Abstract
The novel functionality of aromatic tetrazole derivatives with high nitrogen content predetermines a great interest to tetrazole-containing polymers. Poly(5-vinyltetrazole) is one of the most attractive polymers containing tetrazoles. The 4-chloromethyl styrene (CMS) was copolymerized with acrylonitrile (in various mole ratios) by free radical polymerization method at 70 °C using α,α-azobis(isobutyronitrile) as an initiator. The reaction azide ion with copolymers, simultaneously with replacement of all the chlorine atoms in CMS units, causes the nitrile groups are entirely converted to tetrazole in dimethylformamide at elevated temperatures. The polymers, obtained in quantitative yields, were characterized by FT-IR and 1H NMR spectroscopy, differential scanning calorimetry, and gel permeation chromatograph studies. Thermal properties nitrogen-rich polymers show that explosive thermal degradation takes place at around 260 °C.
Introduction
The design of new energetic molecules is based on compounds exhibiting a high density and an elevated heat of formation. These fundamental properties, achieved through the presence of numerous nitrogen atoms and/or explosophoric groups, ensure high performance levels that can be useful in target applications such as explosives, propellants, or gas generators. The same basics also apply when considering the use of polymers, instead of single molecules, as energetic ingredients. Azaheterocycles are obviously suitable scaffolds for achieving nitrogen-rich polymers. There is a considerable interest in polyvinyltetrazoles (PVT) containing a large amount of nitrogen because of their powerful energetics,[Citation1–Citation3] interpolymer complexity,[Citation4,Citation5] biological activity, high thermostability,[Citation6–Citation8] and good solubility in various solvents,[Citation9] exhibiting wide applications including dynamite, polyelectrolytes,[Citation10,Citation11] distinctive complex,[Citation12] biocompatible material of different natures, and oxygen enriching membrane.[Citation13–Citation15] The investigation on the radical (co)polymerization,[Citation16] radiation-induced bulk polymerization,[Citation17] solution interdiffusion,[Citation18,Citation19] solution properties and kinetics of solution formation in various media,[Citation20] rheological properties,[Citation21] swelling thermodynamics,[Citation22] and thermal degradation and its kinetics and mechanism of the PVT has been reported. Well-known technique for the preparation of the PVT is traditional radical polymerization of 5-vinyltetrazole monomer,[Citation23,Citation24] Considering that the 5-vinyltetrazole monomer is not readily available at the present time because of the absence of commercial source of 5-vinyltetrazole and also the difficulty in the synthesis of the 5-vinyltetrazole,[Citation25–Citation27] a new and powerful technique for the preparation of the PVT by tetrazole cyclization of polyacrylonitrile (PAN) could be a real challenge.
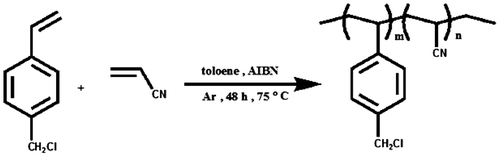
This paper deals with an efficacious preparation and characterization of the PVT by the tetrazolation of the cyano groups in the PAN, that is, polymer-analogous conversion. In this work, we first synthesized the copolymer of 4-chloromethyl styrene (CMS) with acrylonitrile by radical polymerization. Reaction of azide ion with copolymers simultaneously with replacement of all the chlorine atoms in CMS units causes the nitrile groups are entirely converted to tetrazole in dimethylformamide at elevated temperatures. Thermal properties nitrogen-rich polymers were characterized differential scanning calorimetry (DSC).
Experimental
Materials
The CMS (Aldrich, 90%) and acrylonitrile (Merck) were distilled under reduced pressure to remove inhibitors, before use. The initiator α,α-azobis(isobutyronitrile) (AIBN) (Merck) was purified by crystallization from methanol.
Measurements
Infrared spectra were recorded with a 4600 Unicam FT-IR spectrophotometer as KBr pellets. 1H NMR spectra were run on a Bruker 400 MHz spectrometer at room temperature using CDCl3 as a solvent. The molecular weights (MW and Mn) were determined using a Waters 501-gel permeation chromatograph (GPC) fitted with 102 and 103 nm Waters styragel columns. THF was used as an elution solvent at a flow rate of 1 mL/min, and polystyrene standard was employed for calibration. The DSC curves were obtained on a TGA/SDTA 851 calorimeter at heating and cooling rates of 10 °C/min under N2.
Copolymerization of 4-chloromethylstyrene with acrylonitrile: PCSA
For preparing of copolymers (PCSA1 and PCSA2), a mixture of 4-chloromethylstyrene with different amounts of acrylonitrile with molar ratios of 1:1 and 1:2, respectively, was dissolved in 15 mL of toluene and was mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoule was degassed, sealed under vacuum, and maintained at 75 ± 1 °C in a water bath, with stirring for about 48 h. Then, the solutions were poured from ampoules into cooled methanol. The precipitates were collected and washed with methanol and dried under vacuum to yield (approximately 85%) of copolymers (Scheme ). For PCSA1 and PCSA2: 1H NMR (DMSO-d6, ppm) 0.88–2 (CH2–CH), 4.85 (CH2–Cl), 6.9–7.7 (Ar–H). FT-IR (KBr, cm−1): 3085–3026 (aromatic C–H), 2926–2860 (aliphatic C–H), 2239 (CN), 1600–1490 (aromatic C=C).
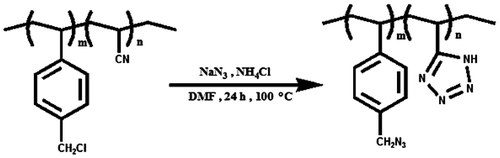
Reaction of sodium azide with copolymers: PAST
The all of reaction with sodium azide and ammonium chloride was carried out in a conical bottle equipped with stirrer and reflux condenser. About 5 g of polymers powder and 100 mL of DMF were added to a 250 mL conical bottle with stirring at ambient temperature. Then to the solution, 6.50 g of NaN3 and 5.35 g of NH4Cl were added with stirring. The bottle was immediately placed into an oil bath and heated to 100 °C and maintained the temperature with stirring for 12 h. The intermediate product was precipitated spontaneously and gradually from the reaction mixture during the course of reaction. The final reaction mixture was added into distilled water for a complete precipitate and also an elimination of DMF. The products obtained were treated in 300 mL of 0.5 M HCl and repeatedly washed with distilled water for a complete removal of Cl−, Na+, and H+. The desired polymer was left to dry in air for several days to constant weight (yield around 95%). 1H NMR (DMSO-d6, ppm): 1–1.7 (CH2–CH), 4.4 (CH2–N3), 6.8–7.7 (Ar–H). FT-IR (KBr, cm−1): 3446, 3027 (aromatic C–H), 2926 (aliphatic C–H), 2099 (azide N3), 1490–1600 (aromatic C=C) (Scheme ).
Results and discussion
The resulting copolymers are white solids and soluble in THF, N,N-dimethylformamide, and dimethylsulfoxide but insoluble in n-hexane, methanol, ethanol, and water.
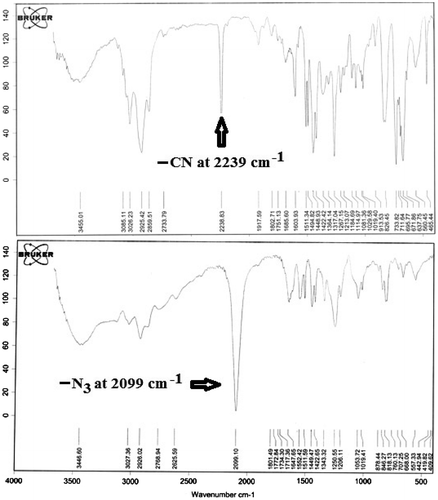
Cycloaddition of C≡N bond of nitriles with sodium azide in the presence of ammonium chloride occurs in one step and results in tetrazole derivatives. To increase the yield of reaction and complete conversion, excess sodium azide was used in this work. In 1H NMR spectra, with replacement of chlorine atoms with azide group, the peak around 4.85 ppm corresponding to two methylene protons of benzyl chloride completely disappeared, and new peaks at 4.4 ppm corresponding to two methylene protons attached to azide group appeared. Analysis of the IR spectra shows that with reaction azide ion with copolymers the band at 2239 cm−1 (the stretching vibrations of the nitrile group) disappears, and in its place, new absorption band appears at 2099 cm−1, which are assigned to the stretching vibrations of the azide (Figure ).
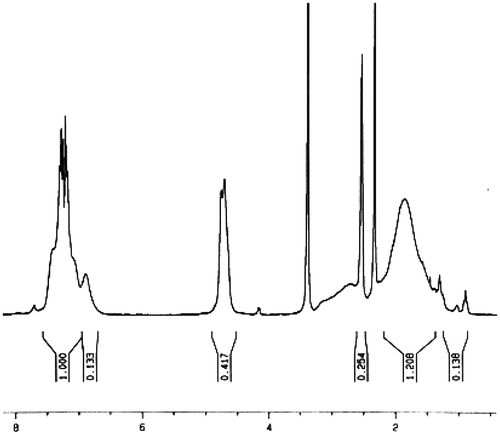
The copolymer compositions were calculated from the 1H NMR spectra data. In the past few decades, 1H NMR spectroscopic analysis has been established as a powerful tool for the determination of copolymer compositions because of its simplicity, rapidity, and sensitivity.[Citation22] Spectrum of copolymer PCSA1 in DMSO-d6 is shown in Figure . The molar compositions of CMS and acrylonitrile in copolymer PCSA2 were calculated from the ratio integrated intensities of the peaks around 4.85 ppm, corresponding to two methylene protons of benzyl chloride in CMS units to the total area between 0.88 and 2 ppm, which were attributed to six protons marked by (#) in CMS and (*) in acrylonitrile. The molar compositions of CMS and acrylonitrile were calculated from Equations (1) and (2) where x and y were the mole fractions of CMS and acrylonitrile, respectively:(1)
(2)
A similar method was used to calculate the molar compositions of monomers in copolymer PCSA1. The compositions of copolymers are presented in Table .
Table 1. Molar composition and GPC data of copolymers.
The study of composition of polymers shows that monomers reactivity ratios are different and CMS is more reactive than acrylonitrile toward propagating species. Therefore, copolymers containing a large proportion of the more reactive monomer (CMS) in random placement.
Thermal behavior
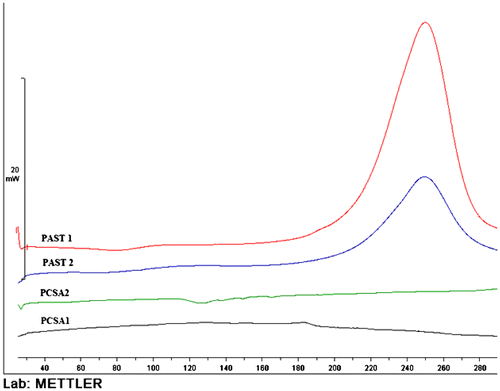
Thermal properties of copolymer were evaluated using the DSC technique. As can be seen from Figure , two copolymers (PCSA1 and PCSA2) are completely stable until 280 °C, while the decomposition nitrogen-rich copolymers (PAST1 and PAST2) occurred about 260 °C, which that is due to the fact that azide groups and tetrazole rings are both destroyed in this stage. With comparing the two copolymers, PAST1 and PAST2, it is observed that in PAST1 with increase in the azide percentage, increasing the amount of energy released. So, the energy content of the azide group is comparably higher than that of the tetrazole ring.
Conclusions
The copolymers were synthesized by free radical solution polymerization. The molar compositions of the obtained copolymers were calculated by the 1H NMR spectral method. The nitrogen-rich copolymers show an explosive thermal degradation together with a release of huge heat and magnitude of heat increased by increases of amount of azide groups in side chains of copolymer.
References
- Shin JA, Lim YG, Lee KH. Synthesis of polymers including both triazole and tetrazole by click reaction. Bull. Korean Chem. Soc. 2011;32:547–552.
- Mohan YM, Mani Y, Raju KM. Synthesis of azido polymers as potential energetic propellant binders. Des. Monomers Polym. 2006;9:201–236.
- Mahkam M. Synthesis and characterization of novel polymers containing pendant silyl ether groups. Des. Monomers Polym. 2010;13:407–413.
- Hosseinzadeh F, Mahkam M, Galehassadi M. Synthesis and characterization of ionic liquid functionalized polymers for drug delivery of an anti-inflammatory drug. Des. Monomers Polym. 2012;15:379–388.
- Annenkov VV, Kruglova VA, Mazyar NL. Complexes of poly-5-vinyltetrazoles with weak polybases. J. Polym. Sci., Part A: Polym. Chem. 1996;34:597–602.
- Gaur B, Lochab B, Choudhary V, Varma IK. Azido polymers–energetic binders for solid rocket propellants. J. Macromol. Sci., Part C: Polym. Rev. 2003;43:505–545.
- Levchik SV, Balabanovich AI, Ivashkevich OA, Gaponik PN. Thermal decomposition of tetrazole-containing polymers. V. Poly-1-vinyl-5-aminotetrazole. Polym. Degrad. Stab. 1995;47:333–338.
- Mahkam M, Massoumi B, Mirfatahi H. Modification of styrene polymer by attaching suitable groups as side chain. e-Polymers. 2009;145:1–7.
- Kizhnyaev VN, Gorkovenko-Spirina OP, Smirnov AI. Solubility of tetrazole-containing polymers in acids. Polym. Sci. Ser. B. 2002;44:171–174.
- Moulay S. Towards halomethylated benzene-bearing monomeric and polymeric substrates. Des. Monomers Polym. 2011;14:179–220.
- Kizhnyaev VN, Gorkovenko OP, Safronov AP, Adamova LV. Thermodynamics of the interaction between tetrazole-containing polyelectrolytes and water. Polym. Sci. Ser. A. 1997;39:366–371.
- Annenkov VV, Kruglova VA, Alsarsur IA, Shvetsova ZhV, Aprelkova NF, Saraev VV. Complexation between poly(5-vinyltetrazole) and copper and cadmium ions in aqueous solutions. Vysokomolekulârnye soedineniâ. Seriâ A i seriâ B A. 2002;44:2053–2057.
- Gaina V, Gaina C. Synthesis and characterization of functional polymers with conjugate chains. Des. Monomers Polym. 2011;14:57–68.
- Li XG, Huang MR. Multilayer ultrathin-film composite membranes for oxygen enrichment. J. Appl. Polym. Sci. 1997;66:2139–2147.
- Li XG, Kresse I, Springer J, Nissen J, Yang YL. Morphology and gas permselectivity of blend membranes of polyvinylpyridine with ethylcellulose. Polymer. 2001;42:6859–6869.
- Mikhailov YuM, Ganina LM, Kurmaz SV, Smirnov VS, Roshchupkin VP. Diffusion mobility of reactants, phase equilibrium, and specific features of radical copolymerization kinetics in the nonyl acrylate/2-methyl-5-vinyltetrazole system. J. Polym. Sci., Part B: Polym. Phys. 2002;40:1383–1389.
- Govorkov AT, Muryshkina YeV, Khokhlova GP, Bannova YeA. Radiation-induced bulk polymerization of 2-methyl-5-vinyltetrazole under γ-irradiation. Polym. Sci. USSR. 1991;33:1138–1142.
- Kukut M, Karal-Yilmaz O, Yagci Y. Synthesis, characterization, and hydrolytic degradation of graft copolymers of polystyrene and aliphatic polyesters. Des. Monomers Polym. 2013;16:233–240.
- Mikhailov YM, Ganina LV, Shapaeva NV. Interdiffusion in solutions of poly(2-alkyl-5-vinyltetrazole). Polym. Sci. Ser. A, Chem., Phys. 1995;37:642–645.
- Li Y, Zhou H, Yanpeng E, Wan L, Huang F, Du L. Synthesis and characterization of a new series of rigid polytriazole resins. Des. Monomers Polym. 2013;16:556–563.
- Kizhnyaev VN, Petrova TL, Smirnov AI. Rheological properties and gel formation of aqueous salt-containing solutions of sodium poly(5-vinyltetrazolate) in the presence of Cr3+ ions. Polym. Sci. Ser. A, Chem. Phys. A. 2001;43:566–571.
- Kizhnyaev VN, Tsypina NA, Adamova LV, Gorkovenko OP. Thermodynamics of swelling of poly(5-vinyltetrazole) salts in water. Polym. Sci. Ser. B. 2000;42:175–179.
- García T, Carreón-Castro MP, Gelover-Santiago A, Ponce P, Romero M, Rivera E. Synthesis and characterization of novel amphiphilic azo-polymers bearing well-defined oligo(ethylene glycol) spacers. Des. Monomers Polym. 2012;15:159–174.
- Gaponik PN, Ivashkevich OA, Karavai VP, Lesnikovich AI, Chernavina NI, Sukhanov GT, Gareev GA. Polymers and copolymers based on vinyl tetrazoles, part 1. Die Angewandte Makromolekulare Chemie. Macromol. Chem. Phys. 1994;219:77–88.
- Gaponik PN, Ivashkevich OA, Chernavina NI, Lesnikovich AI, Sukhanov GT, Gareev GA. Polymers and copolymers based on vinyl tetrazoles, part 1. Die Angewandte Makromolekulare Chemie. Macromol. Chem. Phys. 1994;219:89–99.
- Wouters G, Smets G. Copolymerization of C-vinyltriazoles and C-vinyl tetrazole with vinyl monomers. Macromol. Chem. Phys. 1982;183:1861–1868.
- Lin CH. Synthesis and characterization of segmented copolymers of polystyrene and thermotropic liquid crystalline poly(4-oxybenzoate-co-2,6-oxynaphthoate). Des. Monomers Polym. 2013;16:537–542.