Abstract
Hydantoins represent a group of compounds which over the past few years has attracted great attention because of their diverse biological activities. The synthetic methods for 5,5-dimetyhyl hydantoin (well-known biocidal material) available in literature are particularly difficult, and the yield of the synthesis after purification does not exceed 40%. On the other hand, styrene-type monomers containing spirohydantoin moieties were successfully synthesized by N-alkylation reaction. A simple and efficient preparation of N-chlorinated (N-halamine) monomers is presented, and the experimental data are supported by DFT calculations.
1. Introduction
During the past decade, the great interest has been devoted to biocidal polymers, which are able to kill pathogenic microorganisms or at least are characterized by the high resistance to microbial attack.[Citation1–Citation10]
Polystyrene, is the thermoplastic material applied in numerous industrial branches, and its properties are well known since many years and are often modified for manufacturing the modern plastics with completely different properties than origin polymer.[Citation11–Citation14] On the other hand, hydantoins represent a group of compounds which over the past few years has also attracted great attention because of their diverse biological activities including antibacterial, antiviral, fungicidal, herbicidal, analgesic, antiarythmic, antihypertensive, antidiabetic, HDL/cholesterol modulating, anticonvulsant, and cancer chemotherapeutic activity.[Citation15–Citation17] A particular kind of such compounds is spirohydantoins, which have hydantoin moiety chemically bonded to alicyclic ring condensed with aromatic group. There is only one type of monomer containing spirohydantoin moiety described in literature: allyl monomer (7,8-benzo-3-allyl-1,3-diazaspiro[4.5]decane-2,4-dione),[Citation9] and there is no such styrene-types monomers synthesis and characterization in literature yet.
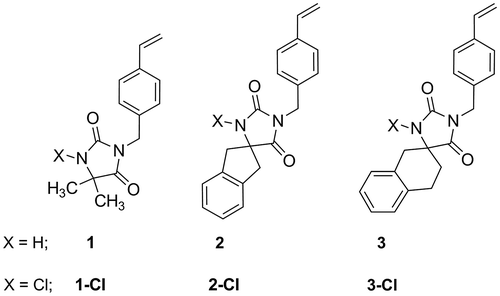
The present study is to report the synthesis of novel free radical polymerizable spirohydantoin-containing styrene-types monomers designed for biocidal materials preparation : 5,5-dimethyl-3-(4-vinylbenzyl)imidazo-lidine-2,4-dione (1), 1-(4-vinylbenzyl)-1′,3′-dihydrospiro-[imidazolidine-4,2′-indene]-2,5-dione (2), and 1-(4-vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (3) and their N-chlorinated form(1-Cl, 2-Cl, 3-Cl). Monomers 1 and 1-Cl have been prepared as a model compounds to verify the procedure.
2. Experimental
Ketones (acetone, 2-indanone, 2-tetralone), 4-vinylbenzyl chloride, potassium cyanide, ammonium carbonate, potassium carbonate anhydrous, trichloroisocyanuric acid (TCICA), and all solvents used in this research were purchases from Sigma Aldrich (Saint Louis, MO). All reagents were of high purity and were used without further purification.
1H and 13C NMR spectra were recorded at room temperature with Bruker Avance III 700 and 400 MHz spectrometers. Chemical shifts (in ppm) were determined relative to TMS. Melting temperature (mp), has been determined by capillary method using DigiMelt MPA161 melting point apparatus (Standford Research Systems, USA) and was corrected.
All glassware used for reactions with air sensitive compounds was dried at 150 °C for several hours, assembled hot, and cooled in a stream of nitrogen.
2.1. Hydantoins synthesis
2.1.1. General procedure
A mixture of ketone (0.04 M) in 50% aqueous ethanol (100 mL), potassium cyanide (0.06 M), and ammonium carbonate (0.4 M) was placed in an autoclave and heated at 60 °C for 6 h. The reaction solution was cooled and acidified of 10% hydrochloric acid until pH = 6.5–7. The resulting precipitate was filtered of and recrystallized from ethyl acetate to give the expected hydantoins with variable yields.
2.1.2. 5,5-Dimethylimidazolidine-2,4-dione (1)
Starting from acetone, this product was isolated as a white solids in 70% yield after crystallization from ethyl acetate; mp 175–177 C (Lit.[Citation18] mp 173 C); IR (HCB): ν 3267, 1775, 1715 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 1.31 (s, 6H, CH3), 8.43 (s, 1H, NH), 10.50 (s, 1H, NH); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 25.2 (CH3), 59.4 (Cq), 156.6 (C=O), 179.8 (C=O).
2.1.3. 1′,3′-Dihydrospiro[imidazolidine-4,2′-indene]-2,5-dione (2)
Starting from 2-indanone, this product was isolated as a white solids in 70% yield after crystallization from ethyl acetate; mp 262–264 C (Lit.[Citation19] mp 260 C); IR (HCB): ν 3194, 1770, 1730 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 3.08 (d, J = 16.8 Hz, 2H, CH2), 3.37 (d, J = 16.8 Hz, 2H, CH2), 7.23 (m, 4H, CHAr), 8.40 (s, 1H, NH), 10.74 (s, 1H, NH); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 43.5 (2 × CH2), 67.8 (Cq), 124.23 (2 × CHAr), 126.9 (2 × CHAr), 139.7 (2 × ) 156.4 (C=O), 178.5 (C=O).
2.1.4. 3′,4′-Dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (3)
Starting from 2-tetralone, this product was isolated as a white solids in 90% yield after crystallization from ethyl acetate; mp 266–268 C (Lit.[Citation19] mp 267 C); IR (HCB): ν 3242, 1784, 1720 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 1.83 (m, 1H, CH2), 1.93 (m, 1H, CH2), 2.78 (d, J = 16.8 Hz, 1H, CH2), 2.89 (m, 2H, CH2), 3.11 (d, J = 16.8 Hz, 2H, CH2), 7.12 (m, 4H, CHAr), 8.30 (s, 1H, NH), 10.70 (s, 1H, NH); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 43.5 (2 × CH2), 67.8 (Cq), 124.23 (2 × CHAr), 126.9 (2 x CHAr), 139.7 (2 × ) 156.4 (C=O), 178.5 (C=O).
2.2. Monomers synthesis
2.2.1. General procedure
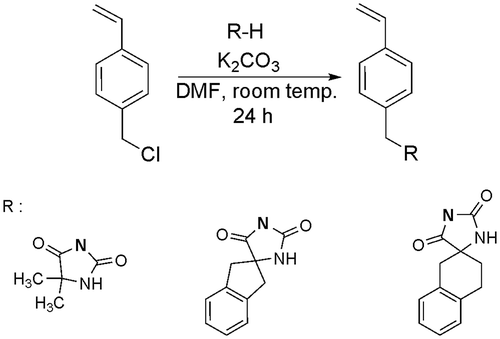
4-Vinylbenzylchloride (10 mM) was added to a stirred solution of hydantoin (10 mM) and potassium carbonate (10 mM) in DMF (30 mL) at room temperature, and the mixture was stirred overnight. Water was added (30 mL) and the mixture was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with water (10 mL) and dried. After the solvent was removed, the solid product was formed and purified by crystallization from appropriate solvent (ethanol or ethyl acetate) to give monomers with variable yields.
2.2.2. 5,5-Dimethyl-3-(4-vinylbenzyl)imidazolidine-2,4-dione (4)
Starting from 1, this product was isolated as a white solids in 60% yield after crystallization from ethyl acetate; mp 89–91 C (Lit.[Citation20] mp 92–93 C); Elemental Analysis: Calc. C, 68.83%; H, 6.60%; N, 11.47%; Found: C 68.81%; H 6.61%, N 11.48%; IR(HCB):ν 3296, 3019, 2982, 2936, 1772, 1710 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 1.31 (s, 6H, CH3), 4.52 (s, 2H, CH2), 5.25 (d, J = 11.2 Hz, 1H,=CH2), 5.82 (d, J = 17.5 Hz, 1H,=CH2), 6.72 (m, 1H,=CH), 7.20 (d, J = 7.7 Hz, 2H, CHAr), 7.44 (d, J = 7.7 Hz, 2H, CHAr), 8.50 (s, 1H, NH); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 25.2 (2 × CH3), 42.3 (CH2), 59.4 (Cq), 114.4 (=CH2), 127.7 (2 × CHAr), 129.0 (2 × CHAr), 135.6 (), 136.5 (=CH),137.4 (
), 156.6 (C=O), 179.7 (C=O).
2.2.3. 1-(4-Vinylbenzyl)-1′,3′-dihydrospiro[imidazolidine-4,2′-indene]-2,5-dione (5)
Starting from 2, this product was isolated as a white solids in 60% yield after crystallization from ethyl acetate; mp 200–201 C; Elemental Analysis: Calc. C, 75.45%; H, 5.70%; N, 8.80%; Found: C 75.46%; H 5.71%, N 8.83%; IR (HCB): ν 3300, 3023, 2925, 2851, 1775, 1714 cm−1; 1H NMR, (700 MHz), CDCl3, δ (ppm): 3.02 (d, J = 16.1 Hz, 2H, CH2), 3.60 (d, J = 16.1 Hz, 2H, CH2), 4.66 (s, 2H, CH2), 5.23 (m, 1H,=CH2), 5.72 (m, 1H,=CH2), 5.73 (s, broad, 1H, NH), 6.68 (m, 1H,=CH), 7.22 (m, 4H, CHAr), 7.36 (m, 4H, CHAr); 13C NMR, (100 MHz), CDCl3, δ (ppm): 42.3 (CH2), 44.4 (2 × CH2), 67.6 (Cq),114.4 (=CH2), 124.8 (2 × CHAr), 126.7 (2 × CHAr), 127.7 (2 × CHAr), 128.9 (2 × CHAr), 135.7 (), 136.5 (=CH),137.4 (
), 139.1 (2 ×
) 156.2 (C=O), 175.7 (C=O).
2.2.4. 1-(4-Vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (6)
Starting from 3, this product was isolated as a white solids in 65% yield after crystallization from ethanol; mp 180–182 C; Elemental Analysis: Calc. C, 75.88%; H, 6.06%; N, 8.43%; Found: C 75.90%; H 6.07%, N 8.45%; IR (HCB): ν 3366, 3160, 3094, 1772, 1716 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 1.83 (m, 1H, CH2), 1.85 (m, 1H, CH2), 2.82 (m, 1H, CH2), 2.90 (m, 2H, CH2), 3.10 (m, 1H, CH2), 4.52 (s, 2H, CH2), 5.26 (m, 1H,=CH2), 5.72 (m, 1H,=CH2), 6.72 (m, 1H,=CH), 7.01 (m, 4H, CHAr), 7.19 (d, J = 8.4 Hz, 2H, CHAr), 7.41 (d, J = 8.4 Hz, 2H, CHAr), 8.40 (s, 1H, NH); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 24.7 (CH2), 30.1 (CH2), 36.8 (CH2), 40.8 (CH2), 60.7 (Cq), 114.4 (=CH2), 125.8 (2 × CHAr), 126.0 (2 × CHAr), 128.7 (2 × CHAr), 129.0 (2 × CHAr), 134.7 (), 136.2 (=CH), 136.3 (
), 136.5 (2 ×
) 155.6 (C=O), 176.3 (C=O).
2.3. N-Chlorination of monomers
2.3.1. General procedure
To the solution of appropriate monomer (5 mM) in acetonitrile (10 mL) TCICA (10 mM) was added in one portion. The resulting slurry was stirred until the mixture became clear and uniform. Then, the solved was removed and obtained white solid was triturated in hot chloroform. The solid was removed by filtration and the chloroform was removed from filtrate to gave the crude chlorinated monomer, which was purified by recrystallization from ethyl acetate.
2.3.2. 1-Chloro-5,5-dimethyl-3-(4-vinylbenzyl)imidazolidine-2,4-dione (4-Cl)
Starting from 4, this product was isolated as a white solids in 90% yield after crystallization from ethyl acetate; mp 75–81 C, Elemental Analysis: Calc. C 60.33%; H 5.42%; N 10.05%; Found: C 60.36%; H 5.43%; N 10.07%; IR (HCB): ν 1768, 1690 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 1.32 (s, 6H, CH3), 4.52 (s, 2H, CH2), 5.27 (d, J = 11.2 Hz, 1H,=CH2), 5.84 (d, J = 17.5 Hz, 1H,=CH2), 6.71 (m, 1H,=CH), 7.21 (d, J = 7.7 Hz, 2H, CHAr), 7.44 (d, J = 7.7 Hz, 2H, CHAr); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 25.2 (2 × CH3), 42.3 (CH2), 59.4 (Cq), 114.4 (=CH2), 127.7 (2 × CHAr), 129.0 (2 × CHAr), 135.6 (), 136.5 (=CH),137.4 (
), 156.6 (C=O), 180.1 (C=O).
2.3.3. 3-Chloro-1-(4-vinylbenzyl)-1′,3′-dihydrospiro[imidazolidine-4,2′-indene]-2,5-dione (5-Cl)
Starting from 5, this product was isolated as a white solids in 85% yield after crystallization from ethyl acetate; mp 185–191 °C; Elemental Analysis: Calc. C 68.09%; H 4.86%; N 7.94%; Found: C 68.06%; H 4.88%; N 7.96%; IR (HCB): ν 1772, 1710 cm−1; 1H NMR, (700 MHz), CDCl3, δ (ppm): 3.02 (d, J = 16.1 Hz, 2H, CH2), 3.58 (d, J = 16.1 Hz, 2H, CH2), 4.65 (s, 2H, CH2), 5.22 (m, 1H,=CH2), 5.70 (m, 1H,=CH2), 6.70 (m, 1H,=CH), 7.22 (m, 4H, CHAr), 7.36 (m, 4H, CHAr); 13C NMR, (100 MHz), CDCl3, δ (ppm): 42.3 (CH2), 44.4 (2 × CH2), 67.6 (Cq),114.4 (=CH2), 124.8 (2 × CHAr), 126.7 (2 × CHAr), 127.7 (2 × CHAr), 128.9 (2 × CHAr), 135.7 (), 136.5 (=CH),137.4 (
), 139.1 (2 ×
) 156.2 (C=O), 180.7 (C=O).
2.3.4 3-Chloro-1-(4-vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′- naphthalene]-2,5-dione (6-Cl)
Starting from 6, this product was isolated as a white solids in 85% yield after crystallization from ethyl acetate; mp 171–179 °C; Elemental Analysis: Calc. C 68.76%; H 5.22%; N 7.64%; Found: C 68.79%; H 4.89%; N 7.98%; IR (HCB): ν 1768, 1711 cm−1; 1H NMR, (700 MHz), DMSO-d6, δ (ppm): 1.82 (m, 1H, CH2), 1.84 (m, 1H, CH2), 2.80 (m, 1H, CH2), 2.88 (m, 2H, CH2), 3.08 (m, 1H, CH2), 4.50 (s, 2H, CH2), 5.24 (m, 1H,=CH2), 5.70 (m, 1H,=CH2), 6.70 (m, 1H,=CH), 7.01 (m, 4H, CHAr), 7.19 (d, J = 8.4 Hz, 2H, CHAr), 7.41 (d, J = 8.4 Hz, 2H, CHAr); 13C NMR, (100 MHz), DMSO-d6, δ (ppm): 24.7 (CH2), 30.1 (CH2), 36.8 (CH2), 40.8 (CH2), 60.7 (Cq), 114.4 (=CH2), 125.8 (2 × CHAr), 126.0 (2 × CHAr), 128.7 (2 × CHAr), 129.0 (2 × CHAr), 134.7 (), 136.2 (=CH), 136.3 (
), 136.5 (2 ×
) 157.6 (C=O), 180.1 (C=O).
3. Results and discussion
All of hydantoins used for monomers preparation were synthesized by improved one-step Bucherer reaction of ketone with variable yields (70–90% yield).[Citation21,Citation22]
Although the 5,5-dimethyl hydantoin is one of the hydantoins most successfully applied for preparation of N-halamines, the methods of synthesis are particularly difficult and the yield does not exceed 40%.[Citation21] From the present approach, 5,5-dimethyl hydantoin was prepared from acetone with 70% yield after crystallization. This optimized method is much easier and more efficient than those available in literature. Spirohydantoins (2-indanone hydantoin and 2-tetralone hydantoin) were obtained in the same way giving products with 70 and 90% yield, respectively.
Novel monomers: 5,5-dimethyl-3-(4-vinylbenzyl)imidazo-lidine-2,4-dione (1), 1-(4-vinylbenzyl)-1′,3′-dihydrospiro-[imidazolidine-4,2′-indene]-2,5-dione (2), and 1-(4-vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (3) and their N-chlorinated forms 1-chloro-5,5-dimethyl-3-(4-vinylbenzyl)imidazolidine-2,4-dione (1-Cl), 3-chloro-1-(4-vinylbenzyl)-1′,3′-dihydrospiro[imidazolidine-4,2′-indene]-2,5-dione (2-Cl), and 3-chloro-1-(4-vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (3-Cl) (Figure ) bearing a polymerizable fragment were synthesized in simple and efficient procedures using early prepared hydantoins and N-alkylation reaction (Figure ).
The N-alkylation reaction is a well-known one-step process to obtain hydantoin-containing compound.[Citation23] The reaction of 5,5-dimethyl hydantoin with 4-vinylbenzyl chloride according to the reference [Citation24] (methanol mixture with potassium hydroxide solution at 60 C) gave the product with yield not exceeding 25% similarly as it was reported by other authors.[Citation25] When the same procedure was used for synthesis of spirohydantoin-containing monomers, the products were not achieved. In order to improve the yield and to obtain novel spirohydantoin-containing monomers, the reaction of hydantoins with 4-vinylbenzyl chloride was modified and carried out in DMF with potassium carbonate at room temperature.(Figure ) After overnight stirring, the monomers were obtained with good yields: 60% (1), 60% (2), and 65% (3). Repeating this procedure always leads to the same products with the same yield and purity.
All of the prepared monomers have been chlorinated to N-halamine compounds. There are three methods of N-chlorination reaction of hydantoins: passing chlorine gas through an alkaline solution of hydantoins,[Citation26] treatment of the hydantoin with an sodium hypochlorite solution,[Citation27] and reaction with TCICA.[Citation28] Reaction of the monomers with aqueous sodium hypochlorite solution gave non-chlorinated substrates; therefore, the TCICA-mediated procedure was employed for N-halamine preparation to gave chlorinated monomers with 85–90% yield after recrystallization.
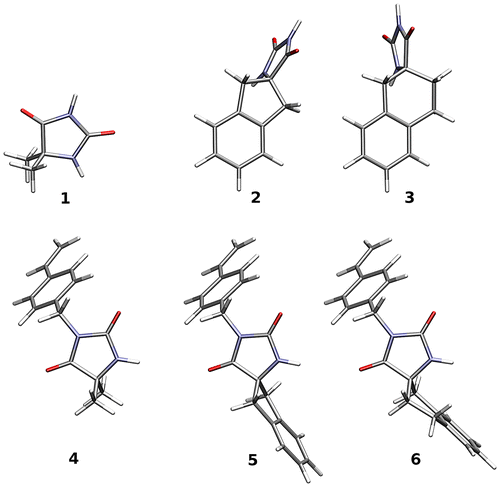
Since the obtained compounds exist in amorphous form in order to investigate the possible conformations, quantum chemical calculations have been performed.
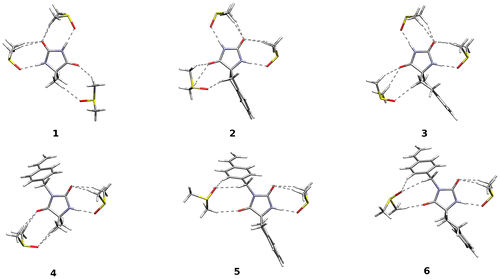
The geometry of all the systems was optimized within the B3LYP/6-311G(d,p) approach, and the nature of stationary points verified by the harmonic vibrational analysis. Different local minima on the potential energy surface were identified for molecules. The lowest energy conformations in the gas phase are presented in Figure . In order to include the solvent influence, the calculations were carried out with hybrid implicit (PCM) and explicit (several DMSO molecules hydrogen-bonded to the N–H and C=O groups) solvent description in addition to vacuum calculations. All the calculations have been performed in Gaussian09 program package.[Citation29]
Though the systematic study would require the subsequent inclusion of the solvent molecules in solvation shells up to the saturation of the results, the chemical intuition prompts that the crucial is the DMSO molecules creating hydrogen bonds with the solute. Thus, only the most important configurations were analyzed: with up to three DMSO molecules for hydantoins and with up to two molecules for non-chlorinated monomers. The comparison of the obtained computational results for various structures with experimental spectroscopic data (see Tables and ) leads to the conclusion that the complexes presented in Figure allow for reliable description of the investigated compounds. The computed frequencies with no scaling factor exhibit a satisfactory correspondence with experimental both in case of N–H and C=O stretching. Calculations of chemical shifts performed with hybrid implicit and explicit solvent model permit to reproduce experimental data even for the labile proton.
Table 1. IR frequencies for N–H and C=O stretching vibrations [cm−1] from experiment and PCM/B3LYP/6-311G(d,p) calculations for structures presented in Figure .
Table 2. 1H NMR chemical shifts [ppm] for investigated compounds from experiment and PCM/B3LYP/6-311G(d,p) calculations for structures presented in Figure .
Since the calculations performed here concern only static structures, any rotation around the single bond is not allowed. Thus, the NMR signals arising from the equivalent protons in experiment are split into more signals in theoretical investigation. For instance, this is the case for the CH3 chemical shifts, where the methyl group rotation in experiment produces only one signal at 1.31 ppm corresponding to the six methyl protons for both molecules 1 and 4, and the theoretical calculations give six separated peak positions outstretched from 0.86 to 2.34 ppm for 1 and from 0.70 to 2.08 ppm for 4. However, the averaging of the theoretical data provides a satisfactory correspondence with the experiment. All of the synthesized monomers have been used in homo- and copolymerization, and the results will be reported soon.
In conclusion: novel styrenic monomers with potential biocidal activity: 5,5dimethyl-3-(4-vinylbenzyl)imidazo-lidine-2,4-dione (1), 1-(4-vinylbenzyl)-1′,3′-dihydrospiro-[imidazolidine-4,2′-indene]-2,5-dione (2), and 1-(4-vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (3) and their N-chlorinated forms 1-chloro-5,5-dimethyl-3-(4-vinylbenzyl)imidazolidine-2,4-dione (1-Cl), 3-chloro-1-(4-vinylbenzyl)-1′,3′-dihydrospiro[imidazolidine-4,2′-indene]-2,5-dione (2-Cl), and 3-chloro-1-(4-vinylbenzyl)-3′,4′-dihydro-1′H-spiro[imidazolidine-4,2′-naphthalene]-2,5-dione (3-Cl) were synthesized in simple and efficient procedures with good yields and high purities. All of the resulting compounds were characterized by FTIR and NMR spectroscopy, and in all cases, the spectra were found to be in consistent with the expected structures.
The experimental work was supported by the theoretical calculations with good correlations between measured and calculated data.
Acknowledgments
Authors are indebted to Prof. Halina Kaczmarek for fruitful discussions and gratefully acknowledge Wrocław Center of Networking and Supercomputing and Academic Computer Centre Cyfronet AGH Kraków for generous allotment of computer time. Financial support from Faculty of Chemistry, Nicolaus Copernicus University, Grant – 7/2012 is gratefully acknowledged.
References
- John Santhosh Kumar S, Subramanian K. Synthesis, characterization, antimicrobial activity, and adhesive property of amino benzyl group substituted acrylic/methacrylic polymers and their copolymer. Int. J. Polym. Mater. Biomater. 2013;62:627–634.
- Shah SSA, Patel URA, Surati KRB. Synthesis, thermal behavior, and antimicrobial activity of polyether based on 4,7-dichloro 6-nitroquinazoline. Int. J. Polym. Mater. Biomater. 2012;61:437–447.
- Chauhan NPS. Spectral and thermal investigation of designed terpolymers bearing p-acetylpyridine oxime moieties having excellent antimicrobial properties. Des. Monomers Polym. 2013;16:543–555.
- Liu ZA, Liao QA, Yang DA, Gao YA, Luo XA, Lei ZA, Li HAB. Well-defined poly(N-isopropylacrylamide) with a bifunctional end-group: synthesis, characterization, and thermoresponsive properties. Des. Monomers Polym. 2013;16:465–474.
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322.
- Tashiro T. Antibacterial and bacterium adsorbing macromolecules. Macromol. Mater. Eng. 2001;286:63–87.
- Kenawy ER, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359–1384.
- Kim SS, Kim J, Huang TS, Whang HS, Lee J. Antimicrobial polyethylene terephthalate (PET) treated with an aromatic N-halamine precursor, m-aramid. J. Appl. Polym. Sci. 2009;114:3835–3840.
- Sun Y, Sun G. Novel regenerable N-halamine polymeric biocides. I. Synthesis, characterization, and antibacterial activity of hydantoin-containing polymers. J. Appl. Polym. Sci. 2001;80:2460–2467.
- Chen Y, Worley SD, Huang TS, Weese J, Kim J, Wei C-I, Williams JF. Biocidal polystyrene beads IV. Functionalized methylated polystyrene. J. Appl. Polym. Sci. 2004;92:363–372.
- Youssef AM, Abdel-Aziz MS. Preparation of polystyrene nanocomposites based on silver nanoparticles using marine bacterium for packaging. Polym. Plast. Technol. 2013;52:607–613.
- Taşdemir M, Uluğ E. Mechanical, morphological and thermal properties of SEBS, SIS and SBR-type thermoplastic elastomers toughened high impact polystyrene. Polym. Plast. Technol. 2012;51:164–169.
- Seeponkai N, Keaitsirisart N, Wootthikanokkhan J, Thanachayanont C, Chuangchote S. Fullerene functionalized polystyrene: synthesis, characterizations, and application in bulk heterojunction polymer solar cells. Int. J. Polym. Mater. Biomater. 2014;63:33–40.
- Namazi H, Heydari A, Pourfarzolla A. Synthesis of glycoconjugated polymer based on polystyrene and nanoporous β-cyclodextrin to remove copper (II) from water pollution. Int. J. Pol. Mater. Biomater. 2014;63:1–6.
- Thenmozhiyal JC, Wong PT, Chiu WK. Anticonvulsant activity of phenylmethylenehydantoins: a structure-activity relationship study. J. Med. Chem. 2004;47:1527–1535.
- Meusel M, Gutshow M. Recent developments in hydantoin chemistry. A review. Org. Prep. Proced. Int. 2004; 36:391–443.
- Abadi AH, Gary BD, Tinsley HN, Piazza GA, Abdel-Halim M. Synthesis, molecular modeling and biological evaluation of novel tadalafil analogues as phosphodiesterase 5 and colon tumor cell growth inhibitors, new stereochemical perspective. Eur. J. Med. Chem. 2010;45:1278–1286.
- Wagner EC, Baizer M. 5,5-Dimethylhydantoin. Org. Synth. 1955;3:323–325.
- Pesquet A, Daich A, Van Hiffte L. General and versatile entry to 4,5-fused polycyclic imidazolones systems. Use of the tandem transposition/pi-cyclization of N-acyliminium species. J. Org. Chem. 2006;71:5303–5311.
- Sun Y, Sun G. Novel refreshable N-halamine polymeric biocides containing imidazolidin-4-one derivatives. J. Polym. Sci., Part A: Polym. Chem. 2001;39:3073–3084.
- Day AR, Kelly CF. N-Menthyl-substituted amides. J. Org. Chem. 1939;4:101–102.
- Zaidlewicz M, Cytarska J, Dzielendziak A, Ziegler-Borowska M. Synthesis of boronated phenylalanine analogues with a quaternary center for boron neutron capture therapy. Arkivoc. 2004;3:11–21.
- Usifoh CO. Anticonvulsant activity of reaction products of 5,5-diphenylhydantoin with substituted methylene bromides. Arch. Pharm. 2001;334:366–368.
- Sun Y, Sun G. Durable and refreshable polymeric N-halamine biocides containing 3-(4′-vinylbenzyl)-5,5-dimethylhydantoin. J. Polym. Sci. 2001;39:3348–3355.
- Chen Y, Wang L, Yu H, Shi Q, Dong X. Synthesis, characterization, and antibacterial activities of novel N-halamine copolymers. J. Mater. Sci. 2007;42:4018–4024.
- Corral RA, Orazi OO. Substitution in the hydantoin ring. III. Halogenation. J. Org. Chem. 1963;28:1100–1104.
- Derosa M, Melenski E, Holder AJ. Reaction of 1-methylpyrrole with 1,3-dichloro-5,5-disubstituted hydantoins: products and AM1 study of intermediates. Heterocycles. 1993;36:1059–1064.
- Whitehead DC, Staples RJ, Borhan B. A simple and expedient method for the preparation of N-chlorohydantoins. Tetrahedron Lett. 2009;50:656–658.
- Gaussian 09, Revision B1, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian Inc.,Wallingford, CT, 2009.