Abstract
Amphoteric terpolymers composed of acrylamide, 2-methylacryloylxyethyl trimethyl ammonium chloride, and 2-acrylamido-2-methyl-1-propane sulfonate were prepared via free-radical polymerization in aqueous solution of ammonium sulfate (AS), using poly(2-methylacryloylxyethyl trimethyl ammonium chloride) as stabilizer to yield terpolymers with random distributions. The particle size of the synthesized amphoteric polyacrylamide (AmPAM) ranged from 1 to 8 μm and the intrinsic viscosity ranged from 5.6 to 14.2 dL/g. The influences of aqueous solution of AS concentration, stabilizer concentration, initiator concentration, monomer concentration on the conversion of monomer, the intrinsic viscosity, and the particle size were systematically investigated. The characteristic of amphoteric polymer was also be studied. Throughout the study, we found that the isoelectric point of the amphoteric polyelectrolyte we synthesized is about 7. With the increase in sodium chloride, the reduced viscosity of AmPAM increased. Among the different type of inorganic salt, the degree of influence of anionic is I > Br > Cl.
1. Introduction
Water-soluble polymers based on acrylamide (AM) have shown significant industrial application and are widely used as flocculants, adhesives and viscosity-control agents for enhanced oil recovery, drainage, and retention aids in the paper manufacturer, etc.Citation[1–5]
Amphoteric polyacrylamide (AmPAM) Citation[6,7] carries anionic and cationic charge group on the polymer backbone, which can adsorb both organic and inorganic suspension substances. So it is more suitable to deal with occasions that other flocculants cannot treat. Comparing with cationic polyacrylamide (CPAM) and anionic polyacylamide (APAM), AmPAM has the advantage of the anti-polyelectrolyte effect Citation[7] and a wide application range of pH value.Citation[8–11]
The main types of methods for producing AM-based polymers include aqueous solution polymerization,Citation[12] dispersion polymerization,Citation[13] inverse emulsion (microemulsion) polymerization,Citation[14,15] precipitation polymerization, and suspension polymerization.Citation[3] For aqueous solution polymerization, the PAM has generally been used as dry powder to reduce transport cost. However, the dissolution of the dry powder requires the consumption of energy intensively. In addition, the fine powder in the air is harmful to the worker. For inverse emulsion polymerization, it is easy to handle. Comparing with other polymerization method, the main advantage of this method is that it could obtain both high molecular weight and high reaction rate. However, there are some drawbacks for this method. For example, the emulsion polymerization may cause environmental pollution because of using organic substance as continues phase and surfactant system.
Dispersion polymerization is an attractive alternative to other polymerization methods in producing polymer particles of 0.1–10 μm in a single step. The dispersions have the property of going instantaneously into solution when diluted with water which is very desirable property for PAM, as the dissolution of dry PAM powder is a slow process.Citation[16–20]
In a typical dispersion polymerization, the monomer, the initiator, and the stabilizer are soluble but the formed polymer is insoluble in the dispersion medium, and therefore, the latter separates out from the media at a critical chain length, the oligomer precipitate and adsorb the stabilizer to form stable particle nuclei, and the resulting polymer particles become stable. The origin of stability of the dispersed particles has been attributed to ‘steric stabilization.’Citation[21]
Most reports on dispersion polymerization deal with polymerization both in non-polar and polar media.Citation[17,22–24] Guha and Ray et al. Citation[22–25] had reported the dispersion polymerization of AM in tert-butyl alcohol water media. However, studies on dispersion polymerization in aqueous without any organic solvent are rather scarce.Citation[26,27] In this kind of dispersion polymerization, instead of using an organic solvent, the system usually contains a high concentration of inorganic salt to induce the Phase separation of the resulting polymer by salting-out effect. Because the aqueous medium is free of organic solvent, the polymer can be directly applied as prepared. The high concentration of inorganic salt in the aqueous medium does not cause any problem in practical application, because the amount of the dispersion employed is very small.
In this study, the amphoteric dispersion terpolymer of AM, 2-methylacryloylxyethyl trimethyl ammonium chloride (DMC), and 2-acrylamido-2-methyl-1-propane sulfonate (AMPS) were synthesized in aqueous solution of ammonium sulfate (AS) by using 2,2-azobis(2-methylpropionconidine) dihydrochloride (V-50) as initiator and poly(2-methylacryloylxyethyl trimethyl ammonium chloride) (PDMC) as steric stabilizer. The positive-charged side groups in the polymer chains of PDMC were anticipated to enhance the stabilization of the lattices by electrostatic repulsion. General dispersion polymerization is highly sensitive to small changes in reaction parameter. The effects of the concentration of salt, stabilizer, initiator, and monomers on the dispersion polymerization and the characteristic of amphoteric polymer were investigated.
2 Experimental
2.1 Materials
DMC was purchased from Wuxi xingyu Chemical Industries Ltd (China) as aqueous solution (74.68 wt%) and was used as received. PDMC was prepared by free radical solution polymerization.Citation[18]
AM (Tianjing Chemical Reagent Co. Ltd China, A.R. grade), AS ((NH4)2SO4) (Sinopharm Chemical Reagent Co. Ltd China, A.R. grade), AMPS (Zhengxing Chemical Industries, China, G.R. grade), the water soluble initiators 2,2-azobis (2-methylpropionconidine) dihydrochloride (V-50) (Aladdin Chemistry Co. Ltd A.R. grade), deionized water was used throughout this study, other reagents were A.R. Grade used without further purification.
2.2 Dispersion polymerization
The polymerization reactions were carried out in a four-neck 250-mL glass reactor. The reactor was charged with AS, AM, AMPS, DMC and PDMC. Then, the system was purged with N2 flow to remove oxygen for 30 min and the temperature was controlled at 55 °C in a water bath. After that, the polymerization was initiated by adding V-50 aqueous solution under continues stirring. After reacting for 8 h, the AmPAM aqueous dispersion copolymer was obtained. In this study, a high viscosity process (Wiesenberger effect) was usually observed during the dispersion polymerization, which was also reported by Ye et al. Citation[28]
As for the synthesis of P(AM-AMPS), it was carried out with the same conditions as above without DMC.
2.3 Characterization
The polymerization product was purified according to the following procedures: the AmPAM dispersion was dissolved in water and then precipitated by a large quality of ethanol and then washed with acetone. The operation was repeated several times to remove the inorganic salts and unreacted monomers.
The intrinsic viscosity and the reduced viscosity of the terpolymer were determined in a 1 mol/L NaCl solution at 30°C with an Ubbelhode capillary viscometer.
The total monomer conversion was obtained by determining the residual contents of AM, AMPS, and DMC with bromating method, and the residual monomer was presumed to be DMC.
The average diameter of the obtained latexes was measured with DLS (Malvern Zetasizer Nano-ZS, UK) Instruments. Each sample was tested for three times to obtain the average particle size. To ensure an extended electrical double layer do not artificially increase the particle size, the concentration of AS solution used to dilute the dispersion was kept constant at 25 wt%, which was similar to the original dispersion samples.
The morphologies of the particles in AmPAM dispersion were obtained with optical microscope (Olympus BX51, Japan).
Rotational viscometer (NXS-11A, China) was used to obtain apparent viscosities of the AmPAM dispersion aqueous solution.
Differential scanning calorimetey (DSC, Q-100, TA Instrument, US) curves of the dried AmPAM samples were obtained at a heating rate of 10°C/min and a scanning range from 10 to 300 °C under nitrogen atmosphere.
Thermogravimetric analysis curves (TG, DIAMOND, PERKIN-ELMER) of the samples were got from the room temperature to 300°C at the heating rate of 10°C/min.
The dispersed product was purified via exhaustive dialysis against DI water, then the terpolymers were dried at 50 °C for 24–48 h. The NMR spectrum of the dried terpolymer sample was obtained in D2O with a Varian (600 MHz, 1H) NMR spectrometer.
3 Results and discussion
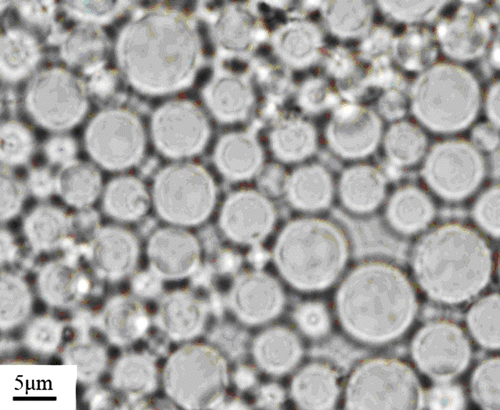
3.1 Micrographs of the dispersed particles
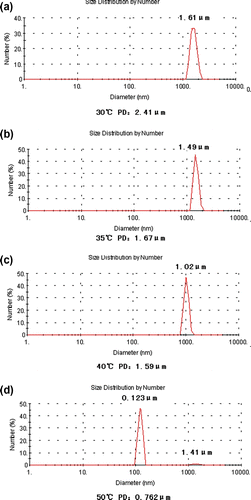
Figure shows the micromorphology of the dispersed particles. Polydisperse spherical particles were formed as shown in Figure , the particle size ranged from 1 to 8 μm. The size of particles varies according to test temperature and the concentration of dilute solution. Figure shows that with the increase in test temperature and the concentration of dilute solution, the particle size would decrease. It can be seen that salting-out effect decreased with the decrease in the salt concentration, that results in a shrink of molecular chain and smaller particles. Figure shows that with the increase in the test temperature the chain of macromolecules assemble leading to the decrease in particle size.
3.2 1H NMR spectrum of AmPAM
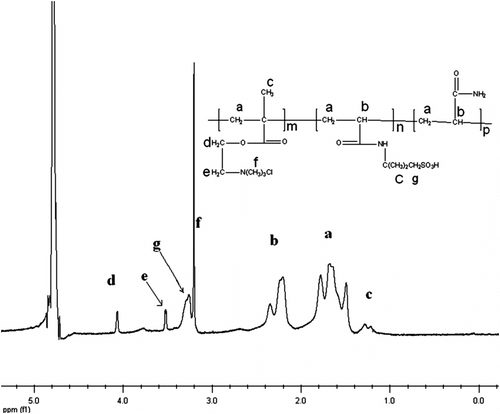
1H NMR spectrum was applied to characterize the structure of AmPAM, as shown in Figure , and the 1H NMR spectrum of AmPAM shows a sharpe singlet at 3.14 ppm (f), which was assigned to the three equivalent methyl groups of ammonium. The singlet at 3.27 ppm (g) was from the –CH2– beside the sulfonic acid group. The analysis of the 1H NMR spectrum provide support for the terpolymer of AM, AMPS, and DMC.
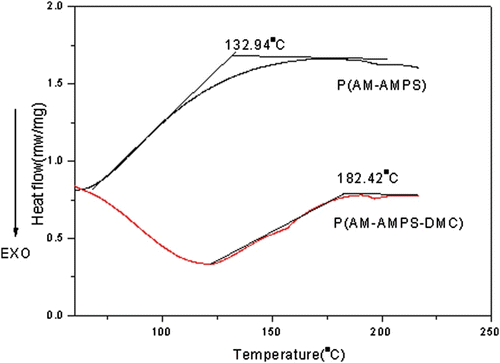
3.3 DSC curve of AmPAM dispersion
The DSC curve of the purified AmPAM product and P(AM-AMPS) is shown in Figure . The picture shows that there is a broad grass transition in the curve of (AM-AMPS) which can be explained that the synthesis of P(AM-AMPS) was through aqueous solution polymerization, the distribution of molecular weight is wider that through dispersion polymerization which leads to a broad grass transition. The glass transition temperature (T g) of P(AM-AMPS) is 132.94 °C, whereas the T g of the AmPAM dispersion product is 182.42 °C, The T g of the AmPAM was higher than the that of the P(AM-AMPS), which would be attributed to the attractive force between cationic group and anionic group of AmPAM molecular chains which decreases the free volume, making the polymer structure more rigid.
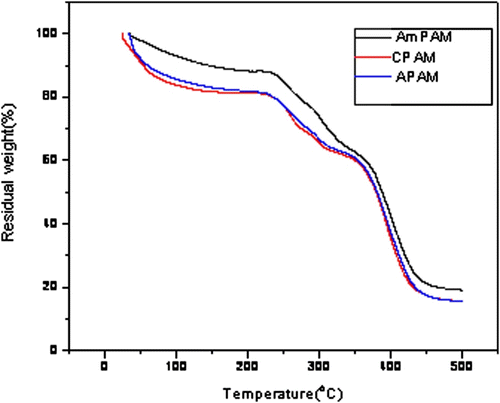
3.4 TG curve of AmPAM dispersion
TG analysis was applied to evaluate the heat-resistant ability of AmPAM, APAM and CPAM, and the results were shown in Figure . We can see from the TG curves that the mass retaining ratio of AmPAM was higher than that of APAM and CPAM at the same temperature. That was due to the more tightening space structure of AmPAM because of the electrostatic attraction between the cation and the anion. From the TG curves, we can draw a conclusion that the heat-resistant ability of AmPAM was better than that of APAM and CPAM.
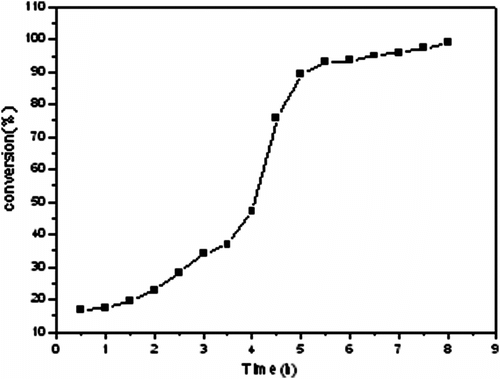
3.5 Analysis of polymerization rate with conversion
Figure shows the typical conversion–time curve, which is generally S-shaped. At the initial stage of reaction, the system is homogeneous, after a certain reaction time, it had been proposed that the nuclei are generated by a self or aggregative nucleation mechanism. The description of the mechanism is as follows: as soon as the polymerization begins, primary radicals, generated by decomposition of the initiator, grow in the continuous phase until they reach their critical chain length before they precipitate to form nuclei, either by a self or by a aggregative mechanism, these nuclei are unstable and aggregate with each other; Concurrently, they absorb the stabilizer and finally become stable (mature) particles. The mature particles capture the oligo-radical and nuclei generated in the continuous phase and thus reduced the rate of particle formation. When enough mature particles are formed to capture all of the oligo-radical and nuclei, no more new particles will be formed and the formation stage is complete. The existing particles continue to grow by capturing the nuclei and oligo-radicals, the captured oligo-radicals will either continue to grow inside the particles or terminated with each other. At the end of the polymerization, nuclei formation stops owing to the exhaustion of either the monomer or the radicals; Mature particles are finally observed.
Based on the mechanism above, the polymerization may happen simultaneously in the solution phase and in the particles phase. The influences of polymerization parameters in the present system are explained in the following section based on the mechanism above.
3.6 Effect of salt concentration
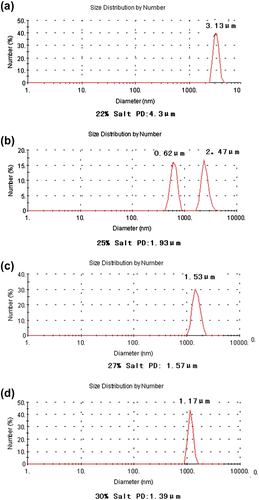
The polymerization experiments were carried out with varying AS concentration from 23 to 28 wt%, whereas the concentrations of monomers, stabilizer and initiator as well as the molar ratio of AM:AMPS/DMC were kept constant at 12 wt%, 3.0 wt%, 15.0 × 10−4 g/g(monomers)−1, and 80:10:10, respectively. The effects of AS concentration on intrinsic viscosity and particle size of terpolymer dispersion were studied as shown in Table .
Table 1. Effect of (NH4)2SO4 concentration on dispersion polymerization.
In this study, when the AS concentration is lower than 24 wt%, unstable dispersion occurred because of the insufficient salting-out effect. Copolymer particles would not precipitate but dissolved in water, resulted in a high in-process viscosity, and yielding a gel-like polymer solution. When the concentration of AS increased, the precipitation of polymer would occur, the stable dispersion could be produced successfully. However, the AS concentration could not be too high. With the increase of the concentration of AS, the stabilization efficiency of PDMC would decrease remarkably because the chains of PDMC shrink too much. In the condition of high concentration of AS, dispersed particles may be formed quickly at the earlier stage, but there are not enough stabilizer molecules to protect the new formed particles, and a high yield of the coagulum was obtained. So, in our system the moderate concentration of AS is 23–27 wt%.
Increasing the concentration of AS, the intrinsic viscosity of the polymer dispersion increased at first and then decreased. At a relatively low AS concentration, the effect of salting-out was feeble, the proportion of polymerization in solution phase increased with the decrease in the concentration of AS. It is known that polymerization in the particle phase favors formation of higher molecular weight polymers than in the solution phase as a result of the decrease in intrinsic viscosity. Increasing the concentration of AS in the reaction system may compress the channels between the particles, as the particles shrink with the increase in the AS concentration. This can hinder the entering of oligo-radicals and monomer to the particles, in this condition the proportion of polymerization in solution phase increased. With the effects mentioned previously, the intrinsic viscosity of the polymer dispersion increased at first and then decreased with the concentration of AS.
It was indicated that the obtained particle size decreased first and then increased with the increase in the salt concentration. Salting-out effect decreased with the decrease in the salt concentration, and the critical oligomer chain length of phase separation became larger, leading to a quickly coalescence of the particle, yielding a larger particle size. However, at a high AS concentration, the salting-out effect became strong, leading to the formation of more primary particles, but the high AS concentration may decrease the stabilizer’s adsorption rate and would hinder the system to get stable dispersion. Too many particles congregate and larger particles were formed. So the particle size reached a minimum when the AS concentration was at 25 wt%.
3.7 Effect of the stabilizer concentration
The polymerization experiments were carried out with PDMC concentration from 1.0 to 5.0 wt%, whereas the concentration of monomer, salt, initiator and the molar ratio of AM/AMPS/DMC were kept constant at 12 wt%, 25 wt%, 15.0 × 10−4 g/g(monomers)−1 and 80:10:10, respectively.
A stable system would not be obtained with too low or high concentration of PDMC. When the concentration of PDMC was lower than 1 wt%, the stabilizer was not enough to stabilize the particles, and an agglomerating system was obtained. Similarly, when the concentration of PDMC was higher than 4.0 wt%, the dispersion became very unstable, some coagulated lumps separated from the medium. This was probably because the extra stabilizer molecules might link between the particles rather than participate in stabilization, inducing agglomeration. The suitable PDMC concentration was from 1.0 to 3.0 wt%.
It was also indicated in Table that increasing the concentration of PDMC, the particle size decreased. Because increasing the stabilizer concentration can prevent the particles from coalescence.
Table 2. Effect of stabilizer concentration on dispersion polymerization.
It was indicated that the obtained intrinsic viscosity increased first and then decreased with the increase of the PDMC concentration. With a high concentration of the stabilizer, there were more small particles to capture the growing oligo-radicals initiated in the solution, leading to a higher rate of polymerization in the particle phase. Because of restricted radical termination in the gel particle, the intrinsic viscosity would increase. On the other hand, the electrostatic repulsion increased with the increase of the concentration of PDMC, under this condition, the particle would be hard, and the oligo-radicals would be difficult to get into the particles. So the intrinsic viscosity decreased.
3.8 Effect of monomer concentration
The polymerization experiments were carried out with monomer concentration from 8 to 18 wt%, whereas the concentration of stabilizer, salt, and initiator and the molar ratio of AM/AMPS/DMC were kept constant at 3.0 wt%, 25 wt%, 15.0 × 10−4 g/g(monomers)−1 and 80:10:10, respectively.
The data of Table indicated that the molecular weight and the particle size increased with the increase in monomer concentration. When the monomer concentration was above 15 wt%, stable dispersion could not be obtained. The polymer dispersion had a very high apparent viscosity and a white gel would be formed with transparent salt solution exuded after some days.
Table 3. Effect of monomer concentration on dispersion polymerization.
With a high monomer concentration, the contact probability between the radicals and the monomers increased, which resulted in the high molecular weight. Congregation of primary particles caused the rise of final particle size.
3.9 Effect of initiator concentration
The polymerization experiments were carried out with initiator concentration from 15.0 to 20.0 × 10−4 g/g(monomers)−1, whereas the concentration of stabilizer, salt, monomer and molar ratio of AM/AMPS/DMC were kept constant at 3.0, 25, and 12 wt%, and 80:10:10, respectively.
Table showed the effect of initiator concentration on polymerization. When the initiator concentration was lower than 15.0 × 10−4 g/g(monomers)−1, the concentration of residual monomer was high and the dispersion was unstable because of the coalescence of particles.
Table 4. Effect of initiator concentration on dispersion polymerization.
It can be seen that particle size and monomer conversion rate increased with the increase in the initiator concentration. The reason was that a high initiator concentration lead to a greater formation rate of unstable oligo-radicals. These primary particles were not stabilized enough by the stabilizer, leading to a greater rate of coagulation and resulting in larger size particles. Table showed that intrinsic viscosity decreased with the increase in initiator concentration. This was an expected behavior. High initial rate of free-radical formation at high initiator concentration led to a high degree of termination, and hence, the low average molecular weight.
3.10 The characteristic of amphoteric polymer
Due to the anionic and cationic charge on the molecular chain, the amphoteric polyelectrolyte show different behavior from the polyelectrolyte. We investigate the effect of pH, the concentration of salt and the type of salt on the water solution of AmPAM.
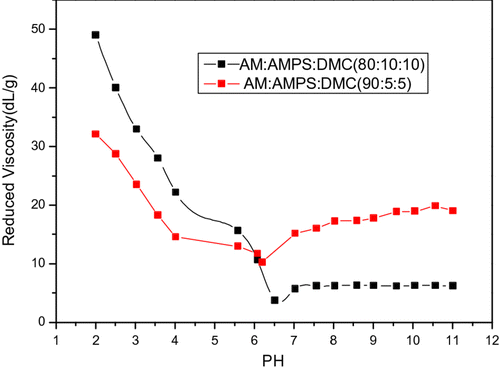
Figure shows that at the pH value of about 7 (6.5, 6.2), the reduced viscosity is the lowest. The lowest point is the isoelectric point of AmPAM. At the isoelectric point, because of the recombination of anionic and cationic charge, the residual charge is the least, leading to the reduce of repulsive force between charges, because of which, the molecular chain shrink, the viscosity decrease. (The concentration of AmPAM is 0.1 g/dL.)
The isoelectric point of the amphoteric polyelectrolyte we synthesized is about 7. If there is no special explanation, the pH of the under test is 7.
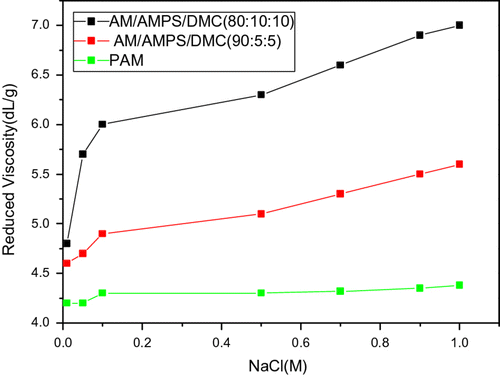
Figure shows that as the concentration of sodium chloride varied from 0.01 to 1 M, the reduced viscosity of AmPAM increased, the reduced viscosity of PAM kept the same. At the isoelectric point, the salt bond between anionic and cationic charge are destroyed with the increase in sodium chloride, the flexibility of molecular chain increase, contributing to the increase in the reduced viscosity. (The concentration of AmPAM is 0.05 g/dL.)
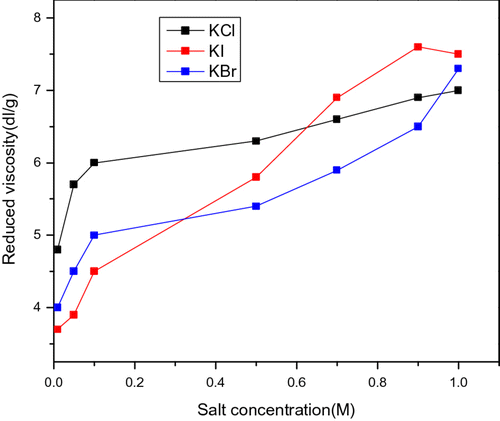
Figure shows the effect of different type of salt on the water solution of AmPAM. We can see that with the same type and concentration of cationic, the degree of influence of anionic is I > Br > Cl. The smaller the radius of anionic is, the stronger the interaction force between the cationic on the AmPAM and anionic is, which results in the break of salt bond, the expansion of the molecular chain and the increase in the reduced viscosity. (The concentration of AmPAM is 0.05 g/dL.)
4 Conclusions
An AmPAM was successfully synthesized through dispersion polymerization in aqueous solution of AS. It is found that the suitable condition for the dispersion polymerization is as follows: concentration were 8–15 wt% for monomer, 1–5 wt% for stabilizer, 15–20 × 10−4 g/g(monomers)−1 for initiator, and 23–27 wt% for AS. The average particle size was between 1 and 8 μm; the intrinsic viscosity was 5.6–14.2 dL/g. In general, the intrinsic viscosity of the resulting AmPAM increased with the increase in monomer concentration, and the decrease in initiator concentration. The size of the resulting AmPAM increased with the increase in monomer concentration, initiator concentration, and the decrease of stabilizer concentration.
The isoelectric point of the amphoteric polyelectrolyte we synthesized is about 7. With the increase in sodium chloride, the reduced viscosity of AmPAm increase. Among the different type of inorganic salt, the degree of influence of anionic is I > Br > Cl.
Acknowledgments
The authors thank the support and funding from the National Nature Science Foundation of China (51203047), Natural Science Foundation of Hubei province (2010CDB04605) and the Foundation of Hubei Provincial Department of Education for Young Scholars (Q20101008).
References
- Wu YM, Chen QF, Xu J, Bi JM. Aqueous dispersion polymerization of acrylamide with quaternary ammonium cationic comonomer. J. Appl. Polym. Sci. 2008;108:134–139.
- Wu YM, Wang YP, Yu YQ, Xu J, Chen QF. Dispersion polymerization of acrylamide with 2-acrylamido-2-methyl-1-propane sulfonate in aqueous solution. J. Appl. Polym. Sci. 2006;102:2379–2385.
- Liu X, Chen Q, Xu K, Zhang W, Wang P. Preparation of polyacrylamide aqueous dispersions using poly (sodium acrylic acid) as stabilizer. J. Appl. Polym. Sci. 2009;113:2693–2701.
- Riahi F, Bouaziz A, Benmesli S, Doufnoune R. Synthesis and characterization of an acrylamide-based resin for coating application. Int. J. Polym. Mater. 2008;57:745–758.
- Hajibevgi M, Shabanian M. Synthesis and characterization of new thermally stable and organosoluble polyamides by direct polycondensation. Des. Monomers Polym. 2012, doi:10.1080/15685551.2012.725212.
- Shen S, Sudol ED, El-aasser MS. Dispersion polymerization of methyl methacrylate: mechanism of particle formation. J. Polym. Sci., Part A: Polym. Chem. 1994;32:1087–1110.
- Fevola MJ, Bridges JK, Kellum MG. pH-responsive ampholytic terpolymers of acrylamide, sodium 3-acrylamido-3-methylbutanoate, and (3-acrylamidopropyl)trimethylammonium chloride. I. Synthesis and characterization. J. Polym. Sci., Part A: Polym. Chem. 2004;42:3236–3251.
- Panbon M, Selb J, Candau F. Polymerization of acrylamide in solution and inverse emulsion: number molecular weight distribution with chain transfer agent. Polymer. 1999;40:3101–3106.
- Paz Y, Kesselman E, Fahoum L. The interaction between poly(N-isopropylacrylamide) and salts in aqueous media: the “salting-out” phenomenon as studied by attenuated total reflection/fourier transform infrared spectroscopy. J. Polym. Sci., Part B: Polym. Phys. 2003;42:33–46.
- Kim , JW , Ryu , JH and Suh , KD . 2001 . Monodisperse micron-sized macroporous poly(styrene-co-divinylbenzene) particles by seeded polymerization . Colloid Polym. Sci. , 279 : 146 – 152 .
- Juna BM, Rao KM, Prasad CV, Rao KC, Subha MCS. Development of triprolidine-hydrochloride-loaded pH-sensitive poly(acrylamide-co-acrylamidoglycolic acid) co-polymer microspheres: in vitro release studies. Des. Monomers Polym. 2011;14:445–459.
- Sarac AS, Cebaci FC. Electroinduced dispersive polymerization of methly methacrylate in aqueous media. Int. J. Polym. Mater. 2004;53:763–776.
- Chiu HT, Yang HM, Liu CS, Hsu HY. Synthesis, stability and properties of polyurethane/acrylic hybrids using m-TMXDI-based anionic poly(urethane-urea) dispersion. Polym. Plast. Technol. Eng. 2012;51:945–953.
- Bhardwaj P, Singh S, Singh V, Aggarwal S, Mandal UK. Nanosize polyacrylamide/SiO2 composites by inverse microemulsion polymerization. Int. J. Polym. Mater. 2008;57:404–416.
- Capek I, Kocsisova T. Micellar aqueous phase polymerization of acrylamide. Des. Monomers Polym. 2011;14:327–345.
- Takahashi K, Miyamori S, Uyama H, Kobayashi S. Preparation of micron-size monodisperse poly(2-hydroxyethyl methacrylate) particles by dispersion polymerization. J. Polym. Sci., Part A: Polym. Chem. 1996;34:175–182.
- Takahashi , K , Miyamori , S and Uyama , H . 1997 . Preparation of monodisperse polymer particles from 4-vinylpyridine . Macromol. Rapid Commun. , 18 : 471 – 475 .
- Ye Q, Zhang Z, Ge X. Formation of monodisperse polyacrylamide particles by dispersion polymerization: particle size and size distribution. Polym. Int. 2003;52:707–712.
- Shan , G and Cao , Z . 2009 . A new polymerization method and kinetics for acrylamide: aqueous two-phase polymerization . J. Appl. Polym. Sci. , 111 : 1409 – 1416 .
- Baran , N , Deniz , S and Akgün , M . 2005 . Dispersion polymerization of styrene in supercritical carbon dioxide using monofunctional perfluoropolyether and silicone-containing fluoroacrylate stabilizers . Eur. Polym. J. , 41 : 1159 – 1167 .
- Chen D, Liu X, Yue Y. Dispersion copolymerization of acrylamide with quaternary ammonium cationic monomer in aqueous salts solution. Eur. Polym. J. 2003;42:1284–1297.
- Guha S, Ray B, Mandal BM. Anomalous solubility of polyacrylamide prepared by dispersion (precipitation) polymerization in aqueous tert-butyl alcohol. J. Polym. Sci., Part A: Polym. Chem. 2001;39:3434–3442.
- Guha S, Mandal BM. Dispersion polymerization of acrylamide: III. Partial isopropyl ester of poly(vinyl methyl ether-alt-maleic anhydride) as a stabilizer. J. Colloid Interface Sci. 2004;271:55–59.
- Lee , KC , Lee , SE and Choi , YJ . 2004 . Dispersion polymerization of acrylamide int-butyl alcohol/water media . Macromol. Res. , 12 : 213 – 218 .
- Ray B, Mandal BM. Dispersion polymerization of acrylamide: part II. 2, 2′‐azobisisobutyronitrile initiator. J. Polym. Sci., Part A: Polym. Chem. 1999;37:493–499.
- Fevola , MJ , Bridges , JK and Kellum , MG . 2004 . pH‐responsive polyzwitterions: a comparative study of acrylamide‐based polyampholyte terpolymers and polybetaine copolymers . J. Appl. Polym. Sci. , 94 : 24 – 39 .
- Cho , MS , Yoon , KJ and Song , BK . 2002 . Dispersion polymerization of acrylamide in aqueous solution of ammonium sulfate: synthesis and characterization . J. Appl. Polym. Sci. , 83 : 1397 – 1405 .
- Ye , Q , Zhang , Z and Ge , X . 2003 . Highly efficient flocculant synthesized through the dispersion copolymerization of water‐soluble monomers induced by γ‐ray irradiation: Synthesis and polymerization . J. Appl. Polym. Sci. , 89 : 2108 – 2115 .