Abstract
Interfacial properties of single-walled carbon nanotubes (SWCNTs) were modified by covalent surface modification of the nanotubes with an ATRP initiator which is able to polymerize acrylic monomers. This methodology starts with the incorporation of 2-phenyl-1-ethanol through a diazotization reaction. Further modification drives to an ATRP initiator, (4-(2-bromo-2-methylpropanoate of 1-hydroxyethyl) phenylidene) which is covalently linked to the surface of the SWCNT. This α-haloester linked to the surface of the SWCNT can be analysed, quantified and used as an initiator in the ATRP polymerization methodology without the addition of the ‘sacrificial initiator’. Well control of the molecular weights of the acrylic polymers grown on the surface of SWCNT was obtained. Furthermore, the polymer characterization by FT-IR, NMR CP-MAS, thermo gravimetric analysis and Transmission Microscopy as well as all the intermediates are reported.
Introduction
Since the discovery of carbon nanotubes (CNTs) in 1991 by Iijima [Citation1] a lot of scientific studies have been developed to understand and characterize their properties and to identify new applications in several fields such as the development of medical, biological, mechanical and electronic devices.[Citation2,Citation3] CNTs are formed by a hexagonal network containing only sp2 carbons, getting the shape of a hollow cylinder integrated by pentagons or hexagons in order to maintain the minimal steric energy generating tough and stable materials since sp2 orbital provide nanotubes with higher strength. CNT are considered as cylinders formed by rolled sheets of graphene and they can be classified according to the number of layers. A single rolled sheet drives to single-walled carbon nanotubes (SWCNTs), while more than one layer corresponds to multi-walled Carbon Nanotube (MWCNT). According to their shapes and number of layers, their electrical and mechanical properties can be predicted. However, both SWCNT and MWCNT have a great disadvantage, they are hydrophobic and do not show wetting properties in protic solvents. They also exhibit poor solubility in most organic solvents, and for that reason they cannot be fully integrated into a wide variety of organic materials such as polymers, in which there exists a lack of interfacial interaction within the surface of the CNT. In order to improve their manipulation in organic polymers matrix, the scientific attention focuses on using the following strategies: (a) surface modification (not covalent functionalization) and (b) chemical functionalization of the CNT (covalent functionalization). By following this concept, different chemical groups can be linked covalently to the surface of CNT. However, after the chemical modification one must be aware that the electrical and mechanical properties of the CNT are irreversibly affected, since the sp2 character of the carbon atoms, from the network of the sidewall have been modified. In general, in most of the scientific work reported in literature, the magnitude of the covalent modification is less than 5% which is mainly due to the type of the CNT and to the chemical reaction used; this fact conduces to a lack of spectroscopic characterization and poor evaluation of the modified surface. Even more when the covalent modification on the surface is an ATRP initiator and due to this poor modification, there is a tendency to use a free initiator named ‘sacrificial initiator’ during the polymerization process.[Citation4–Citation6] Here in this work, we develop a method for the sidewall modification of SWCNT based on the diazonium reaction where the aryl diazonium species are formed in situ by reaction of 2-(4- aminophenyl)ethanol and isopentylnitrite (or sodium nitrite) with the SWNTs in organic solvents. Diazonium salts are known to be reactive with different surfaces of carbon;[Citation7,Citation8] in particular, the reaction of CNTs with aryl diazonium salts are mainly focused on reactions electrochemically induced reactions.[Citation9,Citation10] In situ generation of the diazonium species is a method that avoids the insolation and storage of the unstable and light-sensitive aryl diazonium salts. By applying this procedure, an organic molecule is covalently linked to the surface of the SWCNT which was further modified in order to get an ATRP initiator. Using this compound, a polymer chain was grown on the surface of the SWCNT by the use of copper catalysis through a novel method involving the generation of active species in situ by the reduction of the Cu(II) ion to Cu(I). Since the surface of the SWCNT was highly modified, all the intermediates and final products were successfully characterized by thermo gravimetric analysis (TGA), FT-IR, solid NMR and transmission electron microscopy (TEM).
Experimental
Materials
The SWCNT used in this study were purchased from Bucky USA (9402 Alberene Dr Houston, TX 77074). The diameter of these nanotubes is 5 nm, the length is about 10–30 μm, and they were used without any previous purification. Reagents such as 2-bromo-2-methylpropanoyl bromide (98%), isopentylnitrite [Citation11] (96%), 2-(4-aminophenyl)ethanol (98%), 1,1,4,7,10,10-Hexamethylentrietilentetramine (97%) and 1,2-dichlorobenzene (99%) were obtained from Aldrich.
Characterization
Analytical equipment: FT-IR Nicolet Magna 550, with an optical range between 30 and 7000 cm−1 and wavenumbers with resolution of 0.5 cm−1. A micro attenuated total reflectance device (micro-ATR) has been attached to this equipment. The FT-IR spectra were obtained from a sample of 3 mg and placed directly into the micro-ATR cell. Spectra were collected after 100 scans with resolution of 4 cm−1 between 400 and 4000 cm−1. For the TGA a TG-Q500 from TA instruments, the equipment was used with a heating rate of 10 °C/min between 0–800 °C under nitrogen (before 600 °C) and oxygen (after 600 °C) atmosphere. The cross polarization-magic angle spinning (CP-MAS) studies were carried out in a 500 MHz NMR Bruker Avance III equipped with a solid probe working with the magic angle at 12 kHz. The morphology studies were carried out in a TITAN 300 kV field emission gun microscope, which has a symmetrical condenser objective lens type S-Twin (Cs = 1.3 mm).
Surface modification of SWCNT with diazonium salt
The functionalization of SWCNT was carried out by in situ generation of diazonium salts. For this experiment 0.667 g of SWCNTs were sonicated for 30 min in 20 mL of 1,2-dichlorobenzene. Then, 4.76 g of 2-(4-aminophenyl) ethanol (0.03 mol) dissolved in 20 mL of acetonitrile were added to this suspension. The solution was kept in inert atmosphere for 10 min and then 3.2 g of isopentylnitrite (0.03 mol) was added. The reaction mixture was stirred at 60 °C for 24 h, releasing the pressure every 30 min during the first 5 h of reaction, since nitrogen gas was formed as a side product. After the reaction time was reached, the mixture was dissolved in 50 mL of DMF and filtered over a Nylon (0.2 μm) membrane, and washed extensively with DMF until the filtrate became colourless. The black product was dried in a vacuum (2 mm of Hg) at 60 °C for 24 h and 0.917 g was recovered. As a result of this experiment an alcohol group was attached over the surface of the SWCNT and the product obtained was named as SWCNT-OH. This product was characterized by TGA, IR and Scanning Electron Microscopy.
Preparation of SWCNT-ATRP initiator
In a 100 mL flask, 0.5 g of SWCNT-OH and 10 mL of chloroform were added and sonicated for 10 min. To this suspension, 1.57 g (0.015 mol) of triethylamine in 5 mL of chloroform was added to this suspension. After that, 1.96 g (0.008 mol) of 2-bromo-2-methylpropanoyl bromide was added dropwise at 0 °C. Finally, the mixture was heated at 60 °C for 20 h. The resulting solid was separated from the solution by filtration over a Nylon membrane, and washed with DMF, until the residue was clear. The solid was dried overnight under vacuum at 60 °C, affording 0.5735 g of the product named SWCNT-ATRP initiator.
Polymerization of PMMA on the surface of the SWCNT
In this case, 49.14 mg (0.22 mmol) of copper bromide (II) and 60.82 mg (0.264 mmol) of HMTETA were placed in a 100 mL Schlenk flask under inert atmosphere and dissolved in phenylether. This solution was stirred for 30 min to ensure the formation of the complex Cu(II)/HMTETA. Then, 15 g of MMA monomer (150 mmol) was added slowly to the flask. The SWCNT-initiator was suspended in 20 mL of phenylether and this solution was sonicated for 30 min. This solution was added to the flask, followed by the addition of 134 mg the reducing agent Sn(II) 2-ethyl hexanoate (0.34 mmol). The reaction mixture was degassed by three freezes–thaw cycles with liquid nitrogen. The flask was placed in an oil bath at 75 °C for 2 h. Then, the reaction product was dispersed in THF, and precipitated in hexane, recovering a black solid, which was dried overnight under vacuum at 40 °C to obtain as final product SWNT-PMMA whose characterization was performed by TGA, CP-MAS and FT-IR.
Polymerization of PGMA on the surface of the SWCNT
For the functionalization of SWCNT-Br with the acrylic polymer glycidyl methacrylate, it was used the normal ATRP technique. In this case, 31 mg of Copper bromide (I) (0.22 mmol) and 76.3 mg of HMTETA (0.33 mmol) in 20 mL of methylethyl ketone (MEK) were placed in a 100 mL Schlenk flask under inert atmosphere. The solution was stirred for 30 min to ensure the formation of the complex Cu(I)/HMTETA. After this time 100 equivalents of the monomer were slowly added to the flask. The SWCNT-Br was sonicated in MEK for a period of 30 min and then this solution was slowly added to the flask. This reaction mixture was degassed by three freezes-thaw cycles with liquid nitrogen. The flask was placed in an oil bath at 50 °C for 75 min. The product was dispersed in THF and precipitated in hexane, recovering a black solid which was washed with methanol and the dried overnight under vacuum at 40 °C.
Functionalization of SWCNT with poly (butylacrylate)
The SWCNT-Br was used as initiator for the polymerization reaction of butylacrylate by the activators generated by electron transfer (AGET-ATRP) technique. For this reaction, 0.12 g (0.538 mmol) of copper (II) bromide and 0.134 g (0.5 mmol) of HMTETA in phenylether were placed in a 100 mL Schlenk flask under inert atmosphere and stirred for 30 min. After this time, 26.852 g (0.2148 mol) of butylacrylate was added to the flask, followed by the addition of 0.319 g (0.78 mmol) of the reducing agent Sn(II) 2-ethylhexanoate. The SWCNT-Br was sonicated in a sonic bath for 90 min, and then added to the flask. The reaction mixture was degassed by three freezes-thaw cycles with liquid nitrogen. The flask was placed in an oil bath at 70 °C for 21 h. The product was dissolved in THF and precipitated in hexane, recovering 19.61 g (73% conversion) of a black solid which was dried overnight under vacuum at 40 °C. The product was characterized by TGA.
Results and discussion
The main purpose of this work is to develop a methodology to obtain polymeric composites from SWCNT with improved interaction between CNT and the polymer through the incorporation of polymer chains on the surface of the SWCNT, then the covalent functionalization through the ‘grafting from’ strategy. Therefore, functionalization of the sidewall of the SWCNT with 2-(4-aminophenyl) ethanol was performed on the surface. The resulting material was modified with 2-bromoisobutyryl bromide that was also used as an ATRP initiator. The polymerization of acrylic monomers were carried out by the AGET-ATRP methodology involving the in situ reduction of the copper (II) complexes.
Functionalization with diazonium salt
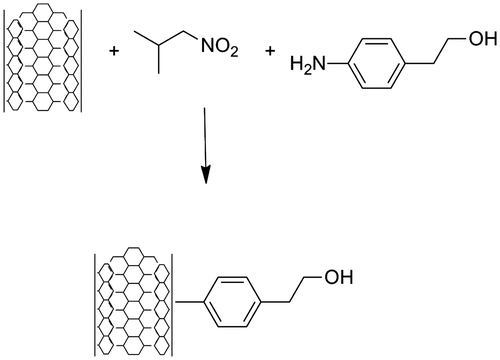
For the introduction of the 2-(4-aminophenyl)ethanol over the surface of the CNT, the reactivity of the sp2 carbons which form the network of hexagons in the CNT must be considered. These carbon atoms have an aromatic character and must have similar reactivity. For the alkylation of an aromatic carbon there are several methodologies reported in literature, however the most successful for graphite, graphene and CNT are those involving free radical addition,[Citation12–Citation14] reaction with diazonium salts [Citation15,Citation16] and cycloadditions [2 + 1] with carbenes or nitrenes.[Citation17,Citation18] Due to the versatility and synthetic reasons, functionalization of the SWCNT with diazonium salts was chosen. Several methods for the aromatic coupling between aromatic diazonium salts and aromatic compounds are reported with high yields.[Citation19,Citation20] There exists a controversy about the pathway for this type of reactions and the most accepted for an aprotic solvent is the free radical mechanism. Here, we report the methodology for the in situ generation of a diazomium salt from an aromatic amine and isoamyl nitrite in the presence of SWCNT using chlorobenzene as a solvent. As a side product from this reaction N2 is released and a new SWCNT-aryl bond is generated on the surface of the SWCNT (see Figure ).
Figure 1. Surface modification of SWCNT with 4-(2-hydroxyethyl)phenylidene via in situ formation of a diazonium salt.
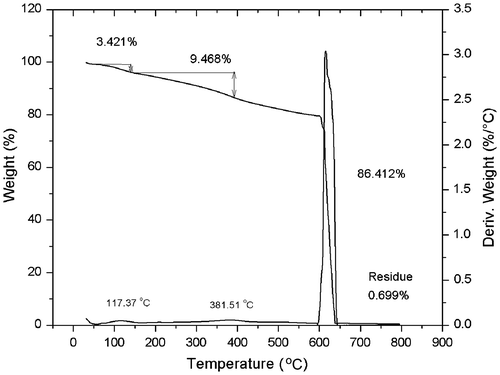
After the reaction took place, a black solid was recovered, the modified SWCNT were purified through washes with DMF using a glass filter fitted with a 0.2 μm membrane, DMF was added until the waste liquid was colourless to ensure that there were no more residual products. Further purification was done by extraction with acetone and ethyl ether to eliminate the remaining DMF in the final product. This product was characterized by thermal gravimetric analysis in an argon atmosphere (at 10 °C/min). In the TGA analysis (Figure ), it was observed a total weight loss of 12.90% at the temperature range between 100 and 400 °C. Most of the weight loss occurs at temperatures >390 °C. With this analysis it was possible to determine the degree of functionalization on the SWCNT.
In order to characterize the surface modification, by the weight losses, stoichiometric calculations were made. From this analysis, different losses were identified including a 3.421% weight loss in the range of 100–150 °C, followed by a loss of 9.468% at 180–390 °C range. After analysing at least five different TGA results, the stoichiometric calculations mechanism of thermolysis were proposed. It was found that first weight lost fitted well with the release of a methanol molecule. The loss of toluene molecule was attributed to the second thermal transition and the loss of the 81.86% weight at approximately 600 °C corresponded to the oxidation of the remaining SWCNT due to the introduction of oxygen to the TGA analyser at this temperature. This mechanism of thermolysis is schematized in Figure .
Figure 3. Proposed mechanism of thermolysis 4-(2-hydroxyethyl)-phenylidene during the thermogravimetric analysis.
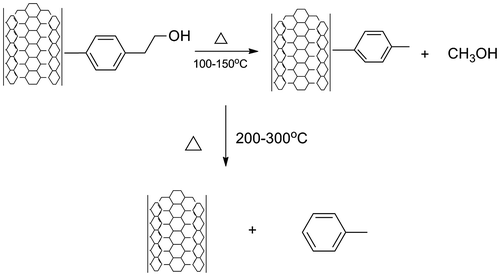
According to the proposed mechanism of thermolysis, it was concluded that the functionalization reaches the 12.90% yield, indicating that each 100 g of modified SWCNT, contains 12.90 g (0.1065 mol) of the 4-(2-hydroxyethyl) phenylidene.
The SWCNT that were modified on their surface with the 4-hydroxyethyl-phenylidene group were also characterized by FT-IR. This analysis showed the characteristic band at 3342 cm−1 from the O–H bond stretch in the alcohol group.
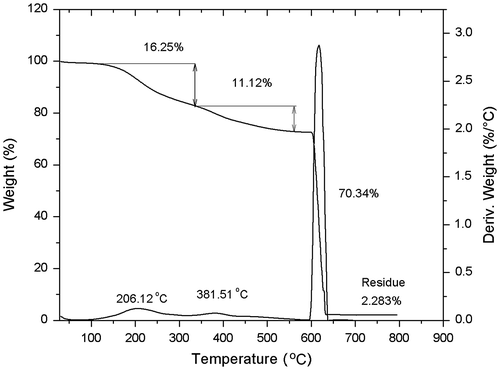
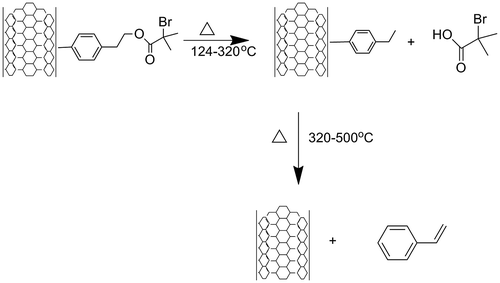
The modified SWCNT was further functionalized with 2-bromo-2-methylpropanoyl bromide to generate a SWCNT-ATRP initiator. As stated before, the exact quantity of 4-(2-hydroxyethyl)phenylidene on the surface of SWCNT was established from the TGA analysis (where it was found 0.1065 mol by 100 g of SWCNT), and the molar ratio between 4-(2-hydroxyethyl) phenylidene and 2-bromo-2-methylpropanoyl bromide was fitted in 1:2. The reaction was carried out in acetone as solvent and triethylamine (6 eq) at 60 °C for 20 h. The reaction product was extensively washed with acetone and ethyl ether, and then dried overnight in vacuum at 40 °C. To determine the degree of functionalization, the product was analysed by TGA (10 °C/min, nitrogen atmosphere, which was changed to oxygen when the temperature reached the 600 °C). The resulting thermogram shows a first weight loss of 16.25% followed by a second weight loss of 11.12% and a third weight loss of 72.5% at approximately 600 °C. The thermogram is showed in Figure .
Figure 4. Thermogram of the nanotubes functionalized with 4-(2-bromo-2-methyl propanoate of 2-hidroxyethyl phenylidene.
As it was previously described, the stoichiometric calculations were based on a set of five different analyses. It was found that for the first weight loss transition and over a transition temperature between 100 and 200 °C, one molecule of 2-bromo-2-methylpropanoic acid was released from the surface, then the second transition corresponds to the loss of styrene. At 600 °C, and higher temperatures, the final loss corresponds to the oxidation of the remaining SWCNT. The schematic mechanism of thermolysis is shown in Figure .
Figure 5. Mechanism of thermolysis of the SWCNT surface functionalized with 4-(2-bromo-2-methylpropanoate of 1-hidroxyethyl) phenhylidene.
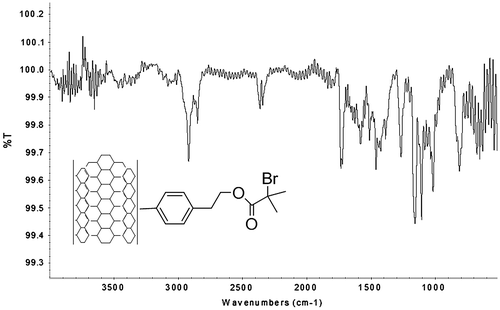
Additionally, FT-IR analysis that is shown in Figure was performed to ensure the presence of the initiator molecule on the surface of the SWCNT. The spectrum shows a band at 1721 cm−1 that corresponds to the stretch of the carbonyl group, and some stretching bands at 1091–1155.48 cm−1, from the C–O bond. With all these results, it was determined a 27% of 4-(2-bromo-2-methylpropanoate of 1-hidroxyethyl) phenylidene functionalization on the sidewall of the SWCNT.
Growing acrylic polymers on the SWCNT surface using the ATRP methodology
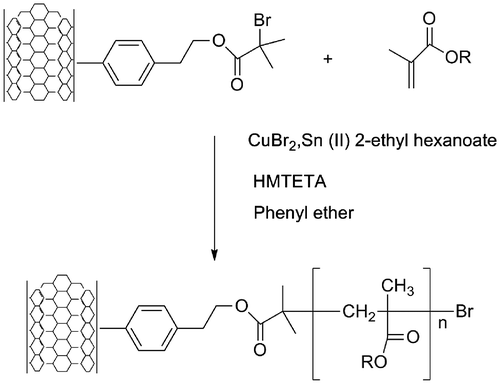
Known as one of the methods for the controlled radical polymerization technique, ATRP is a polymerization technique where the control over the molecular weight can be handled by the ratio between monomer and initiator if the correct system of deactivation of free radicals is employed. The quantification of the ATRP initiator chemically attached to the surface of the SWCNT provide the possibility to establish the ratio of monomer/initiator in order to calculate the polymer added to the surface of the SWCNT that allow us to control the length of the polymer growing in the surface of the nanotube from acrylic monomers such as MMA, BuA and GMA. Additionally, AGET-ATRP conditions were selected due the versatility in the use of the catalytic system which can be manipulated without rigorous experimental conditions. Usually ATRP uses a copper (I) salt, but it can be easily oxidized to the inactive cooper (II) during the storage or incorrect experimental procedures. Instead of considering the normal ATRP, Jakubowski and Matyjaszewsk proposed the usage of the AGET.[Citation21] Under these conditions, a copper (II) ion is reduced in situ donating an electron to an atom or molecule. Here, we have chosen the previously studied experimental conditions for the homopolymerization of acrylic polymers through AGET technique.[Citation22] This implies the use of tin 2-ethylhexanoate (Sn (EH)2) as the reducing agent, HMTETA as the ligand, phenyl ether as solvent and SWCNT surface modified with 4-(2-bromo-2-methylpropanoate of 1-hydroxyethyl) phenylidene as the initiator. The schematic process is shown in the Figure .
In order to prepare polymer chains with different length, several molar ratios between monomer and initiator were tested between 71 and 260, the experimental conditions are reported in Table .
Table 1. Experimental conditions for the polymerization of acrylic monomers on the SWCNT surface.
The polymerization reaction of acrylic monomers was carried out in a Schlenk tube under inert atmosphere. Once the reaction mixture was prepared, the tube and its content were placed to react in an oil bath calibrated at the reaction temperature set for each monomer. Once the reaction time was reached (two hours), the material was removed from the tube and suspended in THF followed by precipitation in hexane. A black solid was obtained which was filtered and washed with methanol. The recovered product was then dried on vacuum for 24 h to remove the remaining solvent from the product. The results for the described polymerization are shown in Table .
Table 2. Results of the polymerization of acrylic polymers on the SWCNT surface.
As it can be seen from Table , the polymerization on the surface of the SWCNT was successfully performed, since it can be deducted from the increase in weight of the final product. Polymerization can only occur on the surface of SWCNT since the initiator molecule is chemically attached to the nanotube surface and no ‘sacrificial initiator’ was added. Yield for methyl methacrylate reached 56% and 90% for glycidyl methacrylate. Unfortunately, molecular weight cannot be evaluated directly with GPC from the composite since the polymer is covalently bonded to the SWCNT.
To determine the molecular weight, the nanocompound must be hydrolysed, however, this determination is not certain at all since both initiator and polymer have the same type of chemical bonds, and the hydrolysis of the acrylic polymer will reduce the molecular weight. Therefore, molecular weight can be calculated from the TGA analysis since the amount of initiator on the surface of the nanotube is known. This data is given on Table .
As can be seen from Table , small differences between the theoretical and calculated molecular weight of the acrylic polymers grown on the surface of the SWCNT was obtained. This remarkable result offers the possibility to accurate design a SWCNT composite with a predicted polymer length.
As an example, we report the characterization of the SWCNT-f-PMMA by FT-IR spectroscopy using a micro-ATR device, where a strong absorption band at 1726 cm−1 can be appreciated. This corresponds to the stretch of the carbonyl from the ester group. In this spectrum, a strong band appears at 1100 cm−1 corresponding to the stretch of the C–O band of the methoxy group.
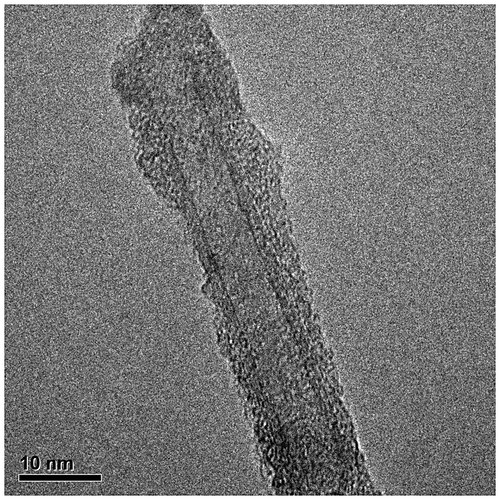
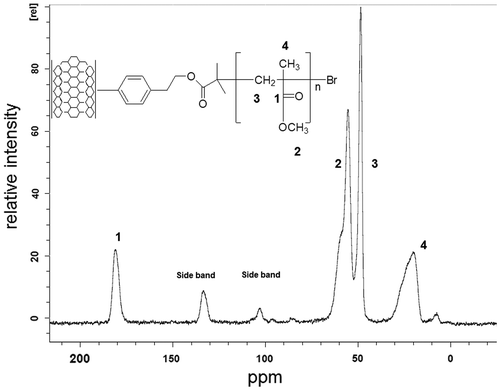
The SWCNT surface modified with poly(methyl methacrylate) was also analysed by TEM. TEM analysis implies the dispersion of the material in toluene that has to be placed in a grid of copper. This material was analysed in a Titan TEM, Figure shows the micrograph to 300,000 magnifications, where it can be seen the modification of the CNT has an inner diameter of 5 nm.
Additionally, solid-state 13C NMR spectroscopy was carried out using MAS, (rotation to an angle of 54.7°) and 1H-13CP pulse sequences, in a 4 mm rotor at a speed of 6.5 kHz. The CP-MAS of the SWCNT-f-PMMA spectrum is shown in Figure , in which resonances at 182, 55, 50 and 24 ppm were identified. These resonances correspond to the carbonyl group, the mehoxy group, the methylene carbons and the methyl carbon atoms, respectively.
The characteristic signals in TGA, FT-IR and CP-MAS were found during the characterization of all the compounds obtained, and therefore it can be concluded that the synthesis of the SWCNT-modified surface with acrylic polymers was successfully performed.
Conclusions
A method for the functionalization of SWCNT through the reaction with the diazonium salt form the 2-(4-aminophenyl)ethanol, further modification generates SWCNT functionalized with 4-(2-bromo-2-methylpropanoate of 1-hidroxyethyl) phenylidene on the surface of the nanotubes. This material was successfully characterized using different analytical techniques. The proposed fraction can act as an ARTP initiator which can polymerize different monomers. This polymerization of acrylic monomers on the surface of the SWCNT follows the controlled radical polymerization concept with a control over the molecular weight which can grow on the surface of the SWCNT. This remarkable result offers the possibility to accurate design a SWCNT composite with a predicted polymer length.
Acknowledgements
The authors wish to thank the National Council of Science and Technology (CONACYT) for the financial support provided through the project number 61061. Also the authors wish to thanks Jorge F. Espinoza, Guadalupe Telles. Lourdes Guillen, Victor Comparan and Julieta Sanchez for the support in the analytical characterization.
References
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58.
- Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chem. Rev. 2006;106:1105–1136.
- Rachmadini Y, Tan VB, Tay TE. Enhancement of mechanical properties of composites through incorporation of CNT in VARTM – a review. J. Reinf. Plast. Compos. 2010;29:2782–2807.
- Tianqi L, Casado-Portilla R, Belmont J, Matyjaszewski K. ATRP of butyl acrylates from functionalized carbon black surfaces. J. Polym. Sci., Part A: Polym. Chem. 2005;43:4695–4709.
- Gao C, Muthukrishnan S, Li W, Yuan J, Xu Y, Muille A. Linear and hyperbranched glycopolymer-functionalized carbon nanotubes: synthesis, kinetics, and characterization. Macromolecules. 2007;40:1803–1815.
- Wang K, Li W, Gao C. Poly(ε-caprolactone)-functionalized carbon nanofibers by surface-initiated ring-opening polymerization. J. Appl. Polym. Sci. 2007;105:629–640.
- Paulus GLC, Wang QH, Strano MS. Covalent electron transfer chemistry of graphene with diazonium salts. Acc. Chem. Res. 2013;46:160–167.
- Jayasundara DR, Cullen RJ, Colavita PE. In situ and real time characterization of spontaneous grafting of aryldiazonium salts at carbon surfaces. Chem. Mater. 2013;25:1144–1152.
- Bahr JL, Yang J, Kosynkin DV, Bronikowski MJ, Smalley RE, Tour JM. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: a bucky paper electrode. J. Am. Chem. Soc. 2001;123:6536–6542.
- Marcoux PR, Hapiot P, Bataila P, Pinson J. Electrochemical functionalization of nanotube films: growth of aryl chains on single-walled carbon nanotubes. New J. Chem. 2004;28:302–307.
- Marek KB. Isoamyl nitrite can cause serious explosions. J. Chem. Educ. 2010;87:583.
- Sung JH, Kim HS, Jin HJ, Choi HJ, Chin IJ. Nanofibrous membranes prepared by multiwalled carbon nanotube/poly(methyl methacrylate) composites. Macromolecules. 2004;37:9899–9902.
- Qin S, Qin D, Ford WT, Herrera JT, Resasco DE. Grafting of poly(4-vinylpyridine) to single-walled carbon nanotubes and assembly of multilayer films. Macromolecules. 2004;37:3965–3967.
- Qin S, Qin D, Ford WT, Zhang YK. Covalent cross-linked polymer/single-wall carbon nanotube multilayer films. Chem. Mater. 2005;17:2131–2135.
- Strano MS. Probing chiral selective reactions using a revised kataura plot for the interpretation of single-walled carbon nanotube spectroscopy. J. Am. Chem. Soc. 2003;125:16148–16153.
- Dyke CA, Tour JM. Unbundled and highly functionalized carbon nanotubes from aqueous reactions. Nano Lett. 2003;3:1215–1218.
- Holzinger M, Abraham J, Whelan P, Graupner R, Ley L, Hennrich F, Kappes M, Hirsch A. Functionalization of single-walled carbon nanotubes with (R-)oxycarbonyl nitrenes. J. Am. Chem. Soc. 2003;125:8566–8580.
- Holzinger M, Steinmetz J, Samaille D, Glerup M, Paillet M, Bernier P, Ley L, Graupner R. [2+1] cycloaddition for cross-linking SWCNTs. Carbon. 2004;42:941–947.
- Milanesi S, Fagnoni M, Albini A. (Sensitized) photolysis of diazonium salts as a mild general method for the generation of aryl cations. Chemoselectivity of the singlet and triplet 4-substituted phenyl cations. J. Org. Chem. 2005;70:603–610.
- Milanesi S, Fagnoni M, Albini A. Cationic arylation through photo(sensitized) decomposition of diazonium salts. Chemoselectivity of triplet phenyl cations. Chem. Commun. 2003:216–217.
- Jakubowski W, Matyjaszewski K. Activator generated by electron transfer for atom transfer radical polymerization. Macromolecules. 2005;38:4139–4146.
- Najera MA, Elizalde LE, Vazquez Y, de los Santos G. Synthesis of random copolymers poly (methylmethacrylate-co-azo monomer) by ATRP-AGET. Macromolecular Symposia (New Trends in Polymer Science). 2009;283–284:51–55.