Abstract
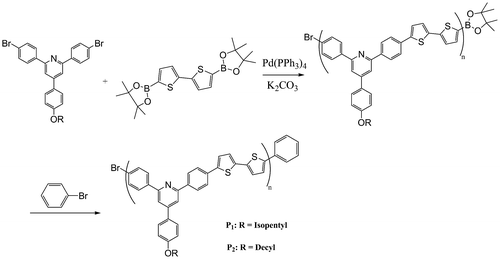
Two new copolymers, P1 and P2, composed of 4[4-(alkoxy)phenyl]-2, 6-bis(4-bromophenyl)pyridine and bithiophene units, have been synthesized via Suzuki cross-coupling reactions. 4[4-(alkoxy)phenyl]-2, 6-bis(4-bromophenyl)pyridines were synthesized starting from condensation reaction of 4-bromoacetophenone and 4-hydroxy benzaldehyde, and subsequent alkoxylation of hydroxyl groups. All of the polymers and intermediates were characterized using FTIR and NMR spectroscopies. The synthesized polymers exhibit good solubility in common organic solvents. The maximum absorption peak for P1 and P2 was 430 and 420 nm, respectively. The optical band gap energy of the polymers was determined by absorption onset and found to be 2.17 eV for P1 and 2.13 eV for P2.
1. Introduction
The research for conjugated polymers in organic electronic and optoelectronic applications with highly stable, highly conductive, and easily processable, has led to extensive studies over the past two decades.[Citation1,Citation2]
In this area, many efforts have been taken to construct and design new polymers with enhanced properties, such as incorporating flexible subtsituents, using electron donating and/or electron-withdrawing substituents, and hybridizing with different conjugated molecules, such as various types of phenylenes.[Citation3–Citation11]
One of the important parts of these researches is tuning HOMO and LUMO levels of polymers by changing the electron donating or withdrawing nature of side chain groups in polymer backbone. Electron donating groups, raising the HOMO levels of polymers and electron acceptors pulling down the LUMO, and therefore, both reduced the final band gap.[Citation12–Citation19] Another important factor of using a polymer in solar cell applications is its solubility in common organic solvents which make it possible to process easily.Citation[20]
The longer side chains caused better solubility of polymers; however, this will influence other phenomena like its conduction. The longer chain have lower conductance, because the electron transfer is less and caught effect will be more.[Citation21–Citation24] In polymers, if the backbone chains are close together, electrons and holes can be transferred from one another. So, long chains have both advantage and disadvantage and the length of these chains must be optimized.
Usually, in the design of new highly electron/hole conductive polymers, meta-linkages between conducting segments are generally excluded because they interrupt conjugation. These meta-linkages have usually been introduced in order to reduce conjugation in a tunable manner, for example, in synthesizing polymers with blue emission.[Citation25–Citation27]
In fact, it appears to be widely accepted that meta-linkages in polymers are something to be avoided if one wishes to produce systems with high conductivity that is generally associated with delocalized carriers. However, there are some reports about using meta-linkages, to produce highly conductive polymers.[Citation28–Citation31]
Polythiophene and its derivatives are the most widely studied conducting polymers.[Citation32,Citation33] Polymers, containing special functional groups like: chromophores, emitters, electrochromophores, ligands, etc. electronically coupled with the conjugated main chain are particularly interesting and important, because of the mutual interactions of the functional group and the conducting chain.[Citation34,Citation35]
Due to high electron affinity, pyridine-containing conjugated polymers have been shown to be promising candidates for light-emitting device (LED).[Citation36,Citation37] The homopolymer of pyridine (Ppy) act as blue emitting material and the other pyridine-containing copolymers have been shown to be highly luminescent.[Citation38–Citation41] The reactivity of pyridine moiety for N-protonation, N-oxidation, and quaternization with RX can also modify the optical and electrical properties.Citation[42] Also in comparison to phenylene-based polymers, they are more resistant to oxidation and show better electron-transporting properties due to higher electron affinity. The capability of using low work function metals, like Al or ITO, makes the pyridine-containing polymers the excellent candidates for polymer LEDs.
Synthesis of polymers containing both thiophene and pyridine rings could be done via some strategies. One type is making a desired thiophene-pyridine-containing monomer via coupling reactions Citation[43] and then its polymerization. The other route is making thiophene-pyridine-containing monomer via modified chichibabin pyridine synthesis [Citation44–Citation46] and then its polymerization via coupling.
In continuation of our works on synthesis of pyridine-containing monomers via modified chichibabin synthesis and related polymers,[Citation47–Citation49] now we used a third route for synthesizing two new copolymers composed of thiophene and pyridine groups via coupling between a synthesized pyridine-containing dibromo compounds and bithiophene with diboronic ester substituents via Suzuki coupling.
All of the compounds and intermediates were characterized using FTIR, 1H NMR, and CHN elemental analysis. The properties of synthesized polymers like thermal stability, molecular weight, HOMO, and LUMO levels were investigated.
2 Experimental
2.1 Materials
p-Bromoacetophenone, p-hydroxybenzaldehyde, and tetrakis (triphenyl phosphine)Pd(0) were obtained from Merck Chemicals. 2, 2′-Bithiophene-5, 5′-dibronic acid bis (pinacol) ester was purchased from Aldrich Chemicals. The other additives and solvents were obtained from Merck Chemicals. Solvents should be degassed using nitrogen atmosphere before use.
2.2 Instrumentation
1H NMR spectra were recorded on a Bruker Avance 400 spectrometer. Molecular weight was obtained using a Knauer gel permeation chromatography (GPC) equipped with Smartine Pump 1000, a differential refractive index detector (Smartin Ri 2300), and PL Gel 10 μm column. THF was used as solvent with flow rate of 1 ml/min at room temperature. GPC was calibrated with standard polystyrene with polydispersity index of 1.09. FTIR spectra were recorded on a JASCO FTIR-6300 spectrometer using KBr pellets. Thermo gravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed on a Mettler TG5 system under nitrogen atmosphere at a heating rate of 10 °C/min. Ultraviolet–visible (UV/Vis) absorption spectra were recorded on a JASCO V-670 spectrophotometer. The electrochemical measurements of the copolymer was carried out on a potentiostat/galvanostat Autolab PGSTAT30 with a platinum electrode at a scan rate of 50 mV/s against Ag/AgCl reference electrode with nitrogen-saturated solution of 0.1 M tetra-butyl-ammonium hexafluorophosphate in acetonitrile. The polymers and TBAPE were dissolved in DMF. The elemental analysis was carried out by a Leco, CHNS-932.
2.3 Monomer synthesis
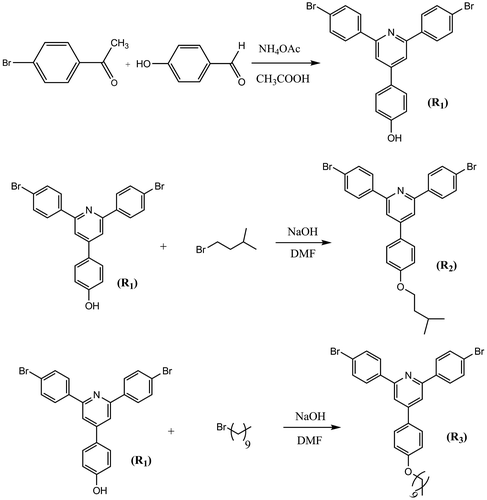
2.3.1 Synthesis of 4(4-hydroxyphenyl)-2, 6-bis (4-bromophenyl) pyridine (R1)
In a round-bottomed 100 mL flask, a solution of p-bromo acetophenone (11.94 g, 60 mmol), p-hydroxy benzaldehyde (3.66 g, 30 mmol), and ammonium acetate (40 g) in acetic acid (80 mL) was prepared. The mixture was heated to reflux for 4 h and cooled to room temperature, and a yellow product was isolated by filtration and washed with methanol followed by recrystallization from DMF. The solid was dried in vacuum at 130 °C for 24 h. A light yellow powder (R1) was obtained.
Yield: 70%; mp: 283 °C
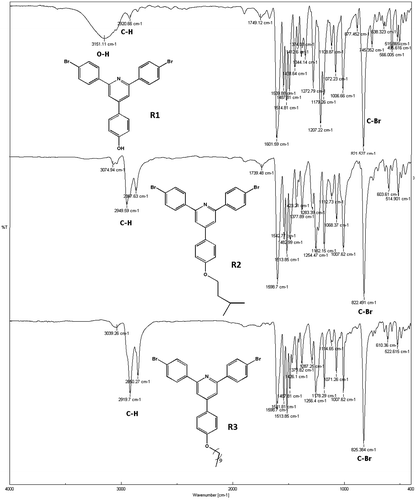
FTIR (KBr, cm−1): 3151, 1601, 1514, 1487, 1207, 1072, 821
CHNS (C23H15Br2NO): C 56.92% (57.41%, calculated), H, 3.62% (3.14%), N, 3.00% (2.91%).
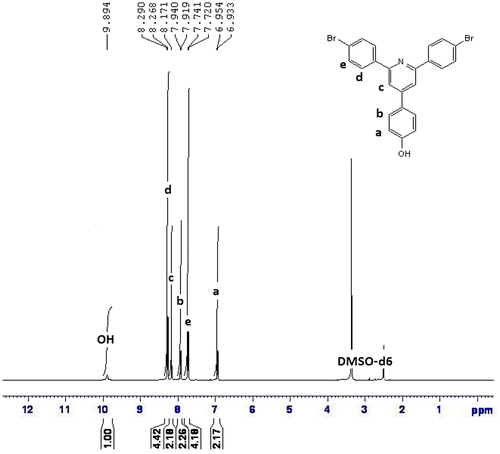
1H NMR (DMSO-d6, ppm): 9.89 (OH, 1H), 8.29–8.26 (aromatic, 4H), 8.17 (aromatic, 2H), 7.94–7.91 (aromatic, 2H), 7.74–7.72 (aromatic, 4H), 6.95–6.93 (aromatic, 2H).
2.3.2 Synthesis of 4[4-(isopentyloxy) phenyl] 2, 6-bis (4-bromophenyl) pyridine (R2)
In a 50 mL one-neck round-bottom flask, a mixture of R1 (2.2 g, 4.56 mmol) and NaOH (0.14 g, 3.51 mmol) in DMF (20 mL) was prepared. The mixture was heated to reflux for 4 h and then 1-boromo-3-methyl butane (0.441 mL, 3.51 mmol) was added and continued for 2 h. Finally, the mixture was cooled and methanol was added to precipitate the product. The mixture was filtered and washed with methanol. A brown product was obtained after recrystallization from chloroform. The solid was dried in vacuum at 40 °C for 24 h.
Yield: 60%; mp: 123 °C
FTIR (KBr, cm−1): 2949, 1598, 1513, 1254, 822
CHN (C28H25Br2NO): C, 61.00% (61.00%, calculated), H, 4.84% (4.57%), N, 2.65% (2.54%)
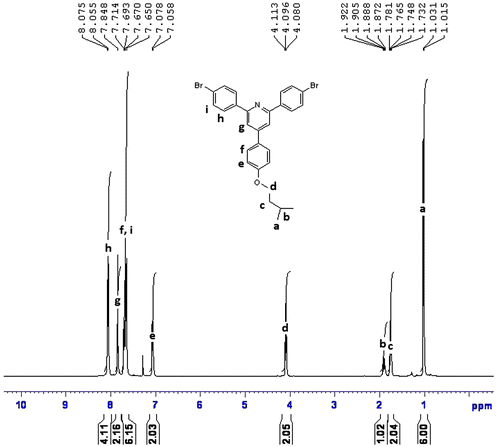
1H NMR (CDCl3, ppm): 8.07–8.05 (aromatic, 4H), 7.84–7.71 (aromatic, 2H), 7.69–7.65 (aromatic, 6H), 7.07–7.05 (aromatic, 2H), 4.11–4.08 (–OCH2–, 2H), 1.92–1.87 (–CH–, 1H), 1.76–1.73 (–CH2–, 2H), 1.03–1.01 (CH3, 6H).
2.3.3 Synthesis of 4[4-(decyloxy) phenyl] 2, 6-bis (4-bromophenyl) pyridine (R3)
This was synthesized according to the same procedure used for synthesis of R2. Only 1-boromodecane was used instead of 1-boromo-3-methyl butane.
Yield: 56%; mp: 77 °C
FTIR (KBr, cm−1): 2919, 1598, 1513, 1256, 1007, 825
CHN (C33H35Br2NO): C, 63.14% (63.78%, calculated), H, 5.85% (5.68%), N, 2.28% (2.25%)
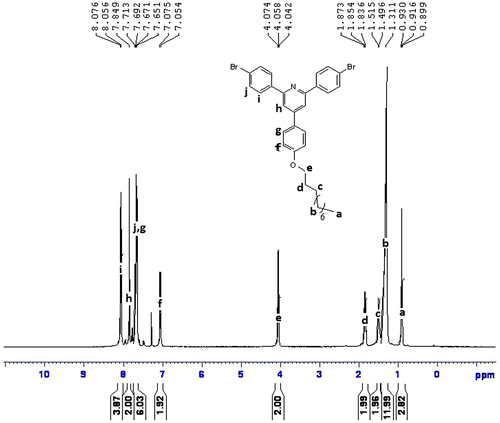
1H NMR (CDCl3, ppm): 8.07–8.05 (aromatic, 4H), 7.84–7.71 (aromatic, 2H), 7.69–7.65 (aromatic, 6H), 7.07–7.05 (aromatic, 2H), 4.07–4.04 (–OCH2–, 2H), 1.87–1.31 (–CH2–, 16H), 0.93–0.89 (–CH3, 3H).
2.4 Polymer synthesis
2.4.1 Polymer from reaction of 4[4-(isopentyloxy)phenyl] 2,6-bis(4-bromophenyl)pyridine with 2,2′-bithiophene-5,5′-dibronic acid bis(pinacol)ester (P1)
In a 100 mL two-neck round-bottom flask, a mixture of R2 (0.137 g, 0.25 mmol), 2,2′-bithiophene-5,5′-dibronic acid bis(pinacol)ester (0.105 g, 0.25 mmol), and tetrakis(triphenyl phosphine)pd(0) (0.003 g) in THF (20 mL) was prepared. The mixture was heated to reflux, and after 20 min, an aqueous solution of K2CO3 (4 M, 3 mL) was added under nitrogen. The mixture was refluxed for 36 h and then bromo benzene was added for end capping. The reaction was allowed to reflux for 24 h and then methanol was added to mixture. The precipitate was filtered and washed with deionized water and methanol. The dark green polymer was obtained after drying in vacuum at 70 °C.
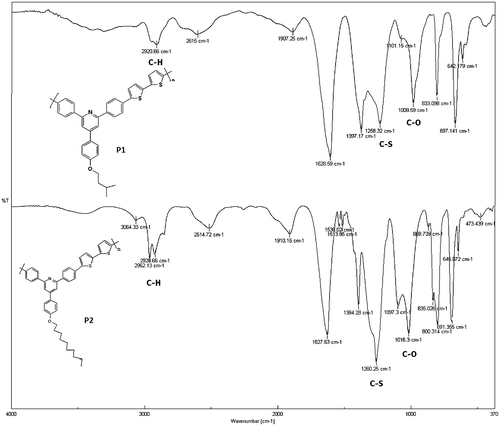
FTIR (KBr, cm−1): 2920, 1628, 1397, 1258, 1008, 833, 697
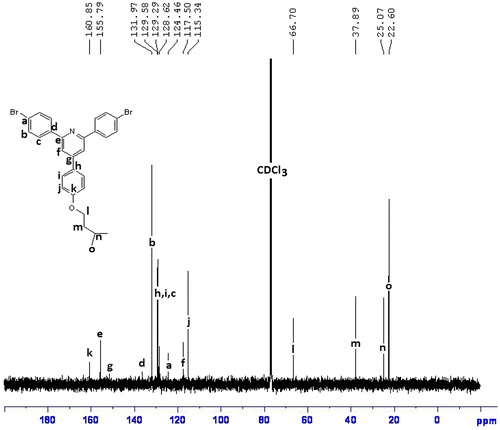
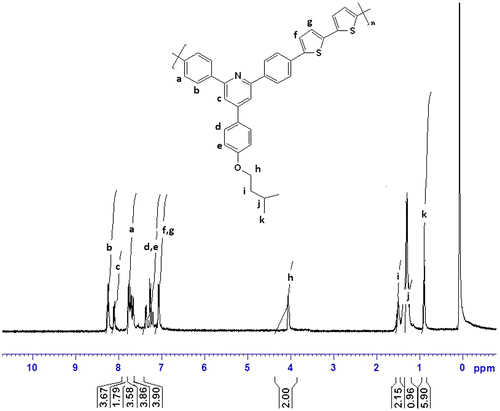
1H NMR (CDCl3, ppm): 8.25–7.11 (aromatic, 18H), 4.03 (–OCH2–, 2H), 1.51–0.93 (aliphatic, 9H)
GPC: Mw = 28,010 g/mol, Mn = 23,130 g/mol, PDI = 1.21
Tg (DSC) = 190 °C
2.4.2 Polymer from reaction of 4[4-(decyloxy) phenyl] 2, 6-bis (4-bromophenyl) pyridine with 2, 2′-bithiophene-5, 5′-dibronic acid bis (pinacol) ester (P2)
This polymer was synthesized according to method reported for synthesis of P1. Only R3 was used instead of R2 in this section.
FTIR (KBr, cm−1): 2962, 1627, 1260, 1016, 800, 691
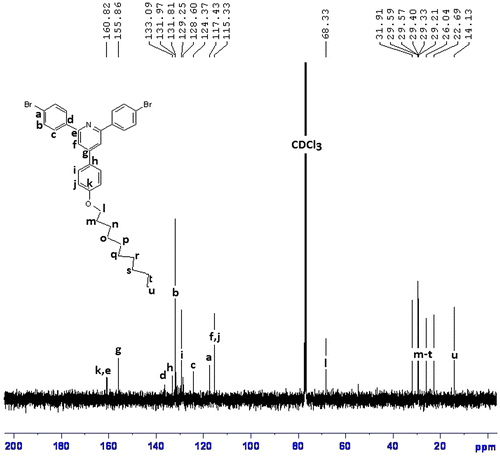
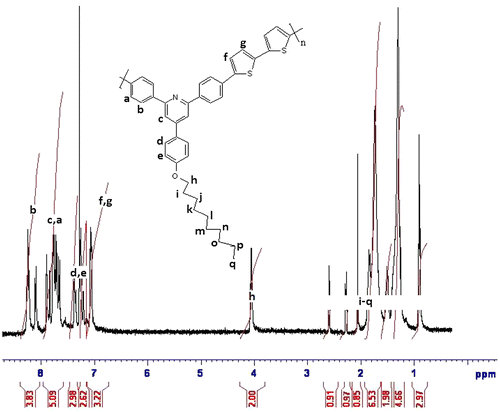
1H NMR (CDCl3, ppm): 8.26–7.05 (aromatic, 18H), 4.07 (–OCH2, 2H), 2.59–0.89 (aliphatic, 19H).
Tg (DSC) = 160 °C
3 Result and discussion
3.1 Monomers and polymers synthesis
First, a pyridine-containing dibromo compound, 4(4-hydroxyphenyl)-2, 6-bis (4-bromophenyl) pyridine, (R1), was synthesized based on reaction of 4-bromo acetophenone and 4-hydroxy benzaldehyde according to a modified chichibabin reaction. Compound R1 was then converted to R2 and R3 by alkoxylation of its hydroxyl group with isopentyloxy and decyloxy groups by reaction with 1-boromo-3-methyl butane and 1-boromodecane, respectively (Scheme ).
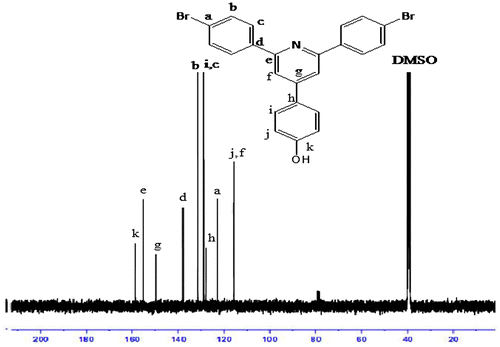
FTIR spectra of synthesized compound, R1, shows peak at 3151 cm−1 for hydroxyl group, and 821 cm−1 for C–Br. The compound did not show carbonyl peak and this is the difference with starting materials. The compound R1 was then converted to R2 and R3 by alkoxylation of its hydroxy group and its peak at 3151 for hydroxy group disappeared (Figure ). The 1H NMR and 13C NMR spectra of synthesized compounds also confirmed their synthesis (Figures ). The synthesized compounds, R1–R3 were also confirmed by CHN elemental analysis (experimental part).
Two polymers, P1 and P2 were synthesized by reaction of R1 and R2 with 2,2′-bithiophene-5,5′-dibronic acid bis(pinacol)ester according to Suzuki coupling in the presence of tetrakis (triphenyl phosphine)pd(0) as catalyst (Scheme ). FTIR spectra of both polymers showed aliphatic C–H, at about 2950 cm−1, a broad peak at about 1627 cm−1 for aromatic rings, a peak of about 1260 cm−1 for C–S, and at about 1010 cm−1 for C–O (Figure ). The structures of these polymers were also characterized using 1H NMR spectroscopy and the results confirmed the synthesis of polymers (Figures).
The number average molecular weight (Mn) and weight average molecular weight (Mw) of these polymers were investigated using GPC and the results showed good chain growth for synthesized polymers (Table ). Because of shielding effect to both solvents of DMF and THF, the results of molecular weight for P2 could not be obtained.
Table 1. Molecular weight of synthesized polymers.
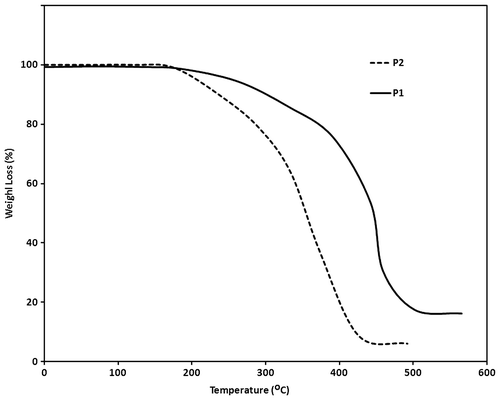
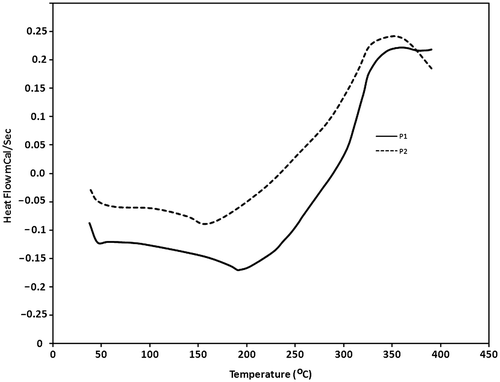
As an important factor, thermal stability of these polymers was studied using TGA. The polymers start to lose weight because of thermal degradation at around 200 °C. These weight losses are because of pendant alkoxy groups in polymer chains. For P2 with larger alkoxy groups, the weight loss is more than P1 and also the percent of remaining polymer at 500 °C is less (Figure ). The temperatures of 5% weight loss of these polymers were 260 and 230 °C, respectively, for P1 and P2. The thermal behavior of these polymers was investigated using DSC. The glass transition temperature (Tg) of these polymers were obtained and it was 190 °C for P1 and 160 °C for P2 (Figure ).
3.2 Photophysical properties
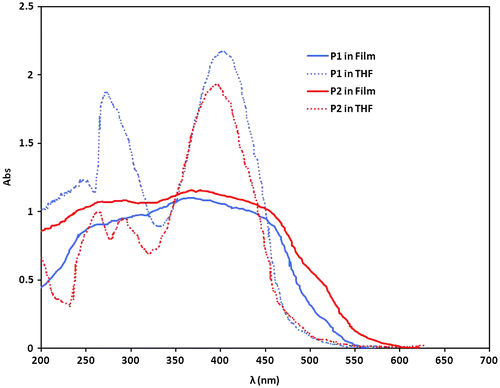
These two synthesized polymers have different properties. The ones with longer side chain (P2) are more electron donating than ones with smaller side chain (P1). So, the polymer P2 has lower band gap. The other effect is mobility of electrons on the polymers and here P1 is better than P2, because chains of P1 are nearer altogether and they can eliminate static charges. To calculate the amount of energy gap of the synthesized compounds, UV–vis spectroscopic method is used. The absorption spectra of the P1 and P2 in THF solution and solid state are shown in Figure . Based on Einstein formula, E = hc/λ, a conductive polymer, absorbs a photon when this photon energy is equal to electron transfer between orbitals. If this energy is less than band gap, the electron could not transfer from HOMO to LUMO. The λonset in UV–vis spectroscopy is an important factor for determining the band gap of compounds. Based on these data of λonset obtained in UV–vis spectra, and using the Einstein formula, the band gaps were measured and the results are summarized in Table .
Table 2. Optical and electrochemical properties of synthesized compounds.
3.3 Electrochemical properties
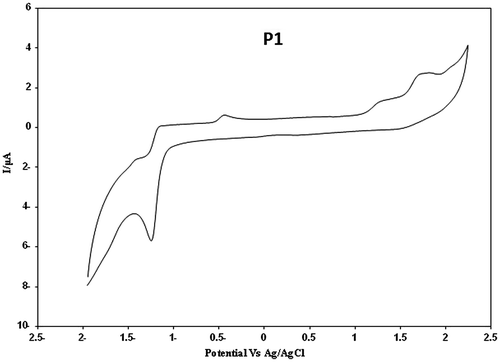
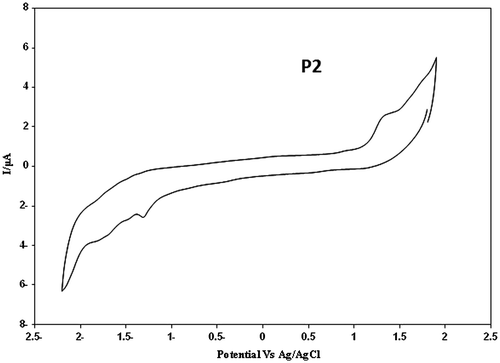
The band gap, HOMO, and LUMO energy levels were estimated from the cyclic voltammetry (CV).
The cyclic voltammogram of polymers are shown in Figures. From the values of the onset oxidation potential (Eox, onset) and onset reduction potential (Ered, onset) of the compounds, the HOMO and LUMO energy levels as well as the electrochemical band gaps (Eg,EC) were calculated. In oxidation potential (Eox, onset), which occurred in positive potentials, polymer looses the electron which is related to HOMO and in reduction potentials (Ered, onset), the polymer obtains the electron related to LUMO. Therefore based on these data obtained in CV, using Figures, the band gap of the polymers were measured and the results are summarized in Table .
The HOMO and LUMO were calculated by these Equations Citation[50]:Here, E1/2, ferrocene = 0.488 eV.
The optical band gap calculated from UV–vis spectroscopy is smaller than the electrochemical band gap, which might be due to the interface barrier of charge injection.
4. Conclusion
In this research, two new polymers, P1 and P2, based on pyridine and thiophene derivatives were introduced. Based on our results, these polymers have low band gap and good solubility in organic solvents, which make them promising for use in active layers in solar cell applications.
Acknowledgments
The authors thank University of Isfahan providing facilities for this work and Central Laboratory of University of Isfahan for FTIR, CHN, NMR, and GPC analysis.
References
- Bredas JL, Silbey R, editors. Conjugated polymers. Dordrecht: Kluwer Academic Publishers; 1991.
- Farchioni R, Grosso G, editors. Organic electronic materials. New York (NY): Springer; 2001.
- Sato M, Tanaka S, Kaeriyama K. Soluble conducting polythiophenes. J. Chem. Soc., Chem. Commun. 1986:873–874. doi:10.1039/c39860000873.
- Jen KY, Miller GC, Elsenbaumer RL. Highly conducting, soluble, and environmentally-stable poly (3-alkylthiophenes). J. Chem. Soc., Chem. Commun. 1986; 1346–1347. doi:10.1039/c39860001346.
- Roncali J. Conjugated poly (thiophenes): synthesis, functionalization, and applications. Chem. Rev. 1992;92:711–738. doi:10.1021/cr00012a009.
- Zotti G, Gallazzi MC, Zerbi G, Meille SV. Conducting polymers from anodic coupling of some regiochemically defined dialkoxy-substituted thiophene oligomers. Synth. Met. 1995;73:217–225. doi:10.1016/0379-6779(95)80019-0.
- Child AD, Sankaran B, Larmat F, Reynolds JR. Charge-carrier evolution in electrically conducting substituted polymers containing biheterocycle/p-phenylene repeat units. Macromolecules. 1995;28:6571–6578. doi:10.1021/ma00123a026.
- Bouachrine M, Bouzakraoui S, Hamidi M, Ayachi S, Alimi K, Lere-Porte J-P, Moreau J. Synthesis and characterization of co-polymers involving various thiophene and phenylene monomers. Synth. Met. 2004;145:237–243. doi:10.1016/j.synthmet.2004.05.008.
- Chen JW, Cao Y. Development of novel conjugated donor polymers for high-efficiency bulk-heterojunction photovoltaic devices. Acc. Chem. Res. 2009;42:1709–1718. doi:10.1021/ar900061z.
- Cheng YJ, Yang SH, Hsu CS. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009;109:5868–5923. doi:10.1021/cr900182s.
- Roncali J. Molecular engineering of the band gap of π-conjugated systems: facing technological applications. Macromol. Rapid Commun. 2007;28:1761–1775. doi:10.1002/marc.200700345.
- Gadisa A, Mammo W, Andersson LM, Admassie S, Zhang F, Andersson MR, Inganas O. A new donor–acceptor–donor polyfluorene copolymer with balanced electron and hole mobility. Adv. Funct. Mater. 2007;17:3836–3842. doi:10.1002/adfm.200700441.
- Thompson BC, Frechet MJ. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008;47:58–77.
- Wienk MM, Struijk MP, Janssen RA. Low band gap polymer bulk heterojunction solar cells. Chem. Phys. Lett. 2006;422:488–491. doi:10.1016/j.cplett.2006.03.027.
- Handa S, Giacalone F, Haque SA, Palomares E, Martin N, Durrant JR. Solid film versus solution-phase charge-recombination dynamics of exTTF–bridge–C60 dyads. Chem. Eur. J. 2005;11:7440–7744. doi:10.1002/chem.200401312.
- Yoshihara T, Druzhinin SI, Zachariasse KA. Fast intramolecular charge transfer with a planar rigidized electron donor/acceptor molecule. J. Am. Chem. Soc. 2004;126:8535–8539. doi:10.1021/ja049809s.
- Kirk ML, Shultz DA, Depperman EC, Brannen CL. Donor−Acceptor biradicals as ground state analogues of photoinduced charge separated states. J. Am. Chem. Soc. 2007;129:1937–1943. doi:10.1021/ja065384t.
- Ramos AM, Meskers SCJ, Beckers EHA, Prince RB, Brunsveld L, Janssen RAJ. Supramolecular control over donor-acceptor photoinduced charge separation. J. Am. Chem. Soc. 2004;126:9630–9644.
- Peng Q, Park K, Lin T, Durstock M, Dai L. Donor-π-Acceptor conjugated copolymers for photovoltaic applications: tuning the open-circuit voltage by adjusting the donor/acceptor ratio. J. Phys. Chem. B. 2008;112:2801–2808. doi:10.1021/jp7105428.
- Krebs FC, Tromholt T, Jørgensen M. Upscaling of polymer solar cell fabrication using full roll-to-roll processing. Nanoscale. 2010;2:873–886. doi:10.1039/b9nr00430k.
- Zoombelt AP, Leenen MAM, Fonrodona M, Nicolas Y, Wienk MM, Janssen RAJ. The influence of side chains on solubility and photovoltaic performance of dithiophene-thienopyrazine small band gap copolymers. Polymer. 2009;50:4564–4570. doi:10.1016/j.polymer.2009.07.028.
- Piliego C, Holcombe TW, Douglas JD, Woo CH, Beaujuge PM, Frechet JMJ. Synthetic control of structural order in N-Alkylthieno[3,4-c]pyrrole-4,6-dione-based polymers for efficient solar cells. J. Am. Chem. Soc. 2010;132:7595–7596. doi:10.1021/ja103275u.
- Chen MH, Hou J, Hong Z, Yang G, Sista S, Chen LM, Yang Y. Efficient polymer solar cells with thin active layers based on alternating polyfluorene copolymer/fullerene bulk heterojunctions. Adv. Mater. 2009;21:4238–4242. doi:10.1002/adma.200900510.
- Zhang M, Sun Y, Guo X, Cui C, He Y, Li Y. Synthesis and characterization of dioctyloxybenzo[1,2-b:4,3-b′]dithiophene-containing copolymers for polymer solar cells. Macromolecules. 2011;44:7625–7631. doi:10.1021/ma201565f.
- Reddinger JL, Reynolds JR. Ultraviolet-emitting, alkoxy-functionalized poly (m-phenylene). Macromolecules. 1997;30:479–481. doi:10.1021/ma961235r.
- Liao L, Pang Y. Blue-emitting soluble poly (m-phenylenevinylene) derivatives. Macromolecules. 2001;34:7300–7305. doi:10.1021/ma0108923.
- Hong SY, Kim DY, Kim CY, Hoffmann R. Origin of the broken conjugation in m-phenylene linked conjugated polymers. Macromolecules. 2001;34:6474–6481. doi:10.1021/ma010254k.
- Tour JM. Soluble oligo- and polyphenylenes. Adv. Mater. 1994;6:190–197. doi:10.1002/adma.19940060303.
- Kang BS, Seo M-L, Jun YS, Lee CK, Shin SC. Wavelength tuning of light-emitting polyarenes via an m-phenylene interrupting block: π–π∗ band-gap adjustment of thiophene-based conjugated polymers. Chem. Commun. 1996;1167–1168. doi:10.1039/cc9960001167.
- Kang BS, Kim DH, Lim SM, Kim J, Seo ML, Bark KM, Shin SC. Thiophene-based π-conjugated emitting polymers: synthesis and photophysical properties of poly[2-(dodecyloxy)-5-methyl-m-phenyleneethynylene] and poly[2-(dodecyloxy)-5-methyl-m-bis(ethynyl)phenyleneoligo-thienylene]s. Macromolecules. 1997;30:7196–7201. doi:10.1021/ma9709462.
- Song C, Swager TM. Highly conductive poly (phenylene thienylene) s: m-phenylene linkages are not always bad. Macromolecules. 2005;38:4569–4576. doi:10.1021/ma0501702.
- Chan HSO, Ng SC. Synthesis, characterization and applications of thiophene-based functional polymers. Prog. Polym. Sci. 1998;23:1167–1231. doi:10.1016/S0079-6700(97)00032.
- Wang HJ, Tzeng JY, Chou CW, Huang CY, Lee RH, Jeng RJ. Novel polythiophene derivatives functionalized with conjugated side-chain pendants comprising triphenylamine/carbazole moieties for photovoltaic cell applications. Polym. Chem. 2013;4:506–519. doi:10.1039/c2py20477k.
- Roncali J. Chapter 3. Linear π-conjugated systems with tailored electronic properties, Annual Reports, Progress in Chemistry Section C. Phys. Chem. 1999;95:47–88. doi:10.1039/pc095047.
- Perepichka IF, Perepichka DF, Meng H, Wudl F. Light-emitting polythiophenes. Adv. Mater. 2005;17:2281–2305. doi:10.1002/adma.200500461.
- Gebler DD, Wang YZ, Blatchford JW, Jessen SW, Lin LB, Gustagson TL, Wang HL, Swager TM, MacDiarmid AG, Epstein AJ. Blue electroluminescent devices based on soluble poly(p-pyridine). J. Appl. Phys. 1995;78:4264–4267. doi:10.1063/1.359890.
- Wang YZ, Gebler DD, Lin LB, Blatchford JW, Jessen SW, Wang HL, et al. Alternating-current light-emitting devices based on conjugated polymers. Appl. Phys. Lett. 1996;68:894–897. doi:10.1063/1.116222.
- Kanbara T, Kushida T, Saito N, Kuwajima I, Kubota K, Yamamoto T. Preparation and properties of highly electron-accepting poly(pyrimidine-2,5-diyl). Chem. Lett. 1992;21:583–586. doi:10.1246/cl.1992.583.
- Wang YZ, Gebler DD, Fu DK, Swager TM, MacDiarmid AG, Epstein AJ. Light-emitting devices based on pyridine-containing conjugated polymers. Synth. Met. 1997;85:1179–1182. doi:10.1016/S0379-6779(97)80201-9.
- Liaw DJ, Wang KL, Pujari SP, Huang YC, Tao BC, Chen MH, Lee KR, Lai JY. A novel, conjugated polymer containing fluorene, pyridine and unsymmetric carbazole moieties: synthesis, protonation and electrochemical properties. Dyes Pigm. 2009;82:109–117. doi:10.1016/j.dyepig.2008.12.004.
- Yang CM, Lee IW, Chen TL, Chien WL, Hong JL. Enhanced emission of organic and polymeric luminogens containing 2,4,6-triphenylpyridine moieties: crystallization and aggregation-enhanced emission. J. Mater. Chem. C. 2013;1:2842–2850. doi:10.1039/c3tc00851g.
- Yamamoto T, Lee BL, Hayashi H, Saito N, Maruyama T. N-Oxides and N-ylides of π-conjugated poly(arylene)s. Preparation and properties of the polymers. Polymer. 1997;38:4233–4239. doi:10.1016/S0032-3861(96)00988-3.
- Krompiec M, Krompiec S, Ignasiak H, Łapkowski M, Kus P, Stanek L, Penczek R, Lis S, Staninski K, Sajewicz M, Gebarowska K. Synthesis and electropolymerization of 3,5-dithienylpyridines, their complexes and N-methylpyridinium cations. Synth. Met. 2008;158:831–838. doi:10.1016/j.synthmet.2008.05.010.
- Jiang HJ, Zhang JL, Sun J, Huang W. Novel amphipathic photoluminescent copolymers containing fluorene, pyridine and thiophene moieties: synthesis, characterization and self-assembly. Polymer. 2012;53:5684–5690. doi:10.1016/j.polymer.2012.10.007.
- Liu B, Bao Y, Du F, Wang H, Tian J, Bai R. Synthesis and characterization of a fluorescent polymer containing 2,6-bis(2-thienyl)pyridine moieties as a highly efficient sensor for Pd2+ detection. Chem. Commun. 2011;47:1731–1733. doi:10.1039/c0cc03819a/.
- Liu B, Dai H, Bao Y, Du F, Tian J, Bai R. 2,6-Substituted pyridine derivative-containing conjugated polymers: synthesis, photoluminescence and ion-sensing properties. Polym. Chem. 2011;2:1699–1705. doi:10.1039/c1py00149c.
- Koohmareh GA, Mohammadifard N. Synthesis and characterization of some new thermally stable polyimides and copolyimides bearing bipyridine side-chain groups. J. Polym. Res. 2011;18:983–991. doi:10.1007/s10965-010-9498-x.
- Koohmareh GA. New organo-soluble polyimides based on a new dianhydride: 4-(4-t-butyl-phenyl)-2,6-bis(3,4-phenyl dicarboxylic acid anhydride)pyridine. Des. Monomers Polym. 2007;10:517–525. doi:10.1163/156855507782401169.
- Koohmareh GA. Souri.: synthesis and characterization of some new thermally stable crystalline polyamides and co-polyamides bearing bipyridine side-chain groups. Des. Monomers Polym. 2011;14:475–485. doi:10.1163/138577211X587672.
- Feng LJ, Hsu SLC, Lee PI, Yi CH, Lun YM, Sue CJ, Yang CW. Reduced adverse effects on Si thin film solar cells caused by growth chamber air exposure. Sol. Energy Mater. Sol. Cells. 2010;94:1166–1172. doi:10.1016/j.solmat.2010.03.009.