Abstract
In this study, a mechanistic transformation was achieved by taking advantage of coupling reaction of reversible addition–fragmentation chain transfer (RAFT) agents. Polymers prepared by different polymerization modes were coupled to form polystyrene-b-poly(ethylene glycol) (PS-b-PEG) block copolymer. For this purpose, two prepolymers, namely azo and RAFT functional polymers were prepared independently. In the first step, a new macroazoinitiator (MI)-containing PEG units was synthesized by direct esterification of 4,4-azobis(4-cyanopentanoic acid) (ACPA) with poly(ethylene glycol) (PEG) at ambient conditions. The other component, polystyrene (PS) was synthesized by atom transfer radical polymerization and modified with a reversible addition–fragmentation chain transfer (RAFT) agent (xanthate moiety) and used as a polymeric RAFT agent for the block copolymer formation. In the block copolymerization process, polymeric radicals stemming from the thermolysis of PEG-MI were coupled with the RAFT moiety of PS. The PEG-MI, PS, PS-XANTH, and PS-PEG block copolymers were characterized by spectral analysis using FT-IR, 1H NMR, GPC, and DSC.
1. Introduction
Mechanistic transformation is an elegant methodology that combines two or more polymerization methods to form block copolymers of structurally different monomers.[1,2]Citation1Citation2 Generally, such combinations cannot be accomplished by using individual distinct polymerization methods. Macromolecular architectures with certain physical and structural properties can be facilitated by this approach. Two main strategies; (i) the use of dual functional initiators [2,3]Citation2Citation3 and (ii) propagating chain end switches [4,5]Citation4Citation5 have been proposed and successfully applied. In the latter case, a direct switch of the propagating chain ends is not feasible due to the short life of active centers and thermodynamic limitations. The only successful example is reported by Endo and co-workers who prepared block copolymers by switching propagating cations into anionic species directly.Citation[6]
A wide variety of transformation reactions have been reported. Previous efforts in this laboratory have focused on the development of the transformations involving combinations of radical systems with anionic,Citation[7] activated monomer,[8,9]Citation8Citation9 cationic,[10–17]Citation10Citation11Citation12Citation13Citation14Citation15Citation16Citation17 and condensation polymerizations.[18–20]Citation18Citation19Citation20 In some cases,[21–23]Citation21Citation22Citation23 the same polymerization mechanisms but different initiating systems were also employed.[24–27]Citation24Citation25Citation26Citation27
The established controlled radical polymerization methods, namely atom transfer radical polymerization (ATRP),Citation[28] reversible addition–fragmentation chain transfer polymerization (RAFT) [29,30]Citation29Citation30, and nitroxide-mediated radical polymerization Citation[31] are extensively used in the transformation approach in combination with living ionic polymerization routes to form block copolymers with well-defined structures.[10,32–34]Citation10Citation32Citation33Citation34 For example, anionic polymerization was successfully transformed to RAFT polymerization using several strategies. In most cases, anionically prepared polymers equipped with RAFT agents were used in the RAFT polymerization of a second monomer in the presence of a radical initiator.Citation[35] The equilibrium established by the chain transfer–fragmentation steps facilitates the formation of the corresponding block copolymers. Several other coupling processes yielding various macromolecular architectures of structurally different monomers but also were reported in the literature.[36–43]Citation36Citation37Citation38Citation39Citation40Citation41Citation42Citation43
In this study, we introduce a different approach in which anionic polymerization was combined with ATRP through RAFT chemistry. As it will be shown, the RAFT agent incorporated at the chain ends takes part only in radical exchange reaction to form a coupled product.
2 Experimental
2.1 Materials
Ethyl 2-bromopropionate (99%, Aldrich), potassium xanthate (98%, Fluka), copper (I) bromide (98%, Acros), N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA, 99%, Aldrich), poly(ethylene glycol) (PEG) methyl ether (Me-PEG, Mn ∼ 2000 g/mol, Aldrich), N,N′-dicyclohexylcarbodiimide (DCC, 99%, Aldrich), 4-dimethylaminopyridine (DMAP, 99%, Aldrich), N,N′-dimethylformamide, methanol (Merck), toluene (Aldrich) were used as received. 4,4-Azobis(4-cyanopentanoic acid) (ACPA, 98%, Fluka) and 2,2′-azobis(isobutyronitrile) (AIBN, 98%, Fluka) were recrystallized from methanol and acetone, respectively, prior to use. Styrene (99%, Aldrich) was passed through a basic alumina column to remove the inhibitor.
2.2 Characterization
1H NMR spectra of 5–10% (w/w) solutions of the intermediates and final polymers in CDCl3 with Si(CH3)4 as an internal standard were recorded at room temperature at 500 MHz on a Agilent VNMRS 500 spectrometer. FT-IR spectra were recorded on Perkin–Elmer FT-IR spectrum one spectrometer with an ATR Accessory (ZnSe, PikeMiracle Accessory) and cadmium telluride (MCT) detector. Resolution was 4 cm−1 and 24 scans with 0.2 cm/s scan speed. Differential scanning calorimetry (DSC) was performed on a Perkin–Elmer Diamond DSC. Molecular weights and polydispersities of the block copolymers were measured by gel permeation chromatography (GPC) employing an Agilent 1100 instrument equipped with a differential refractometer by using tetrahydrofuran as the eluent at a flow rate of 0.3 ml min−1 at 30 °C. Molecular weights were determined using polystyrene (PS) standards.
2.3 Synthesis of polymers
2.3.1 Synthesis of ω-bromo polystyrene (PS-Br)
PS with ω-bromo functionality was synthesized by ATRP. Thus, A Schlenk tube was charged with CuBr (0.125 g, 0.87 mmol), PMDETA (0.15 g, 0.87 mmol), ethyl 2-bromopropionate (0.16 g, 0.87 mmol) and styrene (9, 09 g, 87 mmol). Three freeze–pump–thaw cycles were performed, and the tube was stirred in oil bath at 110 °C for 30 min. After the given time, the mixture was diluted with THF. Then, the copper complex was removed out by passing through a neutral alumina column, and THF was removed by rotary evaporation. The mixture was precipitated in methanol, and the solid was collected after filtration and dried at room temperature under vacuum for overnight. (Mn,NMR: 1700, Mn,GPC: 1660 g/mol, Mw/Mn: 1.22).
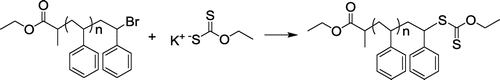
2.3.2 Synthesis of PS with ω-xanthate functionality (PS-Xant)
In a two-necked flask equipped with a stirrer, PS-Br (1.0 g, 1 eq.), potassium xanthate (0.8 g, 10 eq.) and 10 mL DMF was added under N2 atmosphere. The reaction mixture was stirred for 18 h at room temperature and precipitated in 10-fold excess of methanol to yield PS-Xant. (Mn,GPC: 2520 g/mol, Mw/Mn: 1.15).
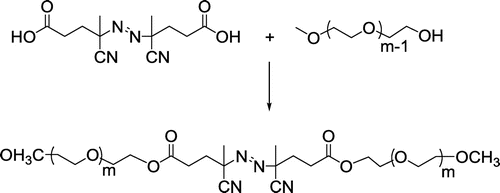
2.3.3 Synthesis of poly(ethylene glycol) macroazoinitiator (Me-PEG-MI)
Me-PEG (Mn:.2000 g/mol, 2.0 g, 1 mmol) and ACPA (1.4 g, 5.00 mmol) were dissolved in 15 mL of CH2Cl2. DMAP (0.61 g, 5.00 mmol) and DCC (1.03 g, 5.0 mmol) in 10 mL of dichloromethane were added to the solution in that order. The reaction mixture was stirred for 22 h at room temperature. The formed product was filtered, and the remaining solution was evaporated to give a viscous liquid. Finally, polymer is precipitated in 100 mL of diethylether to yield Me-PEG-MI. This process was repeated two times to purify the polymer (Mn,NMR: 4300, Mn,GPC: 4100 g/mol, Mw/Mn: 1.16).
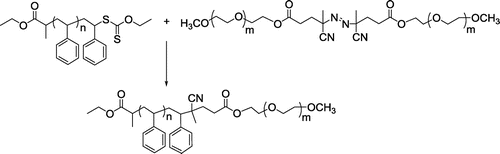
2.3.4 Synthesis of polystyrene-b-poly(ethylene glycol) (PS-b-PEG) block copolymers by thermal coupling
To a Schlenk tube equipped with a magnetic stirrer, PS-Xanth (0.2 g, 1 eq.), Me-PEG-MI (2.05 g, 5 eq.) were added and dissolved in 15 mL DMF. The tube was degassed by three freeze–pump–thaw cycles and placed in a thermostated oil bath at 80 °C for 4 h. The solvent was evaporated, and the resulting concentrated solution is poured into ten-fold excess ice-cold methanol/diethylether mixture (100/10) to precipitate the polymer. The polymer is further dissolved in THF and reprecipitated to purify the polymer and dried under vacuum at room temperature.
3 Results and discussion
As stated in the introduction section, in all reported transformation reactions through RAFT technique, the actual polymerization process is involved. In another words, polymers with RAFT agent functionality at the chain termini were prepared by various polymerization modes are used in the RAFT polymerization of another monomer.
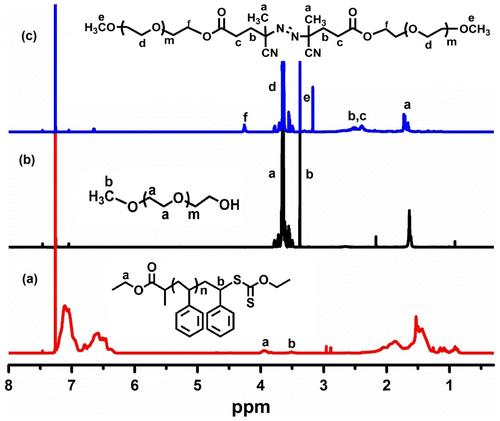
Recently, Moeller and Weberskirch Citation[44] reported a novel methodology for the preparation of telecehelics through radical exchange reaction of terminal RAFT agent and functional azo initiator. The rapid reaction of the primary radicals with RAFT agents prompted us to apply the process with polymeric radicals. Thus, polymers with ω-xanthate groups and radical generating functionalities, such as azo groups, were prepared independently. The overall procedure involves the synthesis of ω-bromo polystyrene (PS-Br) by ATRP, substitution of ω-xanthate polystyrene (PS-Xant), and its reaction with diazo-containing polyethyleneglycol (Me-PEG-MI). Scheme shows the synthesis of PS-Xant, which was reported in previous studies.[45,46]Citation45Citation46 The 1H NMR spectrum confirms the expected structure (Figure ).
In a parallel step, a simple esterification procedure was conducted between a carboxylic acid functional diazo-radical initiator ACPA and commercially available anionically repaired ω-hydroxyl functional PEG to obtain Me-PEG-MI as shown in Scheme .
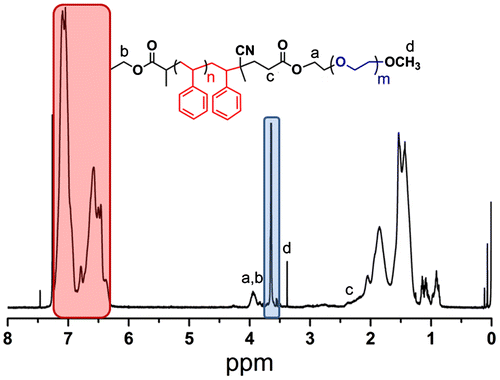
In the 1H NMR spectrum of Me-PEG-MI, the methylene protons next to the hydroxyl groups are shifted to downfield from 3.6 to 4.25 ppm due to the esterification reaction between ACPA and PEG (Figure and (c)).
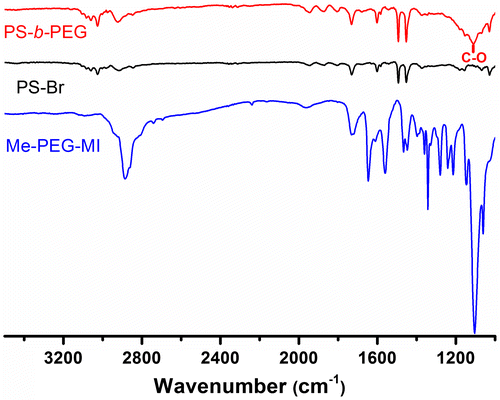
The IR spectrum of Me-PEG-MI further demonstrates the formation of ester linkage by the disappearance of the acidic carbonyl band at 1710 cm−1 of the ACPA and the presence of new ester stretching carbonyl peak –C=O at around 1736 cm−1. The peak about 1100 cm−1 is belonging to PEG –CH2 vibration band.
In the final step, PS-Xant and Me-PEG-MI was coupled by thermal treatment (Scheme ). The mechanism of the coupling process is typical radical exchange reaction of RAFT agents with azo compounds as described in the literature.Citation[44]
The structure of the block copolymer is confirmed by 1H NMR spectroscopy. All characteristic peaks of the main segments are detected as shown in Figure .
The FT-IR analysis further evidences the block copolymer structure. In Figure , the IR spectra of the precursor polymers and final block copolymer are presented. The aliphatic backbone of the PS at 1455 cm−1 and the aromatic ring vibrations observed at around 3027, 3066, and 1608 cm−1 proves that PS chains are in the block copolymers. Additionally, the presence of a new peak located at about 1100 cm−1 indicates successful attachment of PEG segment into block copolymer.
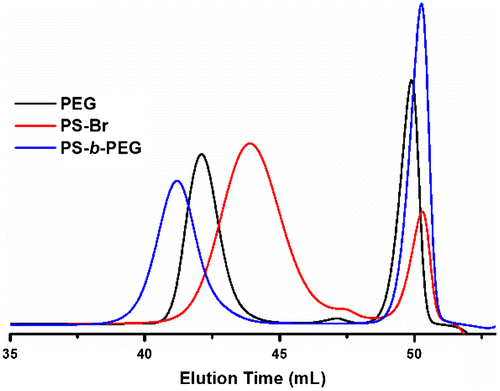
The molecular weight characteristics of the polymers were investigated by GPC. Figure shows the GPC traces of the polymers at different stages. All polymers have unimodal and narrow curves that indicate no side reaction occurred throughout the process. As can be seen, the GPC curve shifts to the higher elution volumes, indicating the increase in the molecular weight after the block copolymer formation.
Notably, all molecular weights obtained from GPC measurements are consistent with those calculated by NMR analysis. The molecular weight characteristics of the polymers at various stages are collected in Table .
Table 1. Molecular weight characteristics of the polymers at various stages.
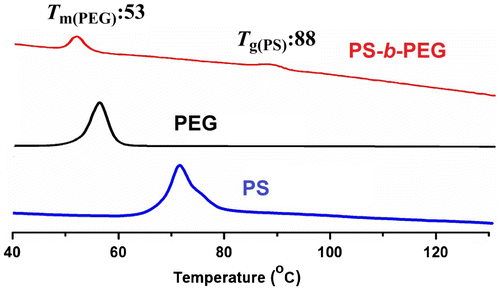
The thermal property of the block copolymer is investigated by DSC under nitrogen atmosphere and compared with that of the precursor polymers (Figure ). The DSC trace of the block copolymer showed an endothermic peak located at 53 °C indicating presence of semicrystalline PEG domain. The crystals extend of PEG segments in such block copolymers strongly depends on the molecular weight of the polyether block. Since the block copolymer is formed from PEG with molecular weight of 2000 g/mol, an intense melting transition is detected. Similar molecular weight effect accounts for the relatively lower Tg value for PS segment at 88 °C. The existence of the individual thermal transitions of both PS and PEG segments clearly indicates occurrence of microphase separation between two domains.
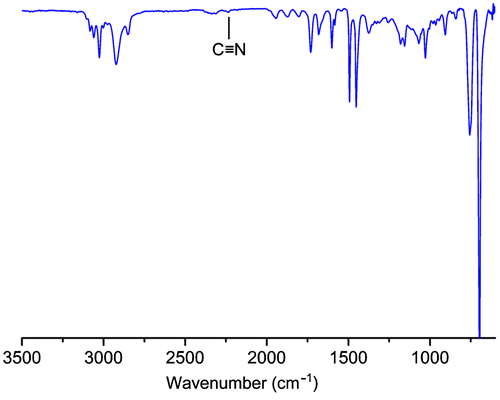
In order to demonstrate the efficiency of the applied radical exchange process, a model reaction was conducted using low molar mass azoinitiator. Thus, AIBN was used as the coupling component together with PS-Xant under identical experimental conditions. The FT-IR spectra of the obtained product exhibits a weak transition around 2200 cm−1, which demonstrates the presence of nitrile functionality at the PS chain end (Figure ).
4 Conclusions
We have demonstrated a new synthetic approach for the preparation of block copolymers through mechanistic transformation on the basis of radical exchange reaction between RAFT agent and polymeric radical initiator. The actual coupling process is simple and does not require polymerization reactions. Since the individual components may be prepared by independently, the described strategy can be adapted to different monomer couples to form corresponding block copolymers. Further, studies in this line are now in progress.
References
- Yagci Y, Tasdelen MA. Mechanistic transformations involving living and controlled/living polymerization methods. Prog. Polym. Sci. 2006;31:1133–1170.
- Bernaerts KV, Du Prez FE. Dual/heterofunctional initiators for the combination of mechanistically distinct polymerization techniques. Prog. Polym. Sci. 2006;31:671–722.
- Hawker CJ, Hedrick JL, Malmstrom EE, Trollsas M, Mecerreyes D, Moineau G, Dubois P, Jerome R. Dual living free radical and ring opening polymerizations from a double-headed initiator. Macromolecules. 1998;31:213–219.
- Hua FJ, Yang YL. Synthesis of block copolymer by “living” radical polymerization of styrene with nitroxyl-functionalized poly(ethylene oxide). Polymer. 2001;42:1361–1368.
- Acar MH, Matyjaszewski K. Block copolymers by transformation of living anionic polymerization into controlled/living atom transfer radical polymerization. Macromol. Chem. Phys. 1999;200:1094–1100.
- Nomura R, Endo T. One-pot transformation of living cationic polymerization into a living anionic one by samarium(II) iodide – synthesis of poly(tetrahydrofuran-b-epsilon-caprolactone) block-copolymer. Macromolecules. 1995;28:1754–1757.
- Cianga I, Senyo T, Ito K, Yagci Y. Electron transfer reactions of radical anions with TEMPO: a versatile route for transformation of living anionic polymerization into stable radical-mediated polymerization. Macromol. Rapid Commun. 2004;25:1697–1702.
- Yagci Y, Serhatli IE, Kubisa P, Biedron T. Synthesis of block copolymers by combination of an activated monomer and free-radical polymerization mechanism. Macromolecules. 1993;26:2397–2399.
- Tasdelen MA, Yagci Y, Demirel AL, Biedron T, Kubisa P. Synthesis and characterization of block-graft copolymers poly(epichlorohydrin-b-styrene)-g-poly(methyl methacrylate) by combination of activated monomer polymerization, NMP and ATRP. Polym. Bull. 2007;58:653–663.
- Kahveci MU, Acik G, Yagci Y. Synthesis of block copolymers by combination of atom transfer radical polymerization and visible light-induced free radical promoted cationic polymerization. Macromol. Rapid Commun. 2012;33:309–313.
- Durmaz YY, Kukut M, Moszner N, Yagci Y. Sequential photodecomposition of bisacylgermane type photoinitiator: synthesis of block copolymers by combination of free radical promoted cationic and free radical polymerization mechanisms. J. Polym. Sci., Part A: Polym. Chem. 2009;47:4793–4799.
- Yagci Y. Block copolymers by combinations of cationic and radical routes. 1. A new difunctional azo-oxocarbenium initiator for cationic polymerization. Polym. Commun. 1985;26:7–8.
- Galli G, Chiellini E, Yagci Y, Serhatli EI, Laus M, Bignozzi MC, Angeloni AS. Block copolymers with crystalline and side-chain liquid-crystalline blocks. Macromol. Rapid Commun. 1993;14:185–193.
- Hizal G, Yagci Y, Schnabel W. N-alkoxy pyridinium ion terminated polytetrahydrofurans – synthesis and their use in photoinitiated block copolymerization. Polymer. 1994;35:4443–4448.
- Yenice Z, Tasdelen MA, Oral A, Guler C, Yagci Y. Poly(styrene-b-tetrahydrofuran)/clay nanocomposites by mechanistic transformation. J. Polym. Sci., Part A: Polym. Chem. 2009;47:2190–2197.
- Yildirim TG, Hepuzer Y, Hizal G, Yagci Y. Synthesis of block copolymers by transformation of photosensitized cationic polymerization to stable free radical polymerization. Polymer. 1999;40:3885–3890.
- Duz AB, Yagci Y. Synthesis of block copolymers by combination of atom transfer radical and promoted cationic polymerization mechanisms. Eur. Polym. J. 1999;35:2031–2038.
- Alkan S, Toppare L, Hepuzer Y, Yagci Y. Block copolymers of thiophene-capped poly(methyl methacrylate) with pyrrole. J. Polym. Sci., Part A: Polym. Chem. 1999;37:4218–4225.
- Tasdelen MA, Beyazit S, Gunes D, Bicak N, Tatar P, Demirel AL, Yagci Y. Poly(p-phenylene methylene)-based block copolymers by mechanistic transformation. J. Polym. Sci., Part A: Polym. Chem. 2011;49:4021–4026.
- Dizman C, Kahveci MU, Yagci Y. Synthesis of polysulfone-b-polystyrene block copolymers by mechanistic transformation from condensation polymerization to free radical polymerization. Polym. Bull. 2013;70:2097–2109.
- Onen A, Yagci Y. Bifunctional initiators – synthesis, characterization, and initiator properties of azo-benzoin initiators. J. Macromol. Sci., Part A: Pure Appl. Chem. 1990;A27:743–753.
- Yagci Y, Onen A. Bifunctional initiators.3. Photochemical-synthesis of block copolymers of styrene and methyl-methacrylate with the aid of azo-benzoin initiators. J. Macromol. Sci., Part A: Pure Appl. Chem. 1991;A28:129–141.
- Tunca U, Serhatli IE, Yagci Y. Polymerization of acrylamide initiated by the redox system ce(iv)-4,4-azobis (4-cyano pentanol). Polym. Bull. 1989;22:483–488.
- Durmaz YY, Karagoz B, Bicak N, Yagci Y. Synthesis of block copolymers by combination of ATRP and photoiniferter processes. Polym. Int. 2008;57:1182–1187.
- Tasdelen MA, Degirmenci M, Yagci Y, Nuyken O. Block copolymers by using combined controlled radical and radical promoted cationic polymerization methods. Polym. Bull. 2003;50:131–138.
- Acik G, Kahveci MU, Yagci Y. Synthesis of block copolymers by combination of atom transfer radical polymerization and visible light radical photopolymerization methods. Macromolecules. 2010;43:9198–9201.
- Erel I, Cianga I, Serhatli E, Yagci Y. Synthesis of block copolymers by combination of photoinduced and atom transfer radical polymerization routes. Eur. Polym. J. 2002;38:1409–1415.
- Matyjaszewski K, Xia JH. Atom transfer radical polymerization. Chem. Rev. 2001;101:2921–2990.
- Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH. Living free-radical polymerization by reversible addition-fragmentation chain transfer: the RAFT process. Macromolecules. 1998;31:5559–5562.
- Moad G, Rizzardo E, Thang SH. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005;58:379–410.
- Hawker CJ, Bosman AW, Harth E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001;101:3661–3688.
- Coca S, Matyjaszewski K. Block copolymers by transformation of “living” carbocationic into “living” radical polymerization. Macromolecules. 1997;30:2808–2810.
- Wang WP, You YZ, Hong CY, Xu J, Pan CY. Synthesis of comb-shaped copolymers by combination of reversible addition-fragmentation chain transfer polymerization and cationic ring-opening polymerization. Polymer. 2005;46:9489–9494.
- Yu XF, Zhang G, Shi TF, Han YC, An LJ. Synthesis of symmetric H-shaped block copolymer by the combination of ATRP and living anionic polymerization. Polymer. 2007;48:2489–2495.
- Pafiti KS, Patrickios CS, Filiz V, Rangou S, Abetz C, Abetz V. Styrene-vinyl pyridine diblock copolymers: achieving high molecular weights by the combination of anionic and reversible addition-fragmentation chain transfer polymerizations. J. Polym. Sci., Part A: Polym. Chem. 2013;51:213–221.
- Aydin M, Tasdelen MA, Uyar T, Jockusch S, Turro NJ, Yagci Y. Polystyrene/clay nanocomposites by atom transfer radical nitroxide coupling chemistry. J. Polym. Sci., Part A: Polym. Chem. 2013;51:1024–1028.
- Aydogan B, Yagci Y. Studies on the preparation of alpha, omega-telechelic polymers by the combination of reverse atom transfer radical polymerization and atom transfer radical coupling processes. Turk. J. Chem. 2007;31:1–10.
- Cianga I, Yagci Y. First polyrecombination reaction via atom transfer radical coupling (ATRC), a new way for the synthesis of poly(p-xylylene). Des. Monomers Polym. 2007;10:575–584.
- Durmaz YY, Aydogan B, Cianga I, Yagci Y. The use of atom transfer radical coupling reactions for the synthesis of various macromolecular structures. In: Matyjasewski K, editor. Controlled/living radical polymerization: progress in ATRP. ACS symposium series. 10232009. Washington (DC): American Chemical Society; 2009. Chapter 12, p. 171–187.
- Durmaz YY, Cianga I, Yagci Y. Studies on the preparation of telechelic polymers by atom transfer radical polymerization and cross coupling processes. e-Polymers. 2006, 50.
- Iskin B, Yilmaz G, Yagci Y. Telechelic polymers by visible-light-induced radical coupling. Macromol. Chem. Phys. 2013;214:94–98.
- Temel G, Aydogan B, Arsu N, Yagci Y. Synthesis of block and star copolymers by photoinduced radical coupling process. J. Polym. Sci., Part A: Polym. Chem. 2009;47:2938–2947.
- Yurteri S, Cianga I, Yagci Y. Synthesis and characterization of alpha, omega-telechelic polymers by atom transfer radical polymerization and coupling processes. Macromol. Chem. Phys. 2003;204:1771–1783.
- Greving N, Keul H, Millaruelo M, Weberskirch R, Moeller M. Synthesis of alpha, omega-isocyanate-telechelic poly(methyl methacrylate). Macromol. Chem. Phys. 2012;213:1465–1474.
- Iskin B, Yilmaz G, Yagci Y. ABC type miktoarm star copolymers through combination of controlled polymerization techniques with thiol-ene and azide-alkyne click reactions. J. Polym. Sci., Part A: Polym. Chem. 2011;49:2417–2422.
- Iskin B, Yilmaz G, Yagci Y. Mono-addition synthesis of polystyrene-fullerene (c60) conjugates by thiol-ene chemistry. Chem. Eur. J. 2012;18:10254–10257.