Abstract
The new allyl((2-oxo-1,3-dioxolan-4-yl)methyl) carbonate (ADC) monomer was synthesized by reacting glycerol carbonate with allylchloroformate. The structure of the monomer was characterized by FT-IR, 1H NMR, UV–visible, and mass spectroscopies. The ADC monomer was copolymerized with Methyl methacrylate and butyl Acrylate by solution polymerization technique. Further, the ADC copolymer was cross-linked with 1,3-diaminopropane which yields polyurethane acrylate copolymer. The cured film was characterized by FT-IR, 1H NMR spectroscopy and for the Gel content. The uncured and cured films were subjected to thermal analysis, and it was observed that the uncured film undergoes two-step thermal decomposition, whereas the cured film undergoes single-step thermal decomposition with comparably high thermal stability. The biodegradability of uncured films is directly proportional to the conc. of ADC monomer and inversely proportional to its cured film. The cured and uncured films of ADC copolymers were subjected to evaluate their optical and mechanical properties. The dramatic improvement was observed in the optical and mechanical properties of cured ADC copolymer at 5–7 wt.% of ADC monomer.
Introduction
Nowaday’s, cyclic carbonates specially five and six members gaining considerable interest because of their biodegradability, high solvency, high boiling and flash points, low odor levels and evaporation rates, and low toxicities. The cyclic carbonates are finding increased utility as diluents for the epoxy and isocyanate resin systems, and they have become the electrolytes of choice in the production of lithium ion batteries. Cyclic carbonates also find utility as a carrier solvent for cosmetics.[1–4]Citation1Citation2Citation3Citation4 The carbonates are prepared by different methods like phosgenation technique,[5–7]Citation5Citation6Citation7 oxidative carbonylation of alcohols,[8–12]Citation8Citation9Citation10Citation11Citation12 reaction of urea with alcohols, reaction of oxiranes with carbon dioxide,[13–20]Citation13Citation14Citation15Citation16Citation17Citation18Citation19Citation20 use of metal carbonates,Citation[21] all these methods have their own drawbacks.
The cyclic carbonates produced react with aliphatic and aromatic amines, alcohols, thiols, and carboxylic acids under certain conditions; they can also undergo ring-opening polymerization. Polymers containing cyclic carbonate functional groups are vitally important in the present context. Use of the cyclic carbonate group is prime interest because it is highly polar and readily reacts with aliphatic and aromatic amines, alcohols, thiols, and carboxylic acids yields thermoset polymers. This reaction can proceed relatively rapidly at ambient or slightly elevated temperatures and does not release a volatile by-product.[22–27]Citation22Citation23Citation24Citation25Citation26Citation27
Presently, polymers containing epoxy, hydroxyl, isocyanate, amine, and carboxylic acid functional groups are widely used for the preparation of thermosetting systems.Citation[28] Thermoset polymers have widespread use in a wide variety of applications. But many of the functional groups currently in use have limitations in terms of reactivity or may have environmental or other hazards associated with them.
In the present context, we report the facile route for the synthesis of polyurethane acrylate copolymer starting from new allyl((2-oxo-1,3-dioxolan-4-yl)methyl) carbonate (ADC) monomer and also studied the optical, thermal, mechanical, and biodegradable properties of polyurethane acrylate copolymer.
Experimental
Materials
Allylchloroformate, 1,3-diaminopropane, triethylamine, benzoyl peroxide, dichloromethane, toluene, ethyl acetate, and cyclohexane. Methyl methacrylate (MMA) and butyl acrylate (BA) purchased from s.d. fine chem. Ltd, Mumbai, and use without any purification.
Synthesis of glycerol carbonate
Glycerol carbonate was synthesized according to Ref. Citation[29].
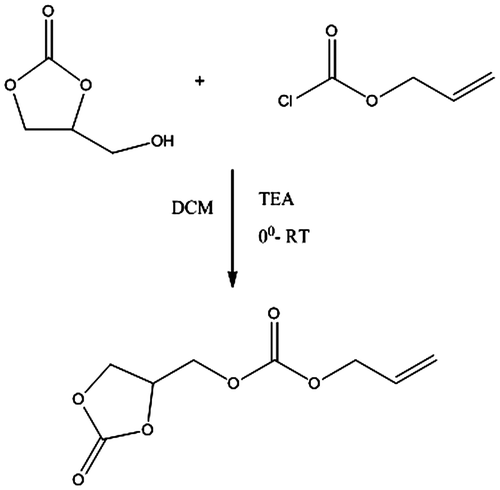
Synthesis of allyl((2-oxo-1,3-dioxolan-4-yl)methyl) carbonate
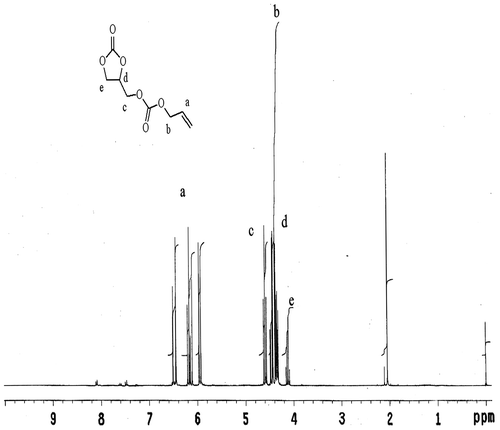
Glycerol carbonate 11.8 gm, triethylamine 11.1 gm, and 100 ml DCM as a solvent were placed in a three-neck round bottom fitted with a magnetic stirrer, condenser, thermometer, and ice bath. Cool the reaction mixture at 0 °C followed by dropwise addition of 12.05 gm of allyl chloroformate for 1 h. After complete addition, stirred the reaction mixture at 0 °C for 30 min, then removed ice bath and allowed the reaction mixture to attained room temperature for 4hrs. Filter the reaction mixture and filtrate was wash with water. Separate out the organic layer and dried over sodium sulfate, and filtrate was evaporated in a rotary evaporator to separate DCM from the product (Figure ). The nature of product is liquid, reaction yield 65%. 1H NMR spectral data: (CDCl3) δ 6.9 (1H d), δ 6.6 (1H dd), δ 5.9 (1H d), δ 4.6 (1H m), δ 4.5 (2H t), δ 4.4 (2H d), δ 4.1 (2H d).
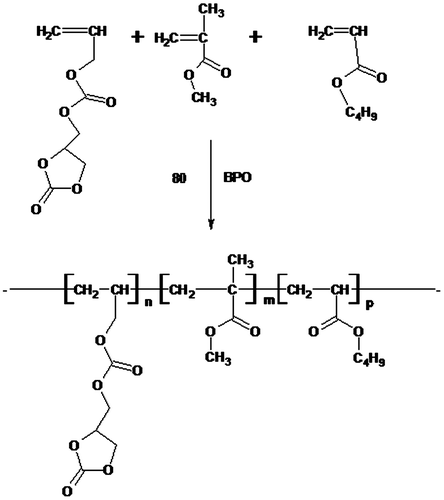
Synthesis of ADC copolymer
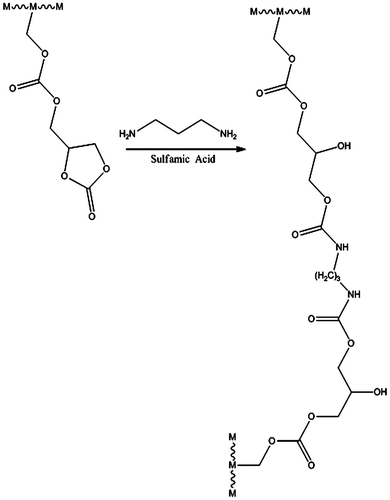
The solution copolymerization of ADC, MMA, and BA was performed in glass reactor equipped with mechanical stirrer, condenser, thermometer pocket, nitrogen inlet, and dropping funnel. The glass reactor was filled with toluene, benzoyl peroxide, and 10% monomer mixture and heated at 80 °C. The remaining monomer mixture and initiator was added dropwise to the reactor over a period of 3hrs. After complete addition, the reaction mixture was further heated at 80 °C for another 1 h in order to achieve maximum conversion. Finally, the reaction mixture was allowed to attained room temperature and filter through mesh. (Figure ) Further the ADC copolymer was then cross-linked with 1,3-diamminopropane at ambient temperature using catalytic amount of sulfamic acid. (Figure ) The recipe of solution copolymerization of ADC, MMA, and BA is summarized in Table .
Table 1. The recipe of solution copolymerization of ADC, MMA, and BA.
Characterization
FT-IR analysis: FT-IR (Shimadzo 8400 s, Japan) of ADC monomer and copolymer was recorded using ATR technique.
HNMR analysis: 1H NMR spectrum of ADC monomer and ADC copolymer was recorded at 500 MHz using Bruker Biospin (Avance AV500WB, Germany) in CDCl3.
MS analysis: 10 ppm solution of ADC monomer was made in dichloromethane and recorded the chromatogram using Shimadzo (QP 2010, Japan).
Thermogravimetric analysis was carried out on SDT Q600 v8.2 build 100 model of TA instruments (USA). Stepwise isothermal ramping up to 600 at 10 °C/min was performed in a nitrogen atmosphere. The change in the weight of the ADC copolymer was measured as a function of temperature.
Differential scanning calorimetry (DSC) (Q100 V9.7 Build 291, USA) at a rate of 10 °C/min. 5–8 mg of sample was in an aluminum pan and pan was kept for thermal analysis in DSC instrument.
Gel contents of the cross-linked film were determined by Soxhlet extraction for 12 h using acetone–toluene mixture (1/1, v/v). Insoluble gel fraction was dried under vacuum for 24 h at 60 °C and weighed to calculate the gel content. The gel content was calculated by following equation.
where W is weight of dried film. W0 is weight of dried film after immersing in solvent Glosses of the cured films were measured on calibrated instrument at 60° angle of reflectance using a digital mini gloss meter (Rhopoint Instruments, ASTM D 523-99).
Testing’s of coatings
Impact resistance was carried out on the both side of coated panels and analyzed accordance to the standard ASTM D 2794. The film results were rated as 0 (best) −10 (poor).
Pencil hardness test was used to observe the hardness values of the films according to ASTM D 3363.
The scratch hardness was determined by the automatic scratch hardness tester as per ASTM D 7027.
Solvent resistance of coating was determined by ASTM D 5402-93 using solvent rub technique for assessing the solvent resistance of an organic coating by rubbing the coating with a cloth saturated with the appropriate solvent.
Adhesion test was performed by crosscut adhesion tester used to check the adhesion of the coated film to metallic substrate, according to ASTM D 3359.
Chemical resistance: alkali resistance: panels were evaluated for blistering according to ASTM D 1647. Acid resistance: panels were evaluated for peeling of coated part according to ASTM D 3260.
Water resistance: the coated panels were immersed in water; the panels were evaluated for blistering according to ASTM D 870-02.
Flexibility of coated M.S. panel was determined by the conical mandrel according to ASTM D 522-93a.
Results and discussion
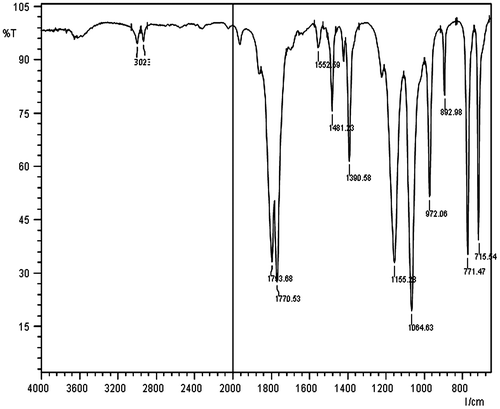
FT-IR analysis of ADC monomer
The FT-IR spectrum of ADC monomer is shown in Figure . The peak at 3023 and 2988 cm−1 corresponds to H–C=C– str and –C–H str, respectively. The cyclic carbonate group and carbonate of ADC monomer show two characteristic absorption peaks at 1793 and 1770 cm−1 str respectively. The peak at 1481 and 1155 cm−1 was assigned to –C=C– str and –C–O str, respectively.
1H NMR of ADC monomer
Figure shows 1H NMR spectra of ADC monomer. 1H NMR spectra of ADC monomer shows characteristic peak at δ 6.9 (1H d), δ 6.6 (1H dd), δ 5.9 (1H d), is typical splitting pattern of HC=CH2, the peak at δ 4.6 m assign to –CH of the ring, the peak at δ 4.5 t corresponds to –CH2 adjacent to the double bond, the peak at 4.4 d assign to CH2–O–, and the peak at δ 4.1 d assign to –CH2 of the ring. Above data of 1H NMR show that ADC was successfully synthesized.
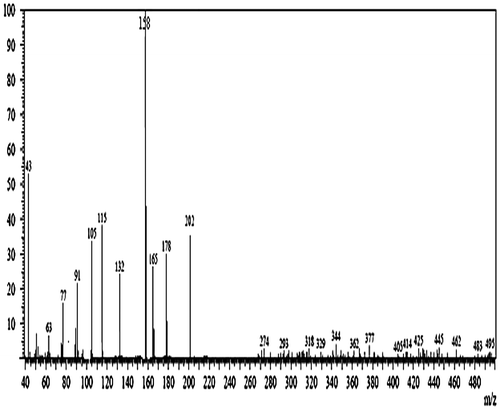
Mass spectrometry of ADC monomer
Figure shows molecular ion peak of ADC monomer at m/z = 202 indicates the formation of DAAM having molecular weight 169, the base peak (100% intensity) at m/z = 158, and other fragments having m/z = 178, 165, 132, 115, 105, and 43, respectively.
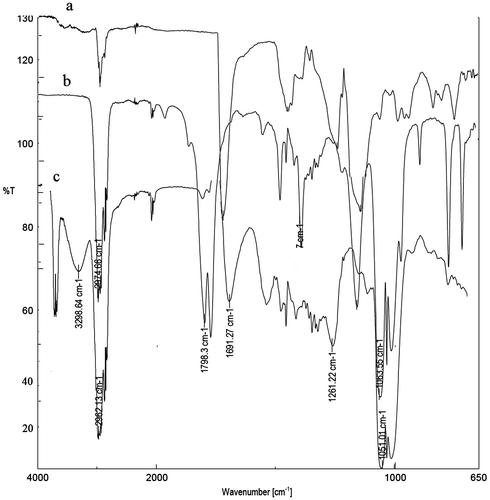
FT-IR analysis of ADC copolymer
Figure show the overlaid FT-IR spectrum of ADC copolymer, where (a) is spectrum of MMA-BA copolymer, (b) is spectrum of ADC-MMA-BA copolymer, and (c) is spectrum cross-linked ADC copolymer. Spectrum (a) shows peak at 1742 cm−1 is characteristic absorption frequency of ester (–COO–) group. Spectrum (b) shows two characteristic peaks at 1798 and 1764 cm−1 and is assigned to cyclic carbonate and carbonate group, respectively. From spectrum (b), it was conclude that the ADC is a part of copolymer. Spectrum (c) shows two peaks at 3298 and 1691 cm−1 was assigned to –OH and –CONH– group, respectively. Thus, spectrum (c) supports the cross-linking reaction took place between ADC copolymer and 1,3-diaminopropane.
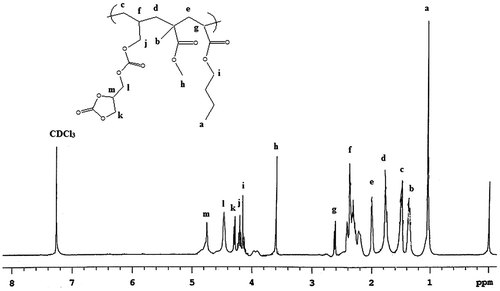
1H NMR spectra of ADC copolymer
The 1H NMR spectra ADC copolymer of is shown in Figure . From Figure of 1H NMR (CDCl3) of ADC copolymer shows characteristics peaks at δ1 t assign for terminal –CH3 of BA, the peak at δ 1.37 s corresponds to –CH3 of MMA, the peak at δ 1.5 d assigns to –CH2 of polymeric chain, the peak at δ 1.78 d corresponds to –CH2, the peak at δ 2 d corresponds to –CH2 of polymeric chain, the peak at δ 2.35 m for –CH of polymeric chain, the peak at δ 2.6 m assigns to –CH of polymeric chain, the peak at δ 3.6 s assigns to –CH3 of MMA, the peak at δ 4.18 t corresponds to –O–CH2 of BA, the peak at δ 4.2 d assigns to –O–CH2 of ADC, the peak at 4.25 d corresponds to –CH2 of cyclic ring, the peak at δ 4.42 d assigns to –O–CH2 adjacent to the cyclic ring, the peak at δ 4.79 m corresponds to –CH of cyclic ring. The above data of 1H NMR show that ADC monomer successfully incorporated in the copolymer.
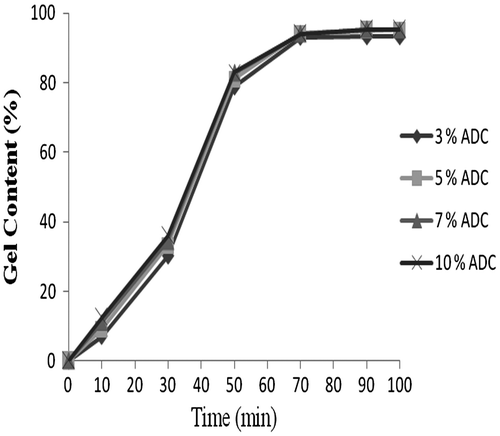
Determination of gel content (%)
The gel contents of the cross-linked films were determined by Soxhlet extraction for 12 h using acetone–toluene mixture (1/1, v/v). Insoluble gel fraction was dried under vacuum for 24 h at 60 °C. The gel content (%) of cross-linked films was calculated at different intervals of time and plots a graph between the gel content (%) against time. From Figure , it is conclude that the 97% of cross-linking reaction was took place in 70 min.
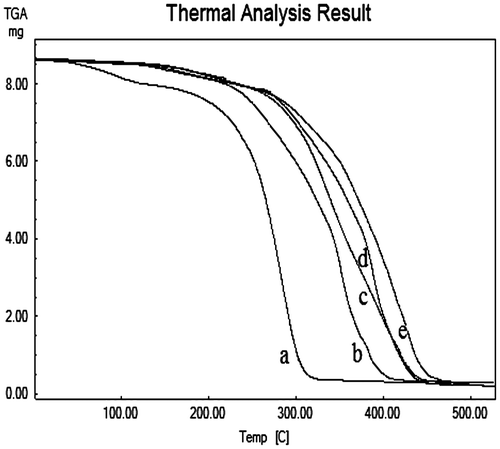
Thermogravimetric analysis
Figure shows thermograms of ADC copolymer and cross-linked ADC copolymer, where thermogram (a) corresponds to 3% ADC copolymer and (b, c, d, and e) for cross-linked ADC copolymer with varying concentration of ADC (3–5 wt.%), respectively. The thermogram (a) undergo two-step thermal decomposition in which the first decomposition was observed in between at temperature 120–150 °C corresponds to elimination of carbon dioxide from the cyclic carbonate group of ADC copolymer. The ADC copolymer decomposed 50% at 312 °C. On the other hand, the rest of the thermograms undergo single-step thermal decomposition. The all cross-linked ADC copolymer decomposed more or less 50% at 380–395 °C, this evident that the cross-linking reaction took place between cyclic carbonate of ADC copolymer and 1,3-diaminopropane, which provide good thermal stability to the copolymer.
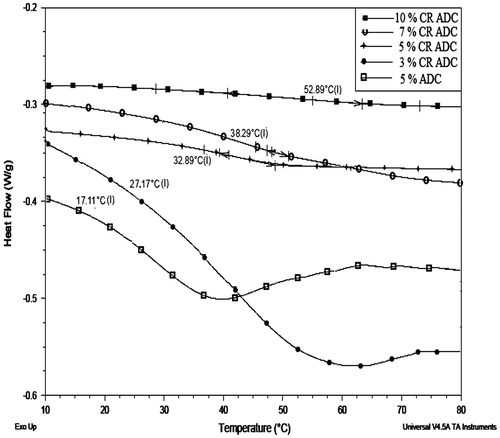
Differential scanning calorimetry analysis
Figure show the glass transition temperature (Tg) of both ADC copolymer and cross-linked copolymer. The Tg of ADC homopolymer is 64 °C taking in to consideration of Tg of ADC homopolymer, the theoretical Tg of ADC copolymer was set at 15 °C using Fox equation. The practical Tg of ADC copolymer was observed at 11.11 °C which is in good agreement with the set Tg. Whereas the Tg of cross-linked ADC copolymer was observed to be increase from 27 to 52 °C with respect to concentration of ADC. The cross-linking restricts the segmental and molecular motion of the copolymer. Thus, the increased in Tg was observed, and also, it supports the cross-linking reaction took place between cyclic carbonate group of ADC copolymer and 1,3-diaminopropane.
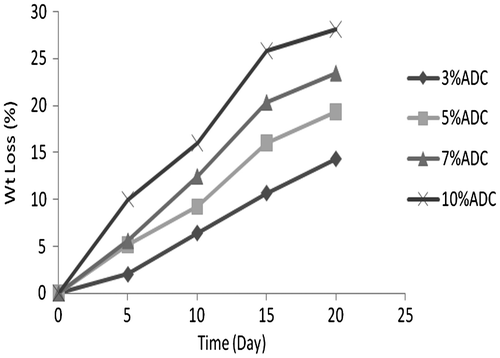
In vitro degradation of ADC copolymer
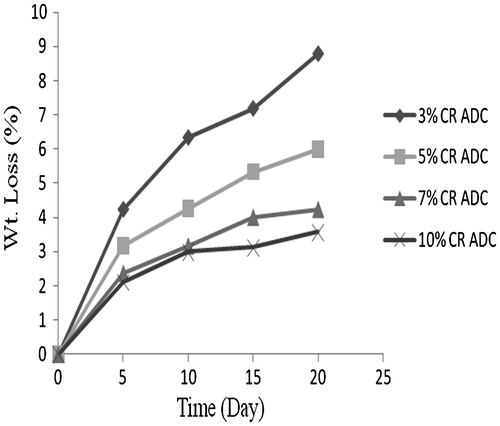
The in vitro degradation of ADC copolymers were done by phosphate buffer (pH 7.4) at 37 °C shown in Figures. The ADC copolymer (10 wt.% of ADC in polymer) was degrade 28% in 20 days. On the other hand, the cross-linked ADC copolymer was degrade only 3.5% in 20 days at 10 wt.% ADC concentration. The ADC copolymer comprised of carbonate ring which was degrade in phosphate buffer (pH 7.4) at 37 °C, but after cross-linking the rate of degradation of ADC copolymer decreased due to lack of carbonate rings.
Gloss measurement
The influences of concentration of ADC monomer on gloss of cured films were measured using a digital glossometer at reflectance of 60°. It was observed that, as the concentration ADC monomer increases, the gloss of cured films was also increased. This improvement in gloss is due to the increase in cross-linking density of the films. The results of gloss measurement are shown Table .
Table 2. Influence of concentration of ADC on gloss of cured films.
Mechanical properties of coatings
The coatings were applied onto metal panels using bar applicator at DFT 100 μm. All mechanical testing of coatings was performed according to their ASTM. The ADC copolymer showed poor water and chemical resistance because the ADC copolymer comprised of cyclic carbonate ring having low hydrolytic stability and susceptible to acid and alkali. After cross-linking with 1,3-diaminopropane, dramatic improvement was observed in water and chemical resistance of cross-linked ADC copolymer as cross-linking reaction formed urethane linkage. The cross-linked ADC copolymers showed improvement in the mechanical properties at 5–7 wt.% ADC concentration. The –NH groups of urethane linkages form hydrogen bonds with the substrate, which enhanced scratch hardness, impact resistance, adhesion, and pencil hardness of coatings.Citation[30] The mechanical properties of ADC copolymer and cross-linked ADC copolymer are shown in Table .
Table 3. The mechanical properties of ADC and cross-linked ADC copolymer.
Conclusion
We synthesized new allyl((2-oxo-1,3-dioxolan-4-yl)methyl) carbonate monomer and suggest simple, isocyanate free route for the synthesis of polyurethane acrylate copolymer. The characterization of the synthesized monomer and copolymer was carried by FT-IR and 1H-NMR spectroscopy. The results were in good agreement with the proposed structures. From gel content analysis, it is evident that the 97% of cross-linking reaction was took place in 70 min. The polyurethane acrylate copolymer shows good thermal stability. The biodegradability of ADC copolymer is directly proportional to the conc. of ADC monomer and inversely proportional to its cross-linked copolymer. The polyurethane acrylate copolymer shows excellent optical, scratch, and chemical resistance at 5–7 wt.% concentration of ADC.
Acknowledgment
The authors are immensely like to thank to UGC (SAP) for providing financial assistance.
References
- Kemin W, Wentao W, Xiaodan Z, Qiang Y. Synthesis and photopolymerization of 2,2-di((acryloyloxy)methyl)butyl bis(2-(acryloyloxy)ethyl)carbamate. Des. Monomers Polym. 2012;15:31–39.
- Clements JH. Reactive applications of cyclic alkylene carbonates. Ind. Eng. Chem. Res. 2003;42:663–674.
- Parzuchowski PG, Kiźlińska M, Rokicki G. New hyperbranched polyether containing cyclic carbonate groups as a toughening agent for epoxy resin. Polymer. 2007;48:1857–1865.
- Chung GC, Kim HJ, Jun SH, Kim MH. New cyclic carbonate solvent for lithium ion batteries: trans-2,3-butylene carbonate. Electrochem. Commun. 1999;1:493–496.
- Choppin AR, Rogers JW. The preparation of di-t-butyl carbonate and t-butyl chlorocarbonate. J. Am. Chem. Soc. 1948;70:2967–2967.
- Burk RM, Roof Burk MB. A safe and efficient method for conversion of 1,2- and 1,3-diols to cyclic carbonates utilizing triphosgene. Tetrahedron Lett. 1993;34:395–398.
- Brunelle DJ, Shannon TG. Preparation and polymerization of bisphenol A cyclic oligomeric carbonates. Macromolecules. 1991;24:3035–3044.
- Fenton DM, Steinwand PJ. Noble metal catalysis III. Preparation of dialkyl oxalates by oxidative carbonylation. J. Org. Chem. 1974;39:701–704.
- Ugo R, Renato T, Marcello MM, Pierluigl R. Synthesis of dimethyl carbonate from methanol, carbon monoxide, and oxygen catalyzed by copper compounds. Ind. Eng. Chem. Prod. Res. Dev. 1980;19:396–403.
- Cavinato G, Toniolo L. New aspects of the synthesis of dimethyl carbonate via carbonylation of methyl alcohol promoted by methoxycarbonyl complexes of palladium(II). J. Organomet. Chem. 1993;444:C65–C66.
- Chalk AJ. Fungicidal and herbicidal alpha-haloaceranilides. United State patent US 4,096,167. 1978 June 20.
- Hallgren JE, Lucas GM, Matthews RO. The palladium-catalyzed synthesis of diphenyl carbonate from phenol, carbon monoxide, and oxygen. J. Organomet. Chem. 1981;204:135–138.
- Zhou X, Zhang Y, Xiangui Jie Y, Gongying W. Hydrated alkali metal halides as efficient catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. Chin. J. Catal. 2010;31:765–768.
- Uemura K, Kawaguchi T, Takayama H, Nakamura A, Inoue Y. Preparation of alkylidene cyclic carbonates via cyclization of propargylic carbonates. J. Mol. Catal. A: Chem. 1999;139:1–9.
- Suriano F. Synthesis of a family of amphiphilic glycopolymers via controlled ring-opening polymerization of functionalized cyclic carbonates and their application in drug delivery. Biomaterials. 2010;31:2637–2645.
- Li Q, Zhang W, Zhao N, Sun Y. Synthesis of cyclic carbonates from urea and diols over metal oxides. Catal. Today. 2006;115:111–116.
- Jonas M, Tim B. Synthesis and polymerization of alkyl halide-functional cyclic carbonates. Polymer. 2011;52:5716–5722.
- Al-Azemi T, Bisht KS. Synthesis of novel bis- and tris-(cyclic carbonate)s and their use in preparation of polymer networks. Polymer. 2002;43:2161–2167.
- Yoshida M, Ihara M. Novel methodologies for the synthesis of cyclic carbonates. Chem. Eur. J. 2004;10:2886–2893.
- Manfred L, Eckehard VD. Phasentransfer-katalytische herstellung von kohlensaureestern ohne verwendung von phosgen [Phase transfer-catalytic production of carbonic acid esters without use of phosgene]. 1981;114:1210–1215.
- Cella JA, Bacon SW. Preparation of dialkyl carbonates via the phase-transfer-catalyzed alkylation of alkali metal carbonate and bicarbonate salts. J. Org. Chem. 1984;49:1122–1125.
- Tomita H, Sanda F, Endo T. Polyaddition of bis(seven-membered cyclic carbonate) with diamines: a novel and efficient synthetic method for polyhydroxyurethanes. J. Polym. Sci., Part A: Polym. Chem. 2001;39:4091–4100.
- Bungo O, Shoko I, Takeshi E. Salt effect on polyaddition of bifunctional cyclic carbonate and diamine. J. Polym. Sci., Part A: Polym. Chem. 2005;43:6282–6286.
- Toshiro A, Toshikazu T, Takeshi E. Cationic ring-opening polymerization of cyclic carbonates with alkyl halides to yield polycarbonate without the ether unit by suppression of elimination of carbon dioxide. Macromolecules. 1997;30:737–744.
- Kim M, Hooi-Sung K, Chang S, Dae-Won P. Syntheses and thermal properties of poly(hydroxy)urethanes by polyaddition reaction of bis(cyclic carbonate) and diamines. J. Appl. Polym. Sci. 2001;81:2735–2743.
- Strage P. Sodium stannate catalyst for hydroxyalkylation of phenols or thiophenols. United State patent US 4,310,707. 1982.
- Teruo Y, Shigeru I, Hajime K, Yoshiharu I. Synthetic studies with carbonates. Part 6. Syntheses of 2-hydroxyethyl derivatives by reactions of ethylene carbonate with carboxylic acids or heterocycles in the presence of tetraethylammonium halides or under autocatalytic conditions. J. Chem. Soc. Perkin Trans.1. 1977;1:1266–1272.
- Roller MB. Themoset and coatings technology: the challenge of interdisciplinary chemistry. Polym. Eng. Sci. 1979;19:692–698.
- Bell JB, Silver L, Arthur V. Method for preparing glycerin carbonate. United State patent US 2,915,529. 1959.
- Tathe DS, Jagtap RN. Synthesis of bio-based polyurethane from modified Prosopis juliflora oil. J. Am. Oil Chem. Soc. 2013;90:1405–1413.