Abstract
Multi-walled carbon nanotube (MWCNT)/amino acid-based poly(amide-imide) (PAI) composites containing pendant thiazole moiety were successfully prepared through ultrasonication-assisted solution blending. In order to improve the compatibility between the PAI and MWCNTs, carboxylated MWCNTs were used in this study. The mechanical and thermal properties and cross-sectional morphology of the composites were investigated. The incorporation of MWCNTs–(COOH)n improved the mechanical properties of the composites compared with that of neat PAI. An improvement in thermal properties of the MWCNT/PAI composites was also observed. This synergetic enrichment in mechanical and thermal properties of the composites is due to the increased interfacial interactions resulted from the improved dispersion state and entanglement of polymer chains, as revealed by electron microscopy observation.
1. Introduction
The supreme and multipurpose characteristics of carbon nanotubes (CNTs) have made them promising filler material to improve mechanical, thermal, and electrical properties of polymers.Citation[1] The key point is to transfer the potential properties of CNTs to the polymer composites. The prerequisite of any composite is a good dispersion of the filler within the hosting matrix.Citation[2] It is also vital to stabilize the dispersion to prevent reaggregation of the filler. In the case of nanofillers, these tasks are particularly challenging since the extremely large surface area that characterizes them is responsible for their strong tendency to form agglomerates.Citation[3] Due to the strong attractive van der Waal’s forces, CNTs tend to aggregate and form bundles or ropes, usually with highly entangled network structure. Thus, despite of their high surface area, CNTs can act as desirable interface for stress transfer, it undesirably induces strong attractive forces between the CNTs themselves, leading to the excessive agglomeration behavior.Citation[4] In this context, two main issues are extremely important to the achievement of polymer composites with improved properties due the addition of CNTs. These issues are the interfacial interaction between filler and polymer and the proper dispersion of the filler in the polymer matrix.[5–7]Citation5Citation6Citation7 Ultrasonication or mechanical mixing is generally used to disperse CNTs, while chemical functionalization can help stabilizing the disperson, since it can prevent reaggregation of CNTs.[8,9]Citation8Citation9 Tailoring of the chemical bonds might lead to an optimized interaction of the reinforcing CNTs with solvent and/or polymer matrix, so that the external stress can be efficiently transferred to the CNT. Furthermore, the functionalized CNTs might reveal properties that differ from those of the pristine CNTs. This goal is currently a great challenge for chemists and materials scientists; however, many approaches have been developed during the last decade.[10,11]Citation10Citation11
Polyimide is well recognized as a high-performance polymer. It is a promising candidate matrix in the manufacture of advanced composite materials due to its unusual good thermal stability, low dielectric constant, high mechanical strength, and chemical inertness.[12–14]Citation12Citation13Citation14 However, the stubborn characteristics have been major problems as a result of high melting point and insolubility. To overcome this problem, various copolyimides and polymer structure modification have been developed. For example, poly(amide-imide) (PAI) has been developed as an alternative materials offering a compromise between excellent thermal stability and processability.[15,16]Citation15Citation16 PAIs have synergetic properties due to characteristics of both polyamides and polyimides existing in the molecular backbone.Citation[17]
In recent years, the effect of adding nanofillers on the physical and mechanical properties of PAIs has been studied by many groups, but a few studies concerned with the fabrication of multi-walled CNT (MWCNT)/PAI composites have been carried out.[18–20]Citation18Citation19Citation20 A number of researchers have undertaken covalent stabilization techniques to molecularly bind (or to functionalize) specific molecular species onto CNT surfaces for enhanced dispersion and functionality;[8–11]Citation8Citation9Citation10Citation11 however, a noncovalent approach via hydrogen interaction of MWCNTs–COOH in polymer solution is selected for this study as this method preserves the mechanical and physical properties of individual CNTs. A small number of studies have also been focused on improving the dispersion of CNTs via the synthesis of polymer with several functional and bulky pendent groups for the matrix polymer itself.
With an aim of expanding the domain of CNT/PAI composites,[18–22]Citation18Citation19Citation20Citation21Citation22 we investigated a series of amino acid-based nanostructured PAI composites containing a range of concentration of MWCNTs and understand the reinforcement contributed by the MWCNTs. The thermal and mechanical properties, including the elastic modulus and tensile strength, as a function of MWCNTs content, were tested. The results from the morphological characterization of the composites were also presented and discussed.
2 Experimental
2.1 Reagents and materials
All chemicals used in this study were obtained commercially from Fluka Chemical Co. (Switzerland), Aldrich Chemical Co. (Milwaukee, WI) and Merck Chemical Co. (Germany). 2-Aminothiazole, 3,5-dinitrobenzoylchloride, acetone, hydrazine hydrate, FeCl3, and propylene oxide from Merck were used for the synthesis of mediators. Propylene oxide was used as acid scavenger. N,N′-dimethylformamide (DMF) (d = 0.94 g cm−3 at 20 °C) and N,N′-dimethylacetamide (DMAc) DMAc as solvent (d = 0.94 g cm−3 at 20 °C) were distilled over barium oxide under reduced pressure. Carboxyl-modified MWCNT (>95% purity and carboxyl content 2.56 wt.%) used in this study was purchased from Neutrino Co. (Iran) and has outer diameter of 8–15 nm and length of ∼50 μm. Other reagents were used without further purification.
2.2 Characterization and instrumentation
The apparatus used for the step-growth polymerization reaction was a Samsung microwave oven (2450 MHz, 900 W) (Korea). Fourier transform infrared (FT-IR) spectra of the composite films were recorded with a Jasco-680 (Japan) spectrometer at a resolution of 4 cm−1, and they were scanned at wavenumber range of 400–4000 cm−1. Band intensities are assigned as weak (w), medium (m), strong (s), and broad (br). Vibration bands were reported as wavenumber (cm−1). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker (Germany) Avance 500 instrument at room temperature (RT) in dimethyl sulphoxide-d6 (DMSO-d6). Multiplicities of proton resonance were designated as singlet (s), doublet (d), triplet (t), and multiplet (m). 13C spectrum is broadband proton decoupled. The chemical shifts were reported in ppm. Elemental analysis was performed in an Elementar Analysensysteme GmbH (Germany). Inherent viscosity was measured by using a Cannon Fenske Routine Viscometer (Germany) at the concentration of 0.5 g/dL in DMF at 25 °C. Optical specific rotations were measured at concentration of 0.5 g/dL in DMF at 25 °C using a quartz cell (1.0 cm) with a Jasco Polarimeter (JASCO Co., Ltd, Japan). Thermal stability of the MWCNT/PAI composites was evaluated by recording thermogravimetric analysis (TGA)/derivative thermogravimetric (DTG) traces (STA503 TGA, Germany) in nitrogen atmosphere (flow rate 60 cm3/min). A heating rate of 10 °C/min and a sample size of 10 ± 2 mg were used in each experiment. The X-ray diffraction (XRD) was used to characterize the crystalline structure of the composites. XRD patterns were collected using a Bruker, D8AVANCE (Germany) diffractometer with a copper target at the wave length (of) λ Cu Kα = 1.54 Å, a tube voltage of 40 kV, and tube current of 35 mA. The samples were scanned at a rate of 0.05°/min from 10° to 80° of 2θ. For XRD studies, rectangular pellets prepared by compression molding were used. The dispersion morphology of the MWCNTs in the PAI matrix was observed using field emission scanning electron microscopy (FE-SEM, HITACHI S-4160, Japan). Transmission electron microscopy (TEM) micrographs were obtained using a Philips CM 120 (Germany) microscope with an accelerating voltage of 100 kV. For TEM studies, ultra-thin sections (30–80 nm) of the composites were prepared using Leica Ultramicrotome. Tensile testing was performed at RT on a Testometric Universal Testing Machine M350/500 (UK), according to ASTM D 882 (standards). Tests were carried out with a cross-head speed of 12.5 mm/min until/to a deformation of 20% and then at a speed of 50 mm/min at break. The dimensions of the test specimens were 35 × 2 × 0.04 mm3. Tensile strength, tensile modulus, and strain were obtained from these measurements. Preparation of the MWCNT/PAI composites was carried out on a MISONIX ultrasonic XL-2000 SERIES, USA. Ultrasonic irradiation was performed with the probe of the ultrasonic horn immersed directly in the mixture solution system with frequency 2.25 × 104 Hz and power 100 W.
2.3 Monomer synthesis
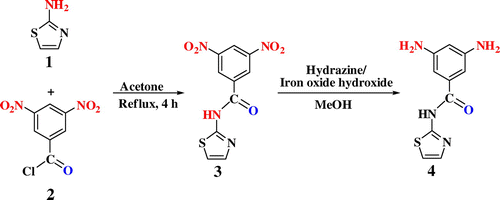
2.3.1 Synthesis of 3,5-diamino-N-(thiazol-2-yl)benzamide (4)
Iron oxide hydroxide catalyst was prepared according to the literature.Citation[23] 3,5-Dinitro-N-(thiazol-2-yl)benzamide (3) was also prepared according to the reported procedure.Citation[24] In an experiment, 14 g iron (III) chloride dissolved in 41 ml of distilled water and adding NaOH to the solution to bring the pH to 7. Then, the suspension was heated to 60 °C and maintained at this temperature for 12 h. After centrifugation and drying, the catalyst was redispersed and milled to a fine powder. Half an hour before the reaction used to be started, the catalyst was activated by adding some drops of distilled water to develop its full catalytic activity in organic solvents. Then, it was filtered off and was washed and reused for further reductions. For synthesis of 3,5-dinitro-N-(thiazol-2-yl)benzamide (3), a solution of 3,5-dinitrobenzoyl chloride (0.01 mol) and 2-aminothiazole (0.01 mol) in anhydrous acetone was refluxed for 4 h. After completion of the reaction, the crude solid product was filtered, washed with water, and purified by recrystallization from ethyl acetate/water. To a suspension of the purified 3,5-dinitro-N-(thiazol-2-yl)benzamide (1.0 g, 3.4 mmol) and iron oxide hydroxide (0.1 g, 1.13 mmol) in methanol (15 mL), hydrazine monohydrate (1.5 mL) was added dropwise to the stirred mixture at 60 °C within 10 min. After complete addition, the mixture was heated at the reflux temperature for another 12 h. The reaction solution was filtered hot to remove iron oxide hydroxide, and the filtrate was then filtered cold to remove the solvent. The crude product was purified by recrystallization from methanol to give 0.68 g of diamine 4 as brown needles (68% yield, m.p. = 208–210 °C).Citation[25]
FT-IR (KBr; cm−1): 3434 (s, N–H stretch), 3385 (s, N–H stretch), 3103 (w, C–H aromatic), 1662 (s, C=O amide stretch), 1560 (m), 1525 (m) 1459 (w), 1326 (w), 1161 (w, C–O stretch), 830 (m), 779 (w, N–H out-of-plane bending). 1H NMR (DMSO-d6; δ, ppm): 4.907 (s, 4H, NH), 6.060 (s, 1H, Ar–H), 6.454 (s, 2H, Ar–H), 7.199–7.208 (d, 1H, Ar–H, J = 3.60 Hz), 7.449–7.507 (d, 1H, Ar–H, J = 3.20 Hz), 11.823 (s, 1H, NH). 13C NMR (DMSO-d6; δ, ppm): 102.55 (CH, Ar), 103.01 (CH, Ar), 113.39 (CH, thiazole ring), 133.61 (C, Ar), 137.66 (CH, thiazole ring), 149.20 (C, Ar), 158.57 (C, Ar), 166.45 (C, C=O). Elemental analysis: calculated for C10H10N4OS: C, 51.27%; H, 4.30%; N, 23.91%; S, 13.69%; found: C, 51.40%; H, 4.330%; N, 23.87%; S, 13.54%.
2.3.2 Synthesis of N,N′-(pyromellitoyl)-bis-L-isoleucine (5)
N,N′-(Pyromellitoyl)-bis-L-isoleucine (5) was prepared according to our previous workCitation[26].
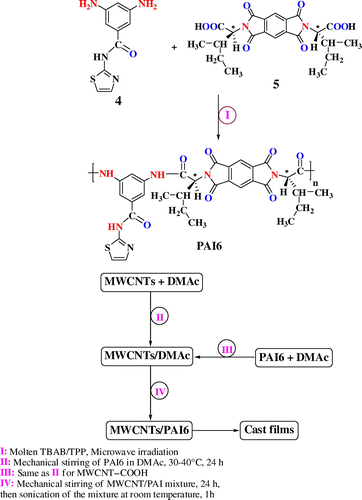
2.4 Synthesis of PAI
A mixture of 0.10 g (2.25 × 10−4 mol) of diacid monomer 5, 0.05 g (2.25 × 10−4 mol) of diamine 4, and 0.29 g of tetrabutylammonium bromide (TBAB) (9.00 × 10−4 mol) were placed in a porcelain dish and were ground completely for 5 min, then 0.23 mL (9.00 × 10−4 mol) of triphenyl phosphite (TPP) was added and the mixture was ground for 3 min. The reaction mixture was irradiated in the microwave oven for 240 s at 100% of power level (900 W). The resulting viscous solution was poured into 30 mL of methanol, filtered, and dried at 80 °C for 6 h under vacuum to give 0.14 g (96%) of yellow powder PAI6. The specific rotation was measured ( = −27.30) at a concentration of 0.5 g/dL in DMF at 25 °C. The inherent viscosity was also measured (ηinh = 0.45 dL/g) under similar conditions.
FT-IR (KBr, cm−1): 3426 (m, br, N–H stretch), 3100 (w, C–H aromatic), 2966 (w, C–H aliphatic), 1775 (m, C=O imide, asymmetric stretching), 1723 (s, C=O imide, symmetric stretching), 1671 (m, C=O amide, stretching), 1602 (s), 1546 (m), 1450 (s), 1382 (m, CNC axial stretching), 1212 (m, CNC transverse stretching), 1107 (m), 725 (s, CNC out-of-plane bending), 620 (w). 1H NMR (DMSO-d6; δ, ppm): 0.917 (t, 6H, CH3, distorted), 1.279–1.297 (d, 6H, CH3, J = 7.2 Hz), 1.563 (m, 4H, CH2), 2.674 (m, 2H, CH), 4.727–4.737 (d, 2H, CH, J = 4.2 Hz), 6.841 (s, 1H, Ar–H), 7.505 (s, 2H, Ar–H), 7.883–7.895 (d, 1H, Ar–H, J = 4.8 Hz), 8.105–8.121 (d, 1H, Ar–H, J = 6.4), 8.522 (s, 2H, Ar–H), 10.348 (s, 2H, NH), 12.689 (s, 1H, NH). Elemental analysis: calculated for (C32H30N6O7S)n: C, 59.80%; H, 4.71%; N, 13.08%; S, 4.99%. Found: C, 59.61%; H, 4.79%; N, 13.18%; S, 4.35%.
2.5 Preparation of the MWCNT/PAI composite films
To attain uniformly mixed MWCNT/PAI solutions with desired weight percentages of MWCNT, a two-step method was used. First, two stock solutions were prepared: PAI was dissolved and MWCNTs were separately dispersed in DMAc solution with stirring for 1 day at 30–40 °C. Then, two stock solutions were mixed to achieve the desired weight percentages of MWCNTs from 5 to 15 wt.%. The MWCNT/PAI solutions were stirred for 1 day at 30–40 °C and then ultrasonicated in water bath for 1 h. To remove the DMAc solvent, MWCNT/PAI solutions were poured into uncovered preheated glass petri dishes and uniformly heated at 60 °C for 1 day; then, the semidried film was further dried in vacuum at 160 °C for 8 h to remove the residual solvent and a solid film was formed. Curly films formed after evaporation of DMAc and could be easily lifted from the glass Petri dishes. Freestanding polymer films 30–40 μm thick were then peeled from the glass plate and were subjected to different tests. Because MWCNTs are black in color, the films containing more MWCNTs look darker: film colors varied from tan (0% MWCNT) to light gray (5% MWCNT) to dark black (10 and 15% MWCNT). Uniform color was observed, an indication of good distribution of MWCNTs in the polymer matrix.
3 Results and discussion
3.1 Synthesis and structural characterization of monomers
Diacid monomer was synthesized by the condensation reaction of an equimolar amount of pyromellic anhydride and S-valine amino acid in reflux acetic acid solution.Citation[26] Iron oxide hydroxide catalyst was prepared according to the literature.Citation[23] 3,5-Dinitro-N-(thiazol-2-yl)benzamide (3) was also prepared according to the reported procedure.Citation[24] Scheme shows the synthetic route to 3,5-diamino-N-(thiazol-2-yl)benzamide by a two-step process. In the first step, nucleophilic displacement of 2-aminothiazole with 3,5-dinitrobenzoylchloride in acetone solvent resulted in 3,5-dinitro-N-(thiazol-2-yl)benzamide as a pale yellow solid.Citation[24] In the second step, this dinitro compound was reduced in methanol in the presence of hydrazine hydrate and a catalytic amount of iron oxide hydroxide at 60 °C to produce brown crystals of the diamine 4. The structure of the diamine 4 was identified by elemental analysis, FT-IR, and NMR spectroscopic methods. In the FT-IR spectrum of diamine 4, the peak attributed to the stretching vibration of the bond C=O appeared at 1662 cm−1. The absorption peaks of amine functions are obvious as two peaks at 3434 and 3385 cm−1. In the 1H NMR spectrum of diamine 4, the signals of aromatic protons are appeared in the range of 6.060–7.507 ppm, and the characteristic resonance signal at 4.907 ppm is due to the amino group. Moreover, the proton for the amide group is observed at 11.823 ppm. Furthermore, in the 13C NMR spectrum, seven peaks corresponding to the seven kinds of aromatic carbons appeared in the range of 102.55–158.57 ppm. This spectrum also exhibited a peak for carbonyl of amide group at 166.45 ppm.
3.2 Synthesis of PAI
In the course of our study on the application of green chemistry principles in polymerization [27,28]Citation27Citation28, we wish to report a fast, simple, safe, and efficient method for the step-growth polymerization of natural S-valine-based diacid 5 with an aromatic thiazole-based diamine (4) in the molten TBAB in the presence of TPP under microwave irradiation. Scheme shows the polycondensation reaction to PAI. The effect of microwave power levels and period of heating were examined to provide the optimum reaction conditions. A series of experiments which were performed with different reaction times under microwave irradiations revealed that the optimal results were obtained after 240 s at 100% of power level. At higher radiation times, dark product was obtained, and on the other hand, under low radiation times, reaction gave low yield and inherent viscosity. This problem could be explained by the fact that molten salts are highly polar media and likely to be strongly microwave absorbing. The inherent viscosity of the synthesized PAI was 0.45 dL/g and the yield was 96%. The resulting polymer showed optical rotation, which indicated that the polymer is optically active and chirality was introduced into the backbone of the polymer. The specific rotation of this polymer was = −27.30 (0.05 g in 10 mL of DMF). The structure of this polymer was confirmed as PAI using FT-IR spectroscopy, 1H NMR, and elemental analysis techniques.
3.3 Fabrication of the composite films
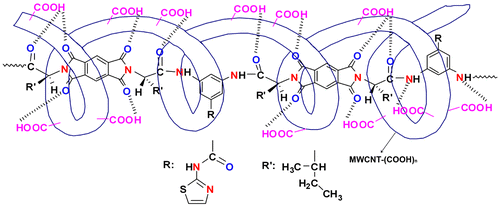
The dispersion of the carboxyl-modified MWCNTs in the 5, 10, and 15 wt.% solutions of PAI in DMAc was achieved by a vigorous stirring with a speed of 15,000 rpm for 1 day using a homogenizer, followed by utrasonication process for one hour to form a new series of chiral composites. The reaction pathways for the preparing MWCNT/PAI composites are shown in Scheme . The effective use of CNTs in composite applications depends on the ability to disperse the CNTs uniformly throughout the matrix without reducing their aspect ratio. Due to the van der Waals attraction, CNTs are held together as bundles and ropes. Therefore, they have very low solubility in solvents and tend to remain as entangled agglomerates. To employ CNTs as effective reinforcement in polymer composites, proper dispersion, and appropriate interfacial adhesion between the CNTs and polymer matrix, several mechanical/physical methods were used.[8,9]Citation8Citation9 So, carboxylated MWCNTs were used in this study. The lower level of aggregation in the modified CNTs can be attributed to the presence of functional groups such as carboxyl groups. This transformation should contribute positively to the good dispersion of CNTs in the PAI matrix. Moreover, the introduction of several functional groups into the matrix of aromatic polymer performs a hydrogen interaction with modified CNTs and a composite based on hydrogen interaction, by which the PAI chains were tightly attached to the surface of MWCNT–(COOH)n, can be result. The interaction between PAI chains and MWCNTs–(COOH)n was described in Figure .
3.4 Characterization of the composites
3.4.1 Spectral data
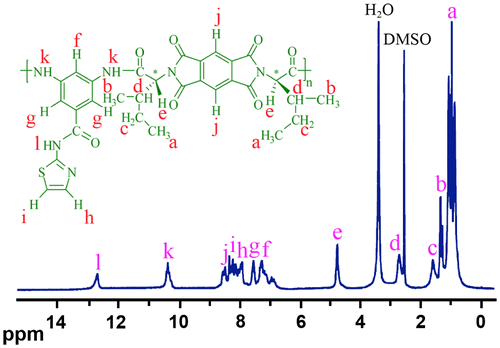
The formation of PAI was confirmed using FT-IR spectroscopy. Strong absorption bands were observed at 1723–1775 cm−1. These are attributed to the asymmetric and symmetric stretching vibrations of the imide carbonyl groups. The bands of C–N bond stretching and ring deformation appear at 1382 and 725 cm−1. Strong bands of absorption characteristic for the new formed amide linkage appeared at around 3426 cm−1 assigned to N–H stretching vibration, at 1671 cm−1 attributed to C=O stretching vibration, and at 1546 cm−1 due to N–H bending vibration. The absorption band at around 3100 cm−1 was attributed to =CH aromatic linkage. The aliphatic C–H stretching peak was also appeared at around 2966 cm−1. The functionalized MWCNTs were also characterized by FT-IR. The spectrum of a pristine MWCNTs/KBr pellet showed a strong, broad absorption band centered at 3433 cm−1, which is attributed to the O–H stretching bands of carboxylic acid moieties from the surface of MWCNTs. The small peak around 2923 cm−1 was ascribed to aliphatic sp3 C–H of MWCNTs.Citation[29] The presence of CNTs in the polymer matrix showed very little changes in the FT-IR spectrum, presumably due to the low MWCNT composition and the weak vibration signals of MWCNTs. The structure of neat PAI was also confirmed with 1H NMR spectroscopy. In the 1H NMR spectrum (Figure ) of this polymer, appearances of the N–H protons of amide groups at 10.348 (k) and 12.689 ppm (l), as three singlet peaks, indicate the presence of amide groups in the polymer’s structure. The resonance of aromatic protons appeared in the range of 6.841–8.522 ppm (f – j). Also, the proton of the chiral center appeared as doublet at 4.727–4.737 ppm (e). The protons of amino acid alkyl group also appeared as multiplet at 2.674 (d), 1.563 (c), 1.279–1.297 (b), and 0.917 (a) ppm in the 1H NMR spectrum.
3.4.2 XRD analysis
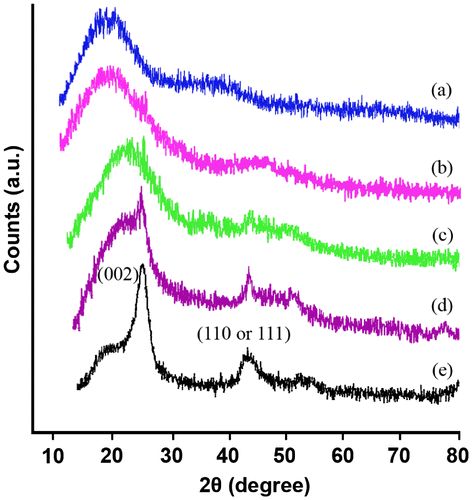
The structure of the MWCNT/PAI composites was examined by XRD, and the resultant curves are shown in Figure . MWCNTs and pristine PAI were also investigated and compared. For the MWCNTs–COOH, two peaks appeared at 2θ = 26° and 44° which are typically associated with the diffraction of metal impurities 2θ = 26° corresponds to the (0 0 2) diffraction plane of the impurity graphite and 2θ = 44° to α-Fe (1 1 0) and/or Ni (1 1 1) diffractions.Citation[30] For the neat PAI, the weak reflection centered at a 2θ value around 20° was characteristic of the amorphous polymer. For the MWCNT composite samples, they showed similar XRD patterns to the pure polymer samples when the MWCNT composition was 5 wt.%. For the MWCNT content higher than 5 wt.%, the MWCNT/PAI composites exhibit peaks of PAI and MWCNTs, as shown in Figure . The reflections at 2θ = 20° and 26° became slightly increased, when the MWCNT composition was increased to 15 wt.%.
3.4.3 Thermal stability
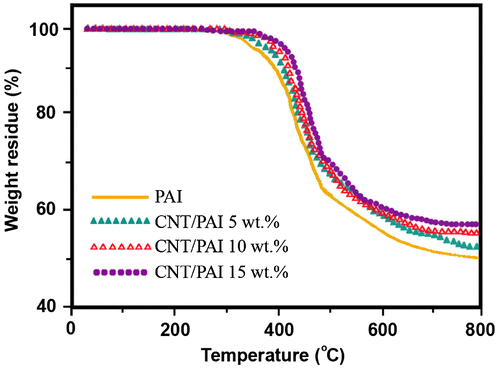
TGA and DTG analysis at a rate of 10 °C/min in a nitrogen atmosphere was utilized to examine the thermal properties of the CNT/PAI composites. TGA results of PAI and their composites in nitrogen at heating rate of 10 °C/min are shown in Figure . The thermal stability of the polymer and composites was studied on the basis of 5 and 10% weight losses (T5 and T10) of the samples and the residue at 800 °C. The onset of decomposition temperature of the composites is higher than that of pure PAI, and it shifts toward higher temperatures as the amount of MWCNTs is increased. The T10 of pure PAI and the MWCNT/PAI composites with increasing amount of the MWCNTs such as 5, 10, and 15 wt.% are 388, 414, 425, and 438 °C, respectively. The T10 of the composite with 15 wt.% MWCNTs exhibits a T10 50 °C higher than that of pure PAI. It is clear that the MWCNT/PAI materials improve thermal stability due to the incorporation of MWCNTs. The end temperature of decomposition is also retarded with increasing MWCNT content. The masses remaining at 800 °C are almost entirely due to the remaining CNTs and are consistent with initial CNT loading. The weight percent remaining after major degradation at 800 °C was higher for composites than for neat PAI. This indicates MWCNT reduces the degradation of PAI at high temperature as the effect was clearly seen in the curves. The results indicate that the addition of CNTs significantly increased the thermal stability of PAI in nitrogen as the effect was clearly seen in the curves.
There are two appealing relationships between the limiting oxygen index (LOI) and the parameters of the combustion process: char yield or char residue (CR) and heat of combustion. LOI also called critical oxygen index or oxygen index (OI) and it is defined as the minimum fraction of oxygen in a mixture of oxygen and nitrogen that will just support combustion (after ignition). On the other hand, OI methods describe the tendency of a material to sustain a flame.
According to Van Krevelen Citation[31], there is a linear relationship between LOI and CR only for halogen-free polymers (Equation (1)):(1)
From this equation, a higher char yield will increase flame retardance. PAI and composites containing 5, 10, and 15 wt.% had LOI values 37.1, 38.3, 39.5, and 40.3, respectively, which were calculated from their char yield. On the basis of the LOI values, such materials can be classified as self-extinguishing materials.
According to Johnson Citation[32], the LOI values of many common materials can be logically well predicted by the expression (Equation (2)):(2) where ΔHcomb is the specific heat of combustion in J/g. So, in the case of this polymer (PAI6) and MWCNT/PAI composites (5, 10, and 15 wt.%), ΔHcomb is 21.6, 20.9, 20.5, and 19.8 kJ/g, respectively.
3.4.4 Microscopy characterization
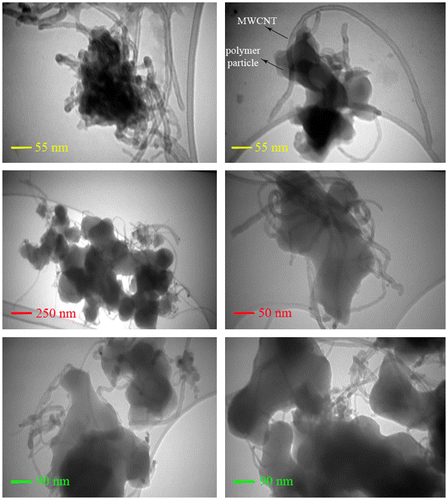
To characterize the morphology and dispersity of MWCNTs in the composites, FE-SEM images of fracture surfaces of neat nanostructured PAI and composites were examined as displayed in Figure . The neat PAI copolymer showed a smooth fracture surface morphology (Figure ). FE-SEM observation revealed that the PAI particles self-organized into nanopatterns. As can be seen from these images, the average diameter of polymeric particles is about 32 nm and the shape of them is about spherical. For MWCNT/PAI composites, MWCNTs well dispersed and embedded in the PAI matrix without showing noticeable MWCNT aggregate (Figure ). In addition, the fracture surface of MWCNT/PAI composites is relatively smooth without exhibiting the pullout of MWCNTs from the PAI matrix, and the boundary between MWCNT and PAI matrix is not discernible clearly. The fracture surface was obtained by cutting in the liquid nitrogen. These results strongly support that due to hydrogen bonding between MWCNT–(COOH)n and functional groups in the PAI matrix, the MWCNTs are wrapped around PAI nanoparticles and MWCNTs are also mixed well with the PAI matrix, eventually leading to the good dispersity of MWCNTs–(COOH)n in the PAI matrix. To assess the dispersion of the CNTs, the thin sections of the composites were also examined in the TEM. For the FE-SEM analysis, three samples with different weight percent of MWCNTs were selected. All samples showed approximately similar observation. So, the composite with 10 wt.% of MWCNT content was selected as representative for TEM study. Figure shows the typical TEM images from the different parts of the composite with 10 wt.% of MWCNTs content at different magnifications for better observation of the polymer and MWCNT interactions. It can be seen that the MWCNTs were almost attached to the polymeric particles, resulting from the increased polarity by the functional groups formed on the surfaces of MWCNTs as well as carbonyl groups in the PAI structure. Possible interactions between the carboxyl groups at the MWCNT–(COOH)n with the oxygen atoms at the carbonyl groups of the PAI are proposed in Figure .
3.4.5 Mechanical properties
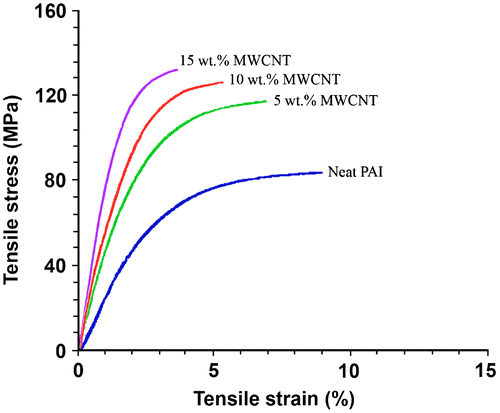
It has been generally accepted that the nanoparticles or nanomaterials benefit the mechanical properties when they are well distributed in the polymer matrix.Citation[6] In this study, MWCNTs–(COOH)n were also found to greatly increase the mechanical properties of PAI due to the nanoreinforcing effect of CNT with high aspect ratio. Figure shows the tensile properties of the MWCNT/PAI composites. It can be seen that, in comparison with neat PAI film, the MWCNT/PAI composite films exhibit higher Young’s modulus and tensile strength but smaller strain at break, and the strain at break decreases gradually with increasing MWCNT feeding content, while a significant increment in Young’s modulus and a moderate increment in tensile strength are observed simultaneously. The Young’s modulus and tensile strength increased from 1.6 GPa and 85.4 MPa for neat PAI film to 2.9 GPa and 117.3 MPa, 3.4 GPa and 126.8 MPa, and 3.7 GPa and 132.9 MPa for MWCNT/PAI composite films with increasing the MWCNT feeding content from 5 to 10 and 15 wt.%, respectively. This increasing effect of CNT on the tensile strength and tensile modulus of composites was more significant at low CNT content when compared with high CNT content, because when the MWCNT content was increased in the composite films, they became somewhat brittle compared to neat PAI film because of the increased stiffness of the composites and decreased the mobility of PAI matrix. Such an embrittlement phenomenon has also been observed in other CNTs reinforced polymer systems.[33,34]Citation33Citation34 The decrease in the elongation at break of the composites with the increasing of MWCNT content could also be attributed to this phenomenon, as shown in Figure . In this study, the reinforcing efficiency of MWCNT–(COOH)n is defined as the normalized mechanical properties of the composites with respect to those of pure PAI. The improvement of the mechanical properties of the MWCNT/PAI composites was attributed to the better interaction between the MWCNTs and the PAI matrix as well as the better dispersion of the MWCNT in the PAI matrix. The incorporation of the MWCNT–(COOH)n and several functional groups into the PAI matrix resulted in the good interfacial adhesion between the MWCNT–(COOH)n and the PAI matrix. This interaction between the MWCNT–(COOH)n and the PAI matrix is crucial for improving the mechanical properties of the composites.
4 Conclusions
In conclusion, PAIs are very attractive candidates for an advanced dispersing agent of CNTs. MWCNT/PAI composites containing thiazole moiety and amino acid group have been fabricated. The composite prepared with combined acid treatment or ultrasonic treatment showed substantially increased mechanical and thermal properties with increasing MWCNT content, indicative of good dispersion of MWCNTs. The significant improvements effects are due to the reinforcement of finely dispersed MWCNTs fillers throughout the matrix as well as the strong hydrogen interaction between carboxyl functional groups in the MWCNTs and carbonyl groups in the PAI matrix, thus being favorable to stress transfer from polymer to CNTs. This study demonstrated that the thermal, mechanical, and morphological behaviors of the composites are strongly dependent on the uniform dispersion of CNTs and the interactions between CNTs and PAI, which can be increased by using of modified CNT and the introduction of several functional groups in the polymer’s chains, providing a design guide of CNT-reinforced composites with a great potential for industrial applications.
Acknowledgements
The study is financially supported by Research Affairs Division Isfahan University of Technology (IUT). Further financial support from National Elite Foundation (NEF) and Center of Excellency in Sensors and Green Chemistry Research (IUT) is gratefully acknowledged.
References
- Grady BP. Carbon nanotube-polymer composites: manufacture, properties, and applications. Hoboken, NJ: John Wiley & Sons; 2011.
- Baughman RH, Zakhidov AA, De Heer WA. Carbon nanotubes-the route toward applications. Science. 2002;297:787–792.
- Rozenberg BA, Tenne R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008;33:40–112.
- Moniruzzaman M, Winey KI. Polymer nanocomposites containing carbon nanotubes. Macromolecules. 2006;39:5194–5205.
- Liao HT, Wu CS. Poly(3-hydroxybutyrate)/multi-walled carbon nanotubes nanocomposites: preparation and characterizations. Des. Monomers Polym. 2013;16:99–107.
- Ji GJJ, Lu H. Electrospinning multiwalled carbon nanotubes-polyurethane composite fibers. Des. Monomers Polym. 2011;14:121–131.
- Li J, Zhang LQ. The addition of carbon nanotube on the tensile properties of carbon fiber-reinforced PEEK composites. Polym.-Plast. Technol. Eng. 2009;48:1176–1179 .
- Moosavi Abdehgah R, Ashouri D, Mousavian S. In situ preparation of high performance polyimide nanocomposites based on functionalized multiwalled carbon nanotubes. Des. Monomers Polym. 2013;16:108–115.
- Hu CY, Xu YJ, Duo SW, Zhang RF, Li MS. Non-covalent functionalization of carbon nanotubes with surfactants and polymers. J. Chin. Chem. Soc. 2009;56:234–239.
- Yu JG, Huang DS, Huang KL, Hong Y. Cross-linking of multi-walled carbon nanotubes with polyethylene glycol. Polym.-Plast. Technol. Eng. 2011;50:328–331.
- Sawada H, Shindo K, Iidzuka JI, Kawase T, Oharu K, Nakagawa H. Dissolution of carbon nanotubes in water and organic media with a variety of fluoroalkyl end-capped oligomers. Int. J. Polymer. Mater. 2005;54:247–256.
- Liu H, Wang T, Wang Q. Synthesis and tribological properties of thermosetting polyimide and its carbon nanotube-containing composites. Polym.-Plast. Technol. Eng. 2012;51:1–5.
- Enhessari M, Ozaee K, Karamali E, Ahmadi MR. Fabrication and thermal properties of polyimide/Ba0.77Sr0.23TiO3 Nanocomposites. Int. J. Polymer. Mater. 2012;61:89–98.
- Vural S, Köytepe S, Seçkin T, Adıgüzel I. Synthesis, characterization and dielectric properties of rodlike zinc oxide-polyimide nanocomposites. Polym.-Plast. Technol. Eng. 2012;51:369–376.
- Afsharian-Moghadam H, Haddadi-Asl V. Synthesis and characterization of a new semi-aliphatic poly(amide-imide) and evaluation of the effect of reaction conditions. Des. Monomers Polym. 2008;11:223–234.
- Rajesh S, Senthilkumar S, Jayalakshmi A, Nirmala MT, Ismail AF, Mohan D. Preparation and performance evaluation of poly (amide-imide) and TiO2 nanoparticles impregnated polysulfone nanofiltration membranes in the removal of humic substances. Colloid Surf. A. 2013;418:92–104.
- Mallakpour S, Zadehnazari A. Synergic effects of molten ionic liquid and microwave irradiation in preparation of optically active nanostructured poly(amide-imide)s containing amino acid and dopamine moiety. Polym.-Plast. Technol. Eng. 2012;51:1090–1096.
- Lee SH, Choi SH, Choi JI, Lee JR, Youn JR. Rheological property and curing behavior of poly (amide-co-imide)/multi-walled carbon nanotube composites. Korean J. Chem. Eng. 2010;27:658–665.
- Lee SH, Choi SH, Kim SY, Young JR. Effects of thermal imidization on mechanical properties of poly (amide-co-imide)/multiwalled carbon nanotube composite films. J. Appl. Polym. Sci. 2010;117:3170–3180.
- Lee SH, Choi SH, Kim SY, Choi JI, Lee JR, Youn JR. Degradation and dynamic properties of poly(amide-co-imide)/carbon nanotube composite films. Polym. Polym. Compos. 2010;18:381–389.
- Mallakpour S, Zadehnazari A. The production of functionalized multiwall carbon nanotube/amino acid-based poly(amide-imide) composites containing a pendant dopamine moiety. Carbon. 2013;56: 27-37.
- Mallakpour S, Zadehnazari A. Synthesize procedures, mechanical and thermal properties of thiazole bearing poly(amid-imide) composite thin films containing multiwalled carbon nanotubes. Colloid Polym. Sci. 2013;291:1525–1534.
- Lauwiner M, Rys P, Wissmann J. Reduction of aromatic nitro compounds with hydrazine hydrate in the presence of an iron oxide hydroxide catalyst. I. The reduction of monosubstituted nitrobenzenes with hydrazine hydrate in the presence of ferrihydrite. Appl. Catal. A.,. 1998;172:141–148.
- Saeed S, Rashid N, Wong WT, Hussain R. 3,5-Dinitro-N-(1,3-thiazol-2-yl) benzamide monohydrate. Acta Cryst. 2011;E67:o660.
- Mallakpour S, Ahmadizadegan H. Poly(amide-imide)s obtained from 3,5-diamino-N-(thiazol-2-yl)-benzamide and dicarboxylic acids containing various amino acid units Production, characterization and morphological investigation. High Perform. Polym. 2013;25:156–164.
- Mallakpour S, Shahmohammadi MH. Microwave-promoted rapid synthesis of new optically active poly(amide imide)s derived from N,N′-(pyromellitoyl)-bis-L-isoleucine diacid chloride and aromatic diamines. J. Appl. Polym. Sci. 2004;92:951–959.
- Mallakpour S, Zadehnazari A. Tailored synthesis of nanostructured polymer thin films from optically active and thermally stable poly(amide-co-imide)s containing hydroxyl pendant groups in a green ionic solvent. Polym.-Plast. Technol. Eng. 2012;51:1097–1105.
- Mallakpour S, Rafiee Z. New developments in polymer science and technology using combination of ionic liquids and microwave irradiation. Prog. Polym. Sci. 2011;36:1754–1765.
- Lee HJ, Oh SJ, Choi JY, Kim JW, Han J, Tan LS, Baek JB. In situ synthesis of poly(ethylene terephthalate) (PET) in ethylene glycol containing terephthalic acid and functionalized multiwalled carbon nanotubes (MWNTs) as an approach to MWNT/PET nanocomposites. Chem. Mater. 2005;17:5057–5064.
- Pérez-Cabero M, Rodríguez-Ramos I, Guerrero-Ruíz A. Characterization of carbon nanotubes and carbon nanofibers prepared by catalytic decomposition of acetylene in a fluidized bed reactor. J. Catal. 2003;215:305–316.
- Van Krevelen DW. Some basic aspects of flame resistance of polymeric materials. Polymer. 1975;16:615–620.
- Johnson PR. A general correlation of the flammability of natural and synthetic polymers. J. Appl. Polym. Sci. 1974;18:491–504.
- Siochi EJ, Working DC, Park C, Lillehei PT, Rouse JH, Topping CC, Bhattacharyya AR, Kumar S. Melt processing of SWCNT-polyimide nanocomposite fibers. Composites Part B. 2004;35:439–446.
- Ge JJ, Zhang D, Li Q, Hou HQ, Graham MJ, Dai L, Harris FW, Cheng SZD. Multiwalled carbon nanotubes with chemically grafted polyetherimides. J. Am. Chem. Soc. 2005;127:9984–9985.