Abstract
Soluble functional copolyimides are of great interest due to their excellent thermal properties and solubility, and the ability to adapt their properties to a wide range of special applications. Therefore, a novel poly(amide-ester-imide)s (PAEI)s was obtained by step-growth polymerization of several optically active diacids (3a–3d) with diol, ‘N,N′-(1,3,5,7-tetraoxo-5,7-dihydropyrrolo[3,4-f]isoindole-2,6(1H,3H)-diyl)bis(4-hydroxybenzamide)’ (4) via tosyl chloride, pyridine, and N,N-dimethylformamide (DMF) as a condensing agent. The obtained polymers were characterized by FT-IR, and 1H-NMR spectroscopy. Also, their physical properties including X-ray diffraction, elemental analysis, specific rotation techniques, field emission scanning electron microscopy (FE-SEM), and thermogravimetric analysis (TGA) were examined. These PAEIs with inherent viscosities in the range of 0.22–0.33 dL/g showed excellent solubility in various solvents such as N-methyl-2-pyrrolidinone, N,N-dimethylacetamide, and DMF. TGA showed that all polymers were stable, with 10% weights loss recorded above 380 °C in nitrogen atmosphere. Preliminary tests on the films of PAEI indicated that these materials are brittle. The FE-SEM of the above-mentioned PAEIs confirmed that these macromolecules show spherical morphology.
1. Introduction
In the past few decades, more consideration has been paid to biodegradable macromolecules. One of the principal aims relating to agricultural, hygienic, and packaging industries in the time coming will be their capacity to improve a high production of easily recyclable and/or biodegradable macromolecules.[1,2]Citation1Citation2 Ecological polymers are also extensively utilized in biomedical applications, such as drug delivery systems, implants, sutures, scaffolds for tissue engineering, and artificial skin.[2–6]Citation2Citation3Citation4Citation5Citation6 Functional polymers derived from monomers carrying optically active amino acids moieties are one kind of the significant non-natural macromolecules, because of great potential applications as biocompatible and biodegradable materials.Citation[7] Between the diverse natural resources that can be simply gained, α-amino acids are highly favorable, because the optically pure L-amino acids are readily obtainable from natural resources. Additional benefit of the macromolecules based on amino acids is the probable low toxicity of their degradation products. But due to the limited thermal stability and solubility of the macromolecules composed of amino acids, these materials would be hard to prepare and use. Therefore, synthesize and application of conventional synthetic polymers containing amino acids are interesting.[8,9]Citation8Citation9
Between the biomaterials, polyesters (PE)s have been well recognized because of their brilliant nontoxicity, biodegradability and biocompatibility.Citation[10] PEs are defined as condensation (or step-growth) macromolecules containing in-chain ester units as their crucial polymer-forming chain linkage. Although it may not be the actual synthetic route followed, PEs are prepared, in a formal sense, either by reaction of a dicarboxylic acid with a diol or by the self-condensation of a ω-hydroxy acid.[11–13]Citation11Citation12Citation13 Higashi and Tobe reported that the reactions promoted by toluenesulfonyl chloride and N,N-dimethylformamide (DMF) were effective for the synthesis of PEs by the reaction of dicarboxylic acids with bisphenols.Citation[14] However, low mechanical, thermal, and processing performances importantly limit the applied utilization of these materials. Development in the mechanical properties can be attained by the introduction of some structure other than ester linkages, for example amide and imide in the main chain.[12–16]Citation12Citation13Citation14Citation15Citation16 Newly, several efforts were made by researchers on high-performance macromolecules, with excellent thermal stability and solubility, and supplied great impetus to the finding of a variety of processable and thermally stable macromolecules. Aromatic PEs are materials showing an excellent physical properties. They are important group of high performance and engineering materials, which apply extensively in the aviation, automobile, and electronic industries. One of the approaches of improving the thermal properties of aromatic PEs is to incorporation some thermally stable groups, such as amide and imide groups into their main chains.[17,18]Citation17Citation18
Aromatic polyimides (PI)s and their copolymers are well known as high-performance macromolecule materials for their exceptional mechanical and electrical properties, outstanding solvent resistance and high thermal and thermooxidative stability.[19–23]Citation19Citation20Citation21Citation22Citation23 However, most aromatic PIs are inflexible materials that do not melt before thermally decomposing, so, would be difficult for processing. To solve these difficulties, amide, and ester groups may be introduced into PI to produce poly(ester-imide)s (PEI)s and poly(amide-ester-imide) (PAEIs) with improved solubility.[24,25]Citation24Citation25 Several strategies in the synthesis of PEIs and poly(amide-ester)s have been reported.[26,27]Citation26Citation27
PEIs are a class of polymers recognized for more than 35 years. They are utilized nowadays in large tonnage as electrical insulating materials. In recent times, novel applications for these macromolecules have been establish, for example adhesives, printed circuit boards, membranes, and engineering thermoplastics. Outstanding properties and easy processing will possibly cause a constant growth of PEIs business.[28,29]Citation28Citation29
In our previous work Citation[30], we reported the synthesis, and properties of a set of copolyesters prepared from dicarboxylic acids and diol. A simple method of the synthesis based on condensation in solution was used. In this article, we wish to describe the successful construction and characterization of novel semiaromatic PAEIs via polycondensation reactions of chiral diacids (3a–3d) with diol 4. All the aforementioned polymers are new, and they are reported for the first time. In the this study, the effect of molar concentration of monomers and tosyl chloride (TsCl), the amounts of DMF, and addition time of diol was examined.
2 Experimental
2.1 Materials
Reagents were purchased from the Fluka Chemical Co. (Buchs, Switzerland), Aldrich Chemical Co. (Milwaukee,WI), Riedel-deHaen AG (Seelze, Germany), and Merck Chemical Co. (Germany). DMF were dried over barium oxide and then were distilled under reduced pressure. S-valine, L-alanine, L-leucine, L-isoleucine, and pyridine (Py) were used as obtained without further purification. Potentially bioactive diol 4 was sold by Vitas-M Laboratory, Ltd.
2.2 Characterization
Infrared spectra of the samples were recorded at room temperature (R.T.) in the range of 4000–400 cm−1, on (Jasco-680, Tokyo, Japan) spectrophotometer. The spectra of solids were obtained using KBr pellets. The vibrational transition frequencies are reported in wave numbers (per centimeter). Band intensities were assigned as weak (w), medium (m), strong (s), and broad (br). Proton nuclear magnetic resonance (1H-NMR spectra, 400 MHz) was recorded in DMSO-d6 solution using a Bruker (Rheinstetten, Germany) Avance 400 instrument. Multiplicities of proton resonance were designated as singlet (s), triplet (t), and multiplet (m). Inherent viscosity was measured by a standard procedure with a Cannon-Fenske (Mainz, Germany) routine viscometer. Specific rotation was measured with a Jasco (Tokyo, Japan) P-1030 polarimeter at the concentration of 0.5 g/dL at 25 °C. Thermal gravimetric analysis (TGA) data were taken on STA503 TA instrument (Hüllhorst, Germany) in a nitrogen atmosphere at a heating rate of 20 °C/min. Elemental analysis was performed by Leco, CHNS-932 (Hanau, Germany). The XRD patterns of the polymers were recorded by employing a Philips X’PERT MPD diffractometer (Rheinstetten, Germany) with a copper target at 40 kV and 30 mA and Cu Kα radiation: l = 1.54 Å in the range of 10–80° at the speed of 0.05°/min. Field emission scanning electron microscopy (FE-SEM) was done using HITACHI (S-4160) (Tokyo, Japan).
2.3 Monomer synthesis
2.3.1 Preparation of optically active diacids
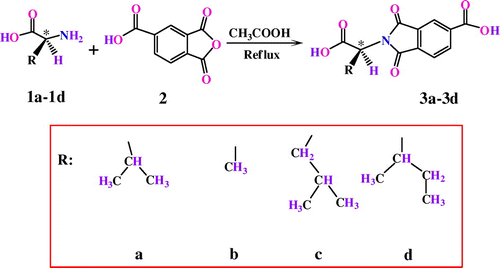
Chiral diacid monomers (3a–3d) for instance: N-trimellitylimido-S-valine,Citation[31] N-trimellitylimido-L-alanine,Citation[32] N-trimellitylimido-L-leucine Citation[33] and N-trimellitylimido-L-isoleucine Citation[34] were prepared according to our previous works (Scheme ).
2.4 Polymer synthesis
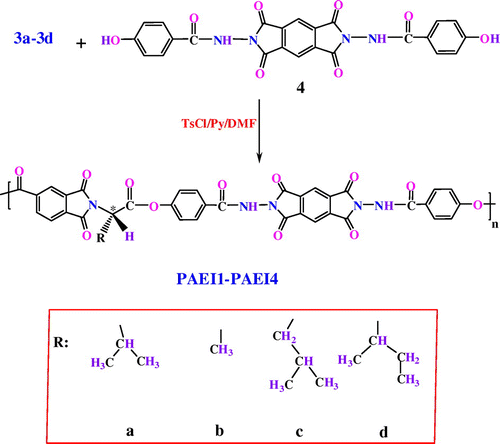
2.4.1 Example: synthesis of PAEI1
In a 10 ml round bottom flask equipped with a magnetic stirrer were placed Py, (0.20 ml) and TsCl (0.1960 g; 1.03 × 10−3 mol). The mixture was stirred to form homogenous solution. After 30 min stirring at R.T., DMF (0.09 ml; 1.03 × 10−3 mol) was added to the reaction mixture, and the stirring was continued for another 30 min. Then, the mixture was added dropwise to a solution of diacid 3a (0.06 g; 2.05 × 10−4 mol) in Py (0.20 ml). The mixture was maintained at R.T. for 30 min, 1 h at 100 °C, and then, diol 4 (0.10 g; 2.05 × 10−4 mol) was added, and the complete solution was stirred at 120 °C for 3 h. The viscous solution was poured into 20 ml of methanol with rapid stirring. The precipitated polymer was filtered off, washed with methanol and dried under vacuum at 80 °C for 6 h. Yield 0.1433 g (94%); ηinh 0.33 dL/g. Other PAEIs were synthesized by the similar procedure (Scheme ).
2.4.2 PAEI1
FT-IR Peaks (KBr, cm−1): 3345 (w, br), 3065 (w), 2940 (w), 1786 (m), 1740 (s), 1720 (s), 1495 (m), 1383 (m), 1266 (m), 1206 (s), 1163 (s), 1059 (m), 726 (w).
1H-NMR (400 MHz, DMSO-d6, δ, ppm): 0.98 (d, 3H, J = 5.6 Hz), 1.15 (d, 3H, J = 6.0 Hz), 2.67–2.69 (m, 1H), 5.15 (distorted d, 1H), 7.31–8.56 (m, 13H, Ar–H), 11.65 (s, br, NH).
Elemental analysis: calcd for (C38H23N5O12): C, 61.54%; H, 3.13%; N, 9.44%. Found: C, 61.28%; H, 3.47%; N, 9.12%.
2.4.3 PAEI2
FT-IR Peaks (KBr, cm−1): 3348 (w, br), 3062 (w), 2953 (w), 1780 (m), 1744 (s), 1719 (s), 1593 (m), 1380 (s), 1253 (s), 1194 (s), 1157 (s), 1053 (m), 727 (w).
2.4.4 PAEI3
FT-IR Peaks (KBr, cm−1): 3339 (w, br), 3067 (w), 2958 (w), 1782 (s), 1738 (s), 1718 (s), 1604 (m), 1381 (s), 1257 (s), 1197 (s), 1158 (s), 1098 (m), 1053 (w), 727 (w).
1H-NMR (400 MHz, DMSO-d6, δ, ppm): 0.89–0.93 (distorted d, 6H), 1.63 (m, 1H), 2.04 (m, 1H), 2.22 (m, 1H), 5.18 (distorted t, 1H), 7.36–8.58 (m, 13H, Ar–H), 11.69 (s, br, NH).
Elemental analysis: calcd. for (C39H25N5O12): C, 61.99%; H, 3.33%; N, 9.27%. Found: C, 61.11%; H, 3.36%; N, 9.19%.
2.4.5 PAEI4
FT-IR Peaks (KBr, cm−1): 3342 (w, br), 3062 (w), 2954 (w), 1781 (m), 1742 (s), 1717 (s), 1600 (m), 1382 (s), 1258 (s), 1194 (s), 1156 (s), 1097 (m), 1052 (w), 726 (w).
3 Results and discussion
3.1 Polymer synthesis
Semiaromatic PAEIs were synthesized by solution polycondensation of an equimolar mixture of the diol 4 with different diacids derived from S-valine, L-alanine, L-leucine, and L-isoleucine (3a–3d) in TsCl/Py/DMF (Vilsmeier adduct) as a condensing agent (Scheme ). The results of the synthesis of PAEIs are presented in Tables. Polycondensation was carried out at various molar ratio of TsCl to diacid, different amount of DMF, various addition times of diol, and consequently the optimum conditions were obtained which are listed in Table . All of these parameters had effect on the macomolecules chain growth. Based on this table, the optimum conditions are as follows: the molar ratio of TsCl to diacid (5/1), the amount of DMF to diacid (5/1), and the addition times of diol (0.5 h R.T. and 1 h 100 °C).
Table 1. The reaction and optimum conditions for the preparation of PAEIs.
Table 2. Synthesis and some physical properties of the obtained PAEIs.a
All of the polymers were obtained in more than 89% of yields. The inherent viscosities of the resulting polymers under optimized conditions were in a ranging of 0.22–0.33 dL/g. All of the PAEIs show optical rotations thus are chiral. The specific rotation of polymers based on different amino acids exhibited random changes, due to the different polymer structures.
3.2 Structural characterization of PAEIs
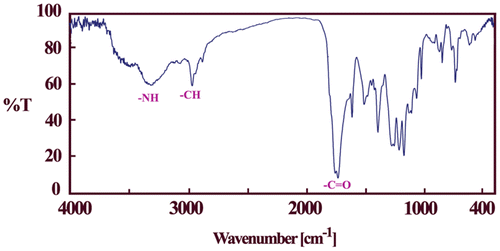
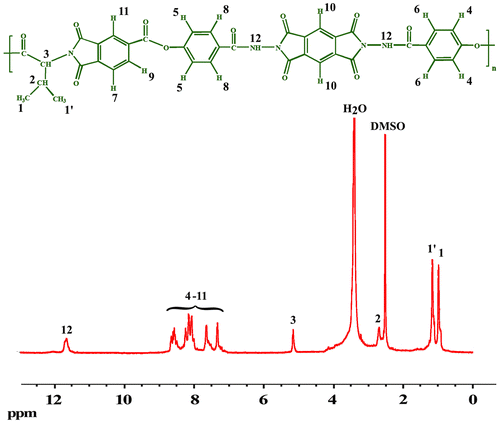
The formation of the PAEIs was confirmed by FT-IR, 1H-NMR spectroscopy techniques, and elemental analyses (Figures, Table ). All PAEIs exhibited characteristic bands of imide and ester groups occurred around 1780, 1740, and 1720 cm−1. All of these PAEIs displayed absorption at 1382 cm−1 and 727 cm−1 that exhibit the presence of the imide heterocycle in these polymers (Figure ). The 1H-NMR spectrum (400 MHz) of PAEI1 has been exhibited in Figure that confirms their chemical structure. In the 1H-NMR spectrum of PAEI1, the absorption of –NH amide group appeared at 11.65 ppm. The resonance of aromatic protons observed in the range of 7.31–8.56 ppm. The peak at 5.15 ppm is assigned for the proton of the chiral center appeared as t. The resonance of aliphatic protons of amino acid (CH2) diastereotopic appeared in the range of 0.98–1.15 ppm.
Table 3. Thermal properties of PAEIs.
The solubility of PAEIs was tested quantitatively in various solvents. All of the PAEIs are soluble in organic solvents such as DMF, DMSO, N,N-dimethyl acetamide (DMAc), N-methyl-2-pyrrolidone, and sulfuric acid at R.T. and are insoluble in solvents, for instance; chloroform, methylene chloride, methanol, ethanol, cyclohexane and water. The excellent solubility of the aforementioned polymers is due to the existence of ester groups in the main chain of them.
3.3 Thermal properties
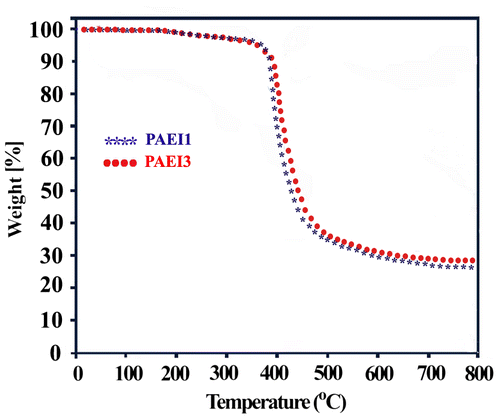
Thermal properties of these polymers were evaluated with thermogravimetry under a nitrogen atmosphere at a heating rate of 20 °C/min (Figure , Table ). The thermal stability has been probed by traditional analysis used in TGA to evaluate the degradation occurring via weight loss. The thermal characteristics such as the temperatures at which 5% (T5) and 10% (T10) degradation occur, char yield at 800 °C and also, limiting oxygen index (LOI), based on Van Krevelen and Hoftyzer equation Citation[35] are given in Table .
T10 on TGA curves of these polymers were stable up to the temperature around 380 °C. The results of the TGA measurements demonstrated that the polymers showed one-stage decomposition patterns. The char yields at 800 °C are in the range of 27–29% indicating their high thermal stability. The PAEI1 and PAEI3 behaved similarly. This shows that the incorporation of amide and imide linkage into the polymer backbone had detrimental effect on the high thermal stability of the obtained PAEIs. These polymers show LOI values that were calculated from their char yield at 800 °C and would be higher than 28.3. On the basis of LOI values, such macromolecules can be classified as self-extinguishing polymers.
3.4 Morphology of polymers
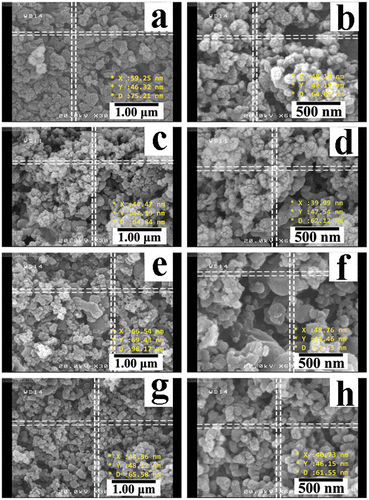
The surface morphology of the macromolecules was observed by FE-SEM via coating the surface of sample with gold (Figure ). As shown in the Figure , the obtained PAEIs have similar and nanoparticle structures with spherical forms are evident. According to FE-SEM pictures of PAEI1 (a,b), PAEI2 (c,d), PAEI3 (e,f), and PAEI4 (g,h), nanostructured PAEIs have the diameter between 39 to 96 nm.
3.5 XRD analysis
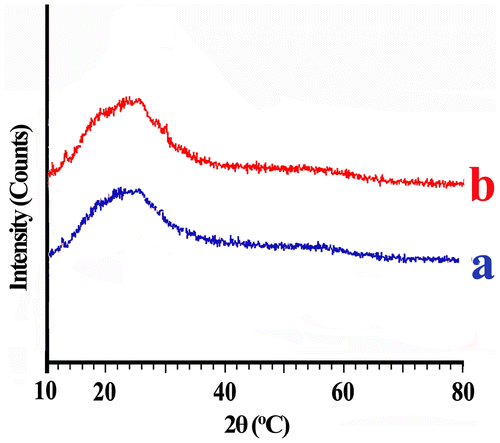
In order to study the crystalline or amorphous nature of the PAEI, X-ray diffraction measurements at R.T. in the region of 2θ = 10–80o were performed. Typical XRD patterns of the powder PAEIs are illustrated in Figure . Based on this Figure, it is revealed that the obtained PAEIs are amorphous in nature, showing broad peak in the region of 2θ = 10–30o. The amorphous behavior is reasonable, because there are asymmetric structures that could increase the disorder in the chains. Hence, the solubility of polymers increases with decreasing crystallinity. Therefore, these macromolecules exhibit excellent solubility behavior.
4 Conclusions
For the first time, the solution polycondensation of diol 4 with diacids (3a–3d) using TsCl/DMF/Py system, for the preparation of a series of moderate molecular weight PAEIs in good yields, was reported. These PAEIs were characterized by FT-IR, and 1H-NMR spectroscopy, inherent viscosity, solubility test, thermal analysis, and XRD. These polymers showed good solubility in aprotic polar solvents. Thermogravimetric analysis data of these PAEIs indicated that all the polymers were thermally stable which could be accredited to multifunctional groups of diol 4. These polymers due to the existence of ester and amino acids linkages maybe considered as potentially biodegradable and biocompatible materials for further applications. Also, due to the presence of amide and imide groups, the aforementioned polymers could be good candidates for high temperature-resistant, high-performance materials.
Acknowledgments
We wish to express our gratitude to the Research Affairs Division of Isfahan University of Technology (IUT), for financial support. Further financial support from National Elite Foundation (NEF) and Center of Excellency in Sensors and Green Chemistry Research (IUT) is gratefully acknowledged.
References
- Roumanet PJ, Laflèche F, Jarroux N, Raoul Y, Claude S, Guégan P. Novel aliphatic polyesters from an oleic acid based monomer. Synthesis, epoxidation, cross-linking and biodegradation. Eur. Polym. J. 2013;49:813–822
- Yoon KR, Hong SP, Kong B, Choi IS. Polycondensation of sebacic acid with primary and secondary hydroxyl groups containing diols catalyzed by Candida antarctica lipase B. Synth. Commun. 2012;42:3504–3512.
- Kim SE, Rha HK, Surendran S, Han CW, Lee SC, Choi HW, Choi YW, Lee KH, Rhie JW, Ahn ST. Bone morphogenic protein-2 (BMP-2) immobilized biodegradable scaffolds for bone tissue engineering. Macromol. Res. 2006;14:565–572.
- Liaw DJ, Chen WH. High glass transitions of novel organosoluble polyamide-imides based on noncoplanar and rigid diimide-dicarboxylic acid. Polym. Degrad. Stab. 2006;91:1731–1739.
- Wang HM, Chou YT, Wu CS, Yeh JT. Polyester/cellulose acetate composites: preparation, characterization and biocompatible. J. Appl. Polym. Sci. 2012;126:E242–E251.
- Zhou XM. Synthesis and characterization of polyester copolymers based on poly(butylene succinate) and poly(ethylene glycol). Mater. Sci. Eng. C. Mater. Biol. Appl. 2012;32:2459–2463.
- Feng L, Hu J, Liu Z, Zhao F, Liu G. Preparation and properties of optically active poly(N-methacryloyl L-leucine methyl ester). Polymer. 2007;48:3616–3623.
- Amass W, Amass A, Tighe B. A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998;47:89–144.
- Mallakpour S, Dinari M. Progress in synthetic polymers based on natural amino acids. J. Macromol. Sci. Part A Pure Appl. Chem. 2011;48:644–679.
- Q Tu, Wang JC, Liu R, Zhang Y. Xu J, Liu J, Yuan MS, Liu W, Wang J. Synthesis of polyethylene glycol- and sulfobetaine-onjugated zwitterionic poly(L-lactide) and assay of its antifouling properties. Colloid Surf. B. 2013;102:331–340.
- McIntyre JE. Synthetic fibres: nylon, polyester, acrylic, polyolefin. Cambridge: CRC Press Woodhead; 2005.
- Yamashita H, Nakano Y. Polyester: properties, preparation and applications. New York, NY: Nova Science; 2008.
- J Scheirs, Long TE. Modern polyesters: chemistry and technology of polyesters and copolyesters. Wiley: Chichester; 2003.
- Higashi F, Tobe A. A new polycondensation involving dicarboxylic acids with differently activated carboxyl groups by TsCl/DMF/Py. Macromol. Chem. Phys. 2001;202:745–749.
- Audic JL, Fourcade F, Chaufer B. Thermodynamics, solubility and environmental issues. In: Letcher TM, editor. Biodegradable material obtained from renewable resource. the Netherlands: Elsevier Science; 2007. p. 369–382.
- Leca M, Segarceanu O, Serban S. Solubility, curing and heat resistance of some ester-imide oligomers as a function of the nature and content of some comonomers. Thermochim. Acta. 1997;299:153–160.
- Mallakpour S, Rafiee Z. Step-growth polymerization of 5-(3-acetoxynaphthoylamino)isophthalic acid with different aromatic diols. Iran. Polym. J. 2008;17:217–226.
- Van der Shuur M, Gaymans RJ. Segmented block copolymers based on poly(propylene oxide) and monodisperse polyamide-6, T segments. J. Polym. Sci., Part A: Polym. Chem. 2006;44:4769–4781.
- Raju MP, Alam S. Synthesis of polyimide nanocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2009;46:1136–1141.
- Chao M, Kou K, Wu G, Zhang J, Li N, Zhang D. Synthesis and properties of semicrystalline copolyimides based on 4,4′-diaminodiphenylether and 1,3-bis(4-aminophenoxy) benzene. J. Macromol. Sci. Part B Phys. 2012;51:2003–2014.
- Zhao L, Yao H, Liu Y, Zhang Y, Jiang Z. Synthesis and properties of novel hyperbranched polyimides end-capped with metallophthalocyanines. J. Appl. Polym. Sci. 2013;128:3405–3410.
- Hsiao SH, Kung YR. Synthesis and properties of new aromatic polyimides containing redox-active anthraquinonemoieties. Polym. Int. 2013;62:573–580.
- Mrsevic M, Düsselberg D, Staudt C. Synthesis and characterization of a novel carboxyl group containing (co)polyimide with sulfur in the polymer backbone. Beilstein J. Org. Chem. 2012;8:776–786.
- Yang HH. Aromatic high-strength fibers. New York, NY: Wiley; 1989.
- Ghosh MK, Mittal KL. Polyimides: fundamentals and applications. New York, NY: Marcel & Dekker; 1996.
- Shabbir S, Zulfiqar S, Ahmad Z, Sarwar MI. Synthesis and characterization of hyperbranched aromatic polyester-imides with goodthermalpropertiesbasedon1,3,5-tris(30,40-carboxyphenyl) benzene trianhydride. Polym. Degrad. Stab. 2010;95:1251–1259.
- Mallakpour S, Rafiee Z. Synthesis and characterization of poly(amide-ester)s containing naphthalene pendent groups and urazole rings. Des. Monomers Polym. 2008;11:283–296.
- Li H, Wang D, Fan L, Yang S. Preparation and characterization of a novel thermally stable unsaturated poly(ester-imide) based on 1,4-bis[2′-trifluoromethyl-4′-(4′-carboxylic acid)trimellitimido phenoxy]benzene. Eur. Polym. J. 2006;42:534–543.
- Behniafar H, Khaje Mirzai AA, Beit Saeed A. Synthesis and properties of new aromatic poly(ester-imide)s derived from 4-p-biphenyl-2,6-bis(4-trimellitimidophenyl) pyridine and various dihydroxy compounds. Polym. Int. 2007;56:74–81.
- Mallakpour S, Zeraatpisheh F. Construction and characterization of bionanocomposites based on optically active poly(ester-imide) containing L-amino acids using nano-ZnO surface-coupled by γ-methacryloxypropyl-trimethoxysilane. Des. Monomers Polym. 2011;14:487–498.
- Mallakpour SE, Hajipour AR, Shahmohammadi MH. Novel optically active poly(amide-imide)s from N-trimellitylimido-S-valine and aromatic diamines by direct polycondensation reaction. Iran. Polym. J. 2002;11:425–431.
- Mallakpour SE, Hajipour AR, Habibi S. Synthesis of novel poly(amide-imide)s containing trimellitylimido-DL/L-alanine moieties via direct polycondensation. J. Appl. Polym. Sci. 2001;80:1312–1318.
- Staubli A, Ron E, Langer R. Hydrolytically degradable amino acid containing polymers. J. Am. Chem. Soc. 1990;112:4419–4424.
- Mallakpour S, Hajipour AR, Shahmohammadi MH. Direct polycondensation of N-trimellitylimido-L-isoleucine with aromatic diamines. J. Appl. Polym. Sci. 2003;89:116–122.
- Van Krevelen DW, Hoftyzer PJ. Properties of polymers. 3rd ed. Amsterdam: Elsevier; 1976.