIs with main-chain chirality were synthesized via direct step-growth polymerization reaction of different chiral diacids (4a-4d) with 4,4′-methylenebis(3-chloro-2,6-diethylaniline) (MCDEA). In vitro, fungal colonization and weight loss of the synthetic polymers were tested to assure their probable biologically activity and biodegradability under natural environment.
2 Experimental
2.1 Materials
The following analytical grade chemicals were purchased from Fluka Chemical Co. (Buchs, Switzerland) and Merck Chemical Co. Pyromellitic dianhydride and TBAB was purchased from Merck Co. (Darmstadt, Germany) and was used without further purification. N,N′-Dimethylformamide (DMF) was dried over barium oxide and then was distilled under reduced pressure. L-Alanine (C3H7NO2), L-isoleucine (C6H13NO2), L-phenylalanine (C9H11NO2), L-valine (C5H11NO2), and MCDEA (C21H28N2Cl2) were used as obtained without further purification.
2.2 Techniques
Cultured samples in Petri plate were photographed with a light digital camera (Canon DS126181, Japan). Inherent viscosities were measured by a standard procedure using a Cannon–Fenske Routine Viscometer (Germany). Specific rotation was measured with a Jasco (Osaka, Japan) P-1030 polarimeter at the concentration of 0.5 g/dL at 25 °C. Surface morphology of polymers was characterized using field emission scanning electron microscopy (FE-SEM; Hitachi S-4160). Proton nuclear magnetic resonance (1H-NMR, 400 MHz) spectra were recorded in dimethylsulfoxide (DMSO-d6 ) solution using a Bruker (Germany) Avance 400 instrument. Multiplicities of proton resonance were designated as singlet (s), doublet (d), and multiplet (m). Infrared spectra of the samples were recorded at room temperature in the range 4000–400 cm−1, on a spectrophotometer (Jasco-680, Japan). Spectra of solids were obtained using KBr pellets. Vibrational transition frequencies are reported in wavenumber (cm−1).
2.3 Polymer synthesis
Optically active monomers, N,N′-(pyromellitoyl)-bis-L-amino acids (4a–4d), were prepared according to our previous published article.Citation[30]
The PAIs were prepared using molten TBAB and triphenyl phosphite (TPP), by the following general procedure: into a 25 mL flask fitted with a mechanical stirrer, diacid (4a–4d) (1.95 × 10−4 mol), MCDEA (1.95 × 10−4 mol), and TBAB (7.80 × 10−4 mol) were placed; then after the mixture was completely ground, TPP (7.80 × 10−4 mol) was added; the reaction temperature was raised to 120 °C and held for 12 h. On cooling to room temperature, the viscous polymer solution was dropped into a 20 mL of methanol giving rise to polymer precipitate.
2.4 In vitro fungal colonization and weight loss test
Environmental compatibility of materials was expressed as the rate of colonization on each specimen in vitro conditions. Samples with 200 mg of each monomer and derived polymers were placed on glass slides in the middle of Petri plates containing Potato Dextrose Agar. Specimens in three replicates were then incubated with saprophytic fungal spores in a dark incubator at 25–28 °C and 80% humidity. Complete colonization of nontoxic specimens and formation of fungal thallus were occurred approximately after 15 days at 25 °C. Thereafter, biodegradability was determined by measuring weight loss of the specimens buried in Petri plates. Each specimen was dug out of the fungal thallus after buried for 60 days, and then dried to a constant weight at 60 °C in a vacuum oven. The dried samples were weighed to measure the weight loss. The weight loss W loss(%) was calculated using the formula: where W initial and W final are the weights of specimen before and after buried in vitro condition, respectively.
3 Results and discussion
The chiral diacid monomers (4a–4d) were synthesized by the condensation reaction of one equimolar of pyromellitic dianhydride (1) and two equimolar of amino acids (L-phenylalanine, L-alanine, L-isoleucine and L-valine) in refluxing acetic acid as shown in Scheme .
Scheme 1 Preparation of amino acid containing diacid monomers, and polycondensation reactions of monomer 5 with different diacids.
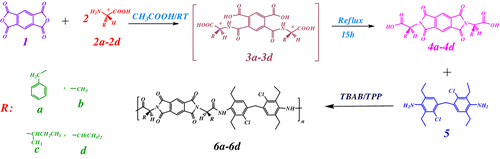
Optically active PAIs (6a–6d) were synthesized by the direct polyamidation reactions of an equimolar mixture of MCDEA with an equimolar of different diacids (4a–4d) in molten TBAB medium (Scheme ). Some properties of these PAIs are listed in Table . Also, the data obtained from specific rotations of polymers confirmed that the polymers are optically active (Table ).
3.1 Structural characterization of PAIs
The structure of PAIs was confirmed by FT-IR and 1H-NMR spectroscopy techniques. The data of FT-IR and 1H-NMR are presented in Table .
Table 1. Synthesis and some physical properties of PAIs.
Table 2. FT-IR and 1H-NMR characterization of PAIs.
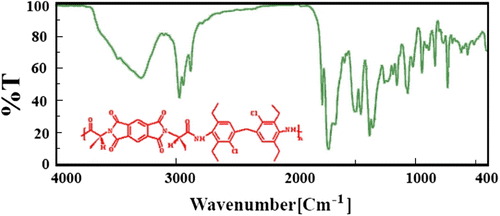
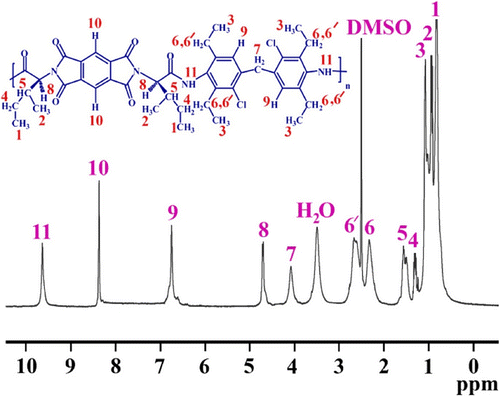
FT-IR spectrum of PAI6b shows absorption of amide, carbonyl and imide groups at 1776 and 1724 cm−1, amide N–H at 3290 cm−1, respectively (Figure ). The other PAIs had similar functional groups. 1H-NMR spectrum (400 MHz) of PAI6c is shown in Figure . In all of the 1H-NMR spectra, the proton of the chiral center appeared around 5.0 ppm (Table ). Also, resonance of the amide protons appeared around 9.6 ppm.
3.2 Morphology of polymers
Nanostructure polymer systems are known to be potential candidates to develop synergistic effects which could yield better properties than those of the pure components.Citation[31] Polymers which have particle size at the nanometer length scale are considered as nanostructured materials.Citation[32] In this investigation, FE-SEM pictures of amino acid-based PAIs showed that the obtained polymers have globular structures in nano-scale morphology (Figure ).
3.3 Fungal colonization and weight loss
The results revealed significant differences among specimens in regard to fungal colonization. Diamine monomer (5) significantly inhibited the growth and colonization of fungal saprophytes while other specimens did not show any inhibition effect on the growth of fungi (Figure ).
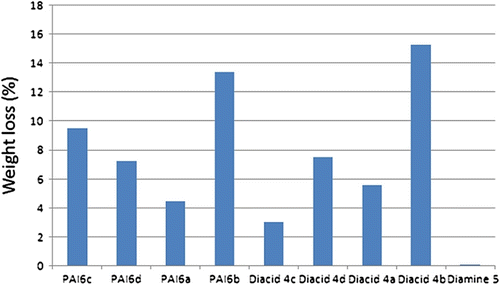
The weight loss of colonized compounds after 60 days ranged from 0.0 to 13.43% where diacid monomer 4b and PAI6b had the maximum rate of weight loss while the weight losses of diamine monomer (5) was the minimum (Figure ). Diacid 4b and PAI6b are based on L-alanine and therefore it may be concluded that the presence of L-alanine in the composite may accelerate the biodegradation of polymer. These results may strongly indicate the biodegradation and biocompatibility of the synthesized polymers containing alanine probably due to susceptibility to fungal enzymatic degradation.
4 Conclusions
The use of ILs in polymer synthesis is green chemical method and they are harmless solvents in the polymerization. Therefore, we determined to synthesize chiral PAIs derivatives of several natural amino acids in high purity and good yields using molten TBAB as an inexpensive and small poisonous IL. Polymers have attractive properties such as biodegradability and biocompatibility due to the presence of amino acids in the polymer main chain. Morphological study of the obtained polymers revealed that all of the polymers possess nanoarchitecture morphology. In vitro fungal colonization and weight loss of the synthetic polymers showed that PAIs are probably biologically active and biodegradable under natural environment.
Acknowledgments
We wish to express our gratitude to the Research Affairs Division, Isfahan University of Technology (IUT) for partial financial support. Further, partial financial support from the National Elite Foundation (NEF) and Center of Excellency in Sensors and Green Chemistry Research (IUT) is gratefully acknowledged.
References
- Eubeler JP, Bernhard M, Zok S, Knepper TP. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. Trends Anal. Chem. 2009;28:1057–1072.
- Funabashi , M , Ninomiya , F and Kunioka , M . 2009 . Biodegradability evaluation of polymers by ISO 14855–2 . Int. J. Mol. Sci. , 10 : 3635 – 3654 .
- Arvanitoyannis , I , Nikolaou , E and Yamamoto , N . 1994 . Novel biodegradable copolyamides based on adipic acid, bis(p-aminocyclohexyl)methane and several a-amino acids: synthesis, characterization and study of their degradability for food packaging applications: 4 . Polymer. , 35 : 4678 – 4689 .
- Vert , M . 2005 . Aliphatic polyesters: great degradable polymers that cannot do everything . Biomacromolecules. , 6 : 538 – 546 .
- Sun , H , Meng , F , Dias , AA , Hendriks , M , Feijen , J and Zhong , Z . 2011 . α-amino acid containing degradable polymers as functional biomaterials: rational design, synthetic pathway, and biomedical applications . Biomacromolecules. , 12 : 1937 – 1955 .
- Li , J , Kong , M , Cheng , XJ , Li , JJ , Liu , WF and Chen , XG . 2011 . A facile method for preparing biodegradable chitosan derivatives with low grafting degree of poly(lactic acid) . Int. J. Biol. Macromol. , 49 : 1016 – 1021 .
- Okamura , A , Hirai , T , Tanihara , M and Yamaoka , T . 2002 . Synthesis and properties of novel biodegradable polyamides containing α-amino acids . Polymer. , 43 : 3549 – 3554 .
- Mitra , T , Sailakshmi , G , Gnanamani , A , Raja , STK , Thiruselvi , T , Gowri , VM , Selvaraj , NV , Ramesh , G and Mandal , AB . 2011 . Preparation and characterization of a thermostable and biodegradable biopolymers using natural cross-linker . Int. J. Biol. Macromol. , 48 : 276 – 285 .
- Almeida , JF , Ferreira , P , Lopes , A and Gil , MH . 2011 . Photocrosslinkable biodegradable responsive hydrogels as drug delivery systems . Int. J. Biol. Macromol. , 49 : 948 – 954 .
- Mallakpour S, Dinari M. Insertion of novel optically active poly(amide-imide) chains containing pyromellitoyl-bis-L-phenylalanine linkages into the nanolayered silicates modified with L-tyrosine through solution intercalation. Polymer 2011;52:2514–2523.
- Mallakpour S, Zadehnazari A. Advances in synthetic optically active condensation polymers–a review. Express Poly. Lett. 2011;5:142–181.
- Liu , Z , Zhou , J , Liang , H and Sun , J . 2012 . Synthesis and characterization of thermoresponsive diblock copolymer bearing amino acid moieties via click reaction . Polym. Bull. , 68 1315–1325
- Creutz , M . 2011 . Confinement chiral symmetry and the lattice . Acta. Phys. Slovaca. , 61 : 1 – 127 .
- Yang , CP , Yang , CC and Chen , RS . 2001 . Synthesis and properties of alternating copolyamide-imides based on 2,6-bis(4-aminophenoxy)naphthalene and comparison with its related isomeric polymers . J. Polym. Sci., Part A: Polym. Chem. , 39 : 2591 – 2601 .
- Liaw , DJ and Chen , WH . 2006 . High glass transitions of novel organosoluble polyamide-imides based on noncoplanar and rigid diimide-dicarboxylic acid . Polym. Degrad. Stab. , 91 : 1731 – 1739 .
- Hu , Z , Li , S and Zhang , C . 2007 . Synthesis and properties of polyamide-imides containing fluorenyl cardo structure . J. Appl. Polym. Sci. , 106 : 2494 – 2501 .
- Tagle , LH , Terraza , CA , Leiva , A , Yazigi , N and Lopez , L . 2010 . Poly(imide-amide)s and poly(imide-ester)s obtained from N, N′-(4,4′-me, R-diphthaloyl)-bis-glycine acid dichloride (R = me, et): synthesis, characterization and thermal studies . J. Appl. Polym. Sci. , 117 : 1526 – 1534 .
- Virkutyte , J and Varma , RS . 2011 . Green synthesis of metal nanoparticles: biodegradable polymers and enzymes in stabilization and surface functionalization . Chem. Sci. , 2 : 837 – 846 .
- Lu , J , Yan , F and Texter , J . 2009 . Advanced applications of ionic liquids in polymer science . Prog. Polym. Sci. , 34 : 431 – 448 .
- Brennecke , JF , Rogers , RD and Seddon , KR . 2007 . Ionic liquids IV not just solvents anymore , Washington , DC : American Chemical Society .
- Kim , DH , Im , JK , Kim , DW , Cheong , M , Kim , HS and Mukherjee , DK . 2011 . Palladium catalysed asymmetric alkylation of benzophenone Schiff base glycine esters in ionic liquids . J. Chem. Sci. , 123 : 4674 – 4681 .
- Mallakpour , S and Rafiee , Z . 2011 . New developments in polymer science and technology using combination of ionicliquids and microwave irradiation . Prog. Polym. Sci. , 36 : 1754 – 1765 .
- Mallakpour S, Dinari M. Novel nanostructure amino acid-based poly(amide–imide)s enclosing benzimidazole pendant group in green medium: fabrication and characterization. Amino Acids. 2012;43:1605–1613.
- Mallakpour , S and Yousefian , H . 2008 . Direct polyamidation in molten tetrabutylammonium bromide: novel and efficient green media . Polym. Bull. , 60 : 191 – 198 .
- Mallakpour , S and Dinari , M . 2010 . High-speed microwave-promoted direct poly-amidation reactions of bulky chiral dicarboxylic acide with different aromatic diamines in imidazolium types ionic liquid as a reaction medium . Design. Monom. Polym. , 13 : 51 – 64 .
- Mallakpour , S and Dinari , M . 2010 . High performance polymers in ionic liquids: a review on prospects for green polymer chemistry. Part I: polyamides . Iran. Polym. J. , 19 : 983 – 1004 .
- Wilpiszewska , K and Spychaj , T . 2011 . Ionic liquids: media for starch dissolution, plasticization and modification . Carbohydrate Polym. , 86 : 424 – 428 .
- Martinez-Palou , R . 2007 . Ionic liquid and microwave-assisted organic synthesis: A green and synergic couple . J. Mex. Chem. Soc. , 51 : 252 – 264 .
- Zhu YJ, Wang WW, Qi RJ, Hu XL. Microwave-assisted synthesis of single-crystalline tellurium nanorods and nanowires in ionic liquids. Angew. Chem. 2004;43:1410–1414.
- Mallakpour S, Dinari M. Progress in synthetic polymers based on natural amino acid. J. Macromol. Sci., Part A: Pure. Appl. Chem. 2011;48:644–679.
- Ania , F , Balta-Calleja , FJ , Flores , A , Michler , GH , Scholtyssek , S , Khariwala , D , Hiltner , A , Baer , E , Rong , L and Hsiao , BS . 2012 . Nanostructure and crystallization phenomena in multilayered films of alternating PP and PA6 semicrystalline polymers . Eur. Polym. J. , 48 : 86 – 96 .
- Curiel MMLG. Polymer-inorganic nanocomposites [dissertation]. University of Twente, Netherlands; 2004.